Search found 37 matches
- Sun Mar 15, 2020 7:49 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Standard enthalpies of formation
- Replies: 7
- Views: 622
Re: Standard enthalpies of formation
I highly doubt that we'll be required to memorize enthalpies of formation, so I assume they'll be given on a test. This would be a good question for Dr. Lavelle himself. Remember which ones can be cancelled because they're in their base state, though. You might have to memorize those, but I believe...
- Sun Mar 15, 2020 5:55 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Heat released/ gained
- Replies: 22
- Views: 1073
Re: Heat released/ gained
In relation to melting / freezing points, energy will be required to break bonds (think heating up an ice cube to make it into water). The energy released when bonds are formed is less obvious, but the climate on a humid day is probably a clue.
- Sun Mar 15, 2020 5:35 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Pre-Equilibrium Approach
- Replies: 2
- Views: 318
Re: Pre-Equilibrium Approach
The pre-equilibrium approach is the method that we choose to approach reaction mechanics in our class. The other options would be..... 1) to directly compute the rate law through raw math, differentials, experimental data, and some brute forcing via guess-and-check methods. 2) to identify the rate l...
- Sun Mar 15, 2020 5:18 am
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: order of reaction
- Replies: 6
- Views: 592
Re: order of reaction
I think the furthest we'll be tested on is the number of reactants.
Zeroth Order: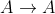
First Order: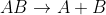
Second Order: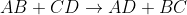
Third Order: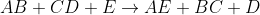
Zeroth Order:
First Order:
Second Order:
Third Order:
- Sun Mar 15, 2020 4:58 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Electrode Mass
- Replies: 10
- Views: 4608
Re: Electrode Mass
I think the conditions are different depending on whether the cell in question is a galvanic cell or a concentration cell. If it's a concentration cell, I think the increased mass might ((key word: MIGHT) change how much of the electrode is being dissolved into the solution, but otherwise it shouldn...
- Sun Mar 15, 2020 4:53 am
- Forum: Balancing Redox Reactions
- Topic: changing half reactions
- Replies: 6
- Views: 541
Re: changing half reactions
You want the cell to transfer electrons from anode to cathode, from negative to positive (it's for this reason that the anode is always on the left and the cathode is always on the right). In order to do that, you usually want the potential difference to be positive. Therefore, the one with the grea...
- Sun Mar 15, 2020 4:42 am
- Forum: First Order Reactions
- Topic: First order vs zero-order
- Replies: 4
- Views: 421
Re: First order vs zero-order
integrated rate laws are designed to express the rate linearly. Always. I'm sure the graphs don't look exactly the same, but if they're both linear and have the same sign of their slope, then that might be why they look so similar. Remember to always take into account what the y-axis is labeled in A...
- Sun Mar 15, 2020 4:27 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Gibbs free energy
- Replies: 18
- Views: 1101
Re: Gibbs free energy
Not only is the standard Gibbs Free Energy measured under standard conditions, it's also kind of the best-case-scenario for the reaction: the most that it will potentially put out. Anything other than that can represent an instantaneous amount, a botched amount, something messing with the experiment...
- Sun Mar 15, 2020 4:20 am
- Forum: Van't Hoff Equation
- Topic: Celcius vs Kelvin for T1 and T2
- Replies: 84
- Views: 7118
Re: Celcius vs Kelvin for T1 and T2
Always always always use Kelvin. Most standard konstants that we use are set for Kelvin, as well -- and I wouldn't suggest that you use the R values (for example) that use Celsius.
- Sun Mar 15, 2020 4:16 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Including H2O in Cell Diagram
- Replies: 3
- Views: 636
Re: Including H2O in Cell Diagram
Correct. I believe by writing (aq) in the first place instead of calling the component (l) implies that that the reactant or product is not purely on its own and is suspended in a liquid medium - in this case, water.
- Sun Mar 15, 2020 4:03 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: A
- Replies: 2
- Views: 320
Re: A
k=Ae^{-E_{a}/RT} The "A" component is a probability out of a total 1.00, and represents the chance that the molecules are in the correct orientation when they collide. I think we're making the experimental assumption that A doesn't adversely affect reactions modeled with the Arrhenius equ...
- Mon Feb 10, 2020 12:47 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Entropy of universe
- Replies: 3
- Views: 305
Re: Entropy of universe
Since S=0 represents a perfectly ordered material ( aka equilibrium ), a positive value for S means that the system has excess energy of some sort that will dissipate through heat, work, phase change, etc. Therefore, since the system will want to somehow decrease its energy to reach a more stable st...
- Mon Feb 10, 2020 12:29 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Reversible and Irreversible Reactions
- Replies: 3
- Views: 454
Re: Reversible and Irreversible Reactions
The lines are blurry, but generally you can call a reaction irreversible if it uses up all (or a considerable amount of) the reaction materials in its reaction, such as an explosion. I'm not sure if you'll usually be told, but so long as the conditions of the reaction environment means that ...
- Mon Feb 10, 2020 12:19 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: % dissociation
- Replies: 5
- Views: 372
Re: % dissociation
% disassociation is a measurement of how much an acid has "dissociated" or disconnected into its constituent parts in a solution. take for example iodic acid HIO\underset{3}{ } + H\underset{2}{ }O \rightleftharpoons IO\underset{3}{ }^{-} + H\underset{3}{ }O^{+} In the solution of H 2 O , t...
- Mon Feb 10, 2020 12:07 am
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Kelvin or Celsius?
- Replies: 86
- Views: 5900
Re: Kelvin or Celsius?
Kelvins are always used when applying the ideal gas law PV=nRT The reason this is done is because Kelvins are more mathematically accurate in terms of chemically applying the excited state of the molecules in a gas to your calculations. zero K is the same as zero energy in the molecules, and is ther...
- Mon Feb 10, 2020 12:02 am
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: constant pressure system
- Replies: 2
- Views: 240
Re: constant pressure system
An example of a constant pressure system is a piston-arranged gas container. The expansion of the gas allows it to push the piston away, maintaining the same internal pressure by expanding its volume. In contrast, a constant volume system has a hard limit on its volume, so if the gas tries to expand...
- Sun Feb 02, 2020 10:46 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Delta H
- Replies: 2
- Views: 213
Re: Delta H
- Sun Feb 02, 2020 10:36 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: State Function
- Replies: 4
- Views: 302
Re: State Function
Internal energy is a quality inherent in a system, whereas heat and work are dependent on other qualities (e.g. mass of substances involved)
- Sun Feb 02, 2020 10:31 pm
- Forum: Phase Changes & Related Calculations
- Topic: Heating Curve
- Replies: 6
- Views: 1054
Re: Heating Curve
The areas on the graph where the lines seem to go flat are areas in which the heat being added to the substance only serves to contribute to the substance's phase change as opposed to increasing the temperature of the substance, whereas areas where the function increases are indicative of temperatur...
- Sun Feb 02, 2020 10:21 pm
- Forum: Phase Changes & Related Calculations
- Topic: Pressure in an Open Beaker
- Replies: 10
- Views: 635
Re: Pressure in an Open Beaker
Pressure of the beaker is not always technically constant, but because the container of the gas is changed to the environment that the experiment is taking place in as opposed to the inside of the beaker itself, then it can be assumed that the reaction makes a negligible difference in the environmen...
- Sun Feb 02, 2020 10:13 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Phase change
- Replies: 20
- Views: 874
Re: Phase change
Because heat (energy) needs to be added to the ice in order to make it undergo a phase change into liquid form,  is positive
is positive
- Sun Feb 02, 2020 10:10 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: q vs H
- Replies: 9
- Views: 498
Re: q vs H
The amount of heat transferred should be a value for H, but its effect on a substance might be measured with respect to volume of said substance. Only then does it become associated with q
- Sun Jan 26, 2020 11:49 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Standard Reaction Enthalpies
- Replies: 3
- Views: 300
Re: Standard Reaction Enthalpies
Most of the time, I think you can assume that either a noble gas or a diatomic halogen are already at their standard state.
Otherwise, they definitely have to tell you.
Otherwise, they definitely have to tell you.
- Sun Jan 26, 2020 11:47 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Heat Capacity of Water
- Replies: 6
- Views: 898
Re: Heat Capacity of Water
Heat capacity is calculated using the equation C=\binom{E}{\Delta T} where " C " is the Heat Capacity, " E " is the energy added per gram of substance , and " ΔT " is the change in temperature. Since the energy added is in the same unit (J), the mass of water heated is ...
- Sun Jan 26, 2020 11:29 pm
- Forum: Phase Changes & Related Calculations
- Topic: Solids, liquids, and gases
- Replies: 4
- Views: 162
Re: Solids, liquids, and gases
They don't? Water is a kind of unique molecule because of how strong its intermolecular interactions are. The heat necessary to break the dense web of Hydrogen Bonds is therefore very high. Any molecule that can sufficiently bond to other molecules in such an efficient way will also be likely to hav...
- Sun Jan 26, 2020 11:09 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Standard enthalpies of formation
- Replies: 7
- Views: 622
Re: Standard enthalpies of formation
I highly doubt that we'll be required to memorize enthalpies of formation, so I assume they'll be given on a test. This would be a good question for Dr. Lavelle himself. Remember which ones can be cancelled because they're in their base state, though. You might have to memorize those, but I believe ...
- Sun Jan 26, 2020 11:05 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: ICE
- Replies: 20
- Views: 940
Re: ICE
An ICE chart is used whenever you're asked to calculate what the effect was of a reaction on the affected substances' concentration or pressure in their container. Notice how whenever you write the "E" (equation) part of the ICE chart that you write products over reactants? Well, the whole...
- Sun Jan 19, 2020 8:13 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Test 1 Acids and Bases Topic
- Replies: 9
- Views: 554
Re: Test 1 Acids and Bases Topic
The most confusing thing that I experienced was telling the difference between pH / pOH and K A / K B . K should be the standard to which the reaction will be measured ((like how a cold/warm object will approach the ambient temperature of a room, so too will an acid/base reaction's Q value approach ...
- Sun Jan 19, 2020 8:01 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Strong Acids/Bases vs. Weak Acids/Bases
- Replies: 9
- Views: 261
Re: Strong Acids/Bases vs. Weak Acids/Bases
In general, the simpler compounds directly containing an H or an OH that can be easily dissociated are the ones that you should be looking at. But if you'd like me to be more specific . . . THE TABLE THAT YOU NEED TO MEMORIZE pg F75, TABLE J.1 ("Common Aqueous Strong Acids and Bases")...
- Sun Jan 19, 2020 7:54 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Stoichiometric coefficients for pH/pOH
- Replies: 3
- Views: 203
Re: Stoichiometric coefficients for pH/pOH
No, you don't need to do that because during the process of calculating [H3O+] or [OH-], you should have already taken the stoichiometric coefficients into account in your ICE chart or KA / KB calculation
- Sun Jan 19, 2020 7:46 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: When do we use the equilibrium sign?
- Replies: 7
- Views: 319
Re: When do we use the equilibrium sign?
Technically almost every reaction doesn't fully dissociate, and will tend to return to its products at least a small amount. If the reaction lies heavily enough one way or another, then we can usually write an arrow going in just that direction.
- Tue Jan 14, 2020 1:13 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Why Ignore Liquid or Solid Volume When Calculating K
- Replies: 7
- Views: 275
Why Ignore Liquid or Solid Volume When Calculating K
Why is it that when calculating for the KP (aka the Equilibrium Constant via pressures), that we don't take into account the volume taken up by liquid or solids in the container with the gas? Since they're hard to compress, wouldn't that reduce the amount of volume available for the gases?
- Sun Jan 12, 2020 8:31 pm
- Forum: Ideal Gases
- Topic: The Ideal Gas Law
- Replies: 3
- Views: 133
Re: The Ideal Gas Law
The non-ideal gas law is best summarized as: (P+\binom{an^2}{V^2})(V-nb)=nRT "a" is a constant accounting for the molecular attraction of individual gas molecules to other gas molecules "b" is a constant accounting for the Volume of the molecules themselves At STP...
- Sun Jan 12, 2020 8:22 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Studying tips
- Replies: 10
- Views: 506
Re: Studying tips
Definitely do all of the homework problems assigned in the syllabus. It's too easy to sorta phone it in and only do 5 for the homework. My suggestion is to do all of them, then choose the 5 that you think were the best or that you were the most proud of yourself for doing. Also, remember to use all ...
- Sun Jan 12, 2020 8:16 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kp
- Replies: 6
- Views: 425
Re: Kp
K P is used to deal with the partial pressures of an equation at equilibrium, (P=Pressure). Since, as explained in lecture, you can't compress solids and it takes an absurd amount of energy to compress liquids a small amount, their affect on the pressure of a reaction container are taken to be negli...
- Sun Jan 12, 2020 8:12 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Inert Gases
- Replies: 4
- Views: 181
Re: Inert Gases
Basically any Group 18 element in a gaseous state is an inert gas. We say this because although they are able to react with other chemical compounds, they only become reactant in those cases usually after extreme pressure, temperature, electricity, or some other kind of energy input. Thus, as explai...
- Sun Jan 12, 2020 8:04 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: stoichiometric coefficients
- Replies: 5
- Views: 360
Re: stoichiometric coefficients
As stoichiometric coefficients represent the quantity of molecules of a chemical that is affecting or being effected by the reaction, the model for calculating Q or K represents the difference in how the amount of those chemicals affects the reaction per amount of chemical added. In this case, when ...

