Search found 116 matches
- Sun Mar 15, 2020 9:28 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Arrhenius eq
- Replies: 6
- Views: 448
Re: Arrhenius eq
And the ln(k) = -Ea/RT + ln(A) version is useful when you're comparing two temperature's k values. The equation becomes ln(k2/k1) = (Ea/R)((1/T1) - (1/T2))
- Sun Mar 15, 2020 9:26 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Fast Reactions
- Replies: 4
- Views: 397
Re: Fast Reactions
Think of it like a bottleneck. The slowest step is what determines the rate, because the reaction can't get any slower (it can get faster, but what we want is the maximum amount of reactant change possible which comes with the slowest step). The slowest step is the rate determining step.
- Sun Mar 15, 2020 9:23 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Different methods
- Replies: 2
- Views: 380
Re: Different methods
Each approach varies in usefulness depending on the context of the problem, the complexity of the reaction, and what information is provided. The differential rate law approach expresses the reaction rate in terms of changes in the concentration of one or more reactants (Δ[R]) over a specific time ...
- Sun Mar 15, 2020 9:23 pm
- Forum: General Rate Laws
- Topic: Overall Order
- Replies: 6
- Views: 422
Re: Overall Order
Another way to say this is that if you have the general rate = k[A]^m[B]^n, the overall order would be m+n
(aka add the exponents of each reactant)
(aka add the exponents of each reactant)
- Sun Mar 15, 2020 9:21 pm
- Forum: First Order Reactions
- Topic: Pseudo first order
- Replies: 2
- Views: 304
Re: Pseudo first order
You would use "pseudo" rate orders when one reactant is in excess so it doesn't really affect the rate. The pseudo constant k' is k[reactant in excess]^m. Using the pseudo constant, you can determine the order of the reactant not in excess and using experimental data, you can determine k'....
- Sun Mar 15, 2020 9:17 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Ea, rate, and temperature
- Replies: 4
- Views: 962
Re: Ea, rate, and temperature
You can see this through the other form of the Arrhenius Equation: k = Ae^(-Ea/RT) ln(k1) = -Ea/RT1 + ln(A) ln(k2) = -Ea/RT2 + ln(A) ln(A) = ln(k2) + Ea/RT2 ln(A) = ln(k1) + Ea/RT1 ln(k2) + Ea/RT2 = ln(k1) + Ea(RT1) ln(k2/k1) = Ea/RT1 - Ea/RT2 ln(k2/k1) = Ea/R((1/T1)-(1/T2)) where a high positive Ea...
- Sun Mar 15, 2020 9:07 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Test 2 Q6 part b ii
- Replies: 5
- Views: 400
Re: Test 2 Q6 part b ii
Additionally, changing the mass of the electrode (the solid) doesn't affect anything. Only changing the concentration of aqueous ions will change E cell (because E cell = E standard - (RT/nF)lnQ
- Sun Mar 15, 2020 9:05 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Athena
- Replies: 34
- Views: 3183
Re: Athena
Thank you for everything you've done these past two quarters, Dr. Lavelle! I know this situation was very unexpected and difficult to manage, but I think you handled it wonderfully. *high five* :) Thank you for all you've done this quarter! I know it must have been really difficult remaking the fin...
- Tue Mar 10, 2020 4:54 pm
- Forum: Administrative Questions and Class Announcements
- Topic: ENDGAME Review Session
- Replies: 71
- Views: 5669
Re: ENDGAME Review Session
Abigail Sanders 1E wrote:Will this review session still be held now that all classes are cancelled? Lyndon's sessions are normally the most helpful thing before the finals/midterms.
Facts! If not hosted in person, maybe livestreamed...?
- Tue Mar 10, 2020 10:12 am
- Forum: Administrative Questions and Class Announcements
- Topic: ENDGAME Review Session
- Replies: 71
- Views: 5669
Re: ENDGAME Review Session
Thank you Lyndon for putting so much time into us. I’m not alone in saying that your weekly workshops, your packets, and your review sessions are probably the only reason I passed 14A and (hopefully!) pass 14B.
P.S. Endgame is a suitable name for replacing Gummy Worms
P.S. Endgame is a suitable name for replacing Gummy Worms
- Mon Mar 09, 2020 12:02 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: n in NFE
- Replies: 64
- Views: 3780
Re: n in NFE
After you balance the redox reactions so that the "number" of electrons from the half reactions cancel, that common "number" of electrons is your n
- Mon Mar 09, 2020 12:00 am
- Forum: Zero Order Reactions
- Topic: Order reactions and rate
- Replies: 2
- Views: 215
Re: Order reactions and rate
The rate law shows the order of each reactant. The order of an individual reactant is the power it's raised to (zero if it's not in the rate law at all), the overall reaction order is the sum of all individual reactant orders. The overall reaction order is N + M in the equation Lavelle showed in cl...
- Sun Mar 08, 2020 11:59 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Adding Pt(s) to a Cell Diagram
- Replies: 14
- Views: 902
Re: Adding Pt(s) to a Cell Diagram
Add Pt(s) whenever there is no solid in the electrodes
- Sun Mar 08, 2020 11:59 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: midterm question// Concentration ratio [ENDORSED]
- Replies: 9
- Views: 724
Re: midterm question// Concentration ratio [ENDORSED]
When pKa is smaller than pH, that means that Ka is larger than the H+ concentration, meaning that the products (H+ concentration included) is larger, so since there is a higher H+ concentration than the neutral pH indicates, this means that the acid protonates.
- Sun Mar 08, 2020 11:56 pm
- Forum: General Rate Laws
- Topic: Amount of product formed
- Replies: 2
- Views: 201
Re: Amount of product formed
I'm not sure it's necessarily easier, but it is standard and lets us know that we're comparing the same thing when looking at different reactions or different concentrations of reactants. If we didn't have this standard, we would be comparing rates from all different points at a reaction, making it...
- Sun Mar 01, 2020 8:03 pm
- Forum: Balancing Redox Reactions
- Topic: Test 2
- Replies: 19
- Views: 982
Re: Test 2
DHavo_1E wrote:Hello,
Will we have to know about entropy and enthalpy? Thank you!
Probably only in connection to the Van'T Hoff Equation.
- Sun Mar 01, 2020 8:02 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: 6M.7 Strength as Reducing Agent
- Replies: 3
- Views: 369
Re: 6M.7 Strength as Reducing Agent
A similar question like this popped up on Test 2 last year, but it clarified which oxidation states were being used (i.e. Fe3+ to Fe2+ instead of Fe3+ to Fe). The UA said that in a scenario like this where you have to order which metal is a better reducing/oxidizing agent, they would clarify, so don...
- Sun Mar 01, 2020 7:54 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: cell diagrams
- Replies: 8
- Views: 499
Re: cell diagrams
Yes, standard convention is that anode will be on the left and cathode on the right
- Sun Mar 01, 2020 7:43 pm
- Forum: Balancing Redox Reactions
- Topic: homework topic 6K
- Replies: 4
- Views: 377
Re: homework topic 6K
Because you have to balance the S2O3 and SO4, you have to add H2O to get the additional oxygens to form the SO4 product, and then since you added hydrogens as part of the H2O, you need to add H+ to the reactants.
- Sun Mar 01, 2020 7:39 pm
- Forum: Balancing Redox Reactions
- Topic: basic solution
- Replies: 4
- Views: 329
Re: basic solution
For basic solutions, you would use H2O to balance the oxygens, then add OH- (instead of H+) to balance the hydrogens, however, since we added additional oxygens from the OH-, we have to add water again, but this time to the other side, to balance the additional oxygens out. Honestly, basic solutions...
- Mon Feb 24, 2020 9:19 pm
- Forum: Balancing Redox Reactions
- Topic: 6K.3 d
- Replies: 2
- Views: 247
Re: 6K.3 d
Yes, there is a typo. It should be Cl2 -> HClO + Cl-
- Mon Feb 24, 2020 9:17 pm
- Forum: Balancing Redox Reactions
- Topic: 6K.3
- Replies: 1
- Views: 153
Re: 6K.3
I'm assuming you're talking about 6K.3 a) That one was hard for me too, but basically, you balance the 5H20 on the reactant side with 10H+ on the products side. Because you have 10H+, there are 10e- along side it. However, there is a charge difference from the reactants to the products where S2O3^2-...
- Mon Feb 24, 2020 9:13 pm
- Forum: Balancing Redox Reactions
- Topic: Flipping Reactions
- Replies: 4
- Views: 298
Re: Flipping Reactions
It's mainly on the overall Ecell voltage. Since Ecell will always be positive, flip the one that will give you that overall positive Ecell.
- Sun Feb 23, 2020 2:43 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: midterm question// Concentration ratio [ENDORSED]
- Replies: 9
- Views: 724
Re: midterm question// Concentration ratio [ENDORSED]
What I did was I found Ka and used the pH given to find the concentration of H+. Since the equation for Ka is [H+][A-]/[HA], you can find the ratio of conjugate base to acid by dividing Ka by the concentration of H+. But how would you know if it stays as a salt in the stomach or becomes something e...
- Sun Feb 23, 2020 2:40 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: midterm question// Concentration ratio [ENDORSED]
- Replies: 9
- Views: 724
Re: midterm question// Concentration ratio [ENDORSED]
You have to think about how Ka=[A][H+]/[CB] (CB being conjugate base). By rearranging the equation, you can get Ka/[H+] = [A]/[CB], which is a ratio. Were we given this equation anywhere? I mean like the original, not manipulated, equation? And I don't recall using it before the midterm but I may b...
- Sun Feb 23, 2020 2:28 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: positive E naught
- Replies: 2
- Views: 175
Re: positive E naught
You can think about the relationship between 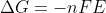 and since you know a spontaneous reaction is when
and since you know a spontaneous reaction is when  is negative, this only happens when
is negative, this only happens when 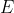 is positive (since n and F are always positive).
is positive (since n and F are always positive).
- Sun Feb 23, 2020 2:25 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagrams
- Replies: 2
- Views: 175
Re: Cell Diagrams
So Dr. Lavelle mentioned this in lecture. You need a solid electrode for the cell diagram and when there isn't for one side you need to have a element that wouldn't interact with the solution . An inert electrode such as Pt is necessary when both oxidized and reduced species are in the same solutio...
- Sun Feb 16, 2020 9:04 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: spontaneity
- Replies: 39
- Views: 1929
Re: spontaneity
As everyone above said, Gibbs free energy is how you determine if a reaction is spontaneous or not. However, it might be important to remember that the reason Gibbs free energy is so clear cut is that it’s because it’s based off total entropy which is always increasing.
- Fri Feb 14, 2020 9:53 am
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: reaction entropy
- Replies: 7
- Views: 598
Re: reaction entropy
You can also calculate deltaG the same way. I think for any state function you can calculate it this way because all we care about is the change from initial to final so in reactions, the “final” stage is the products and the “initial” stage is the reactants, so you would do products - reactants, w...
- Fri Feb 14, 2020 9:44 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs Free energy concept
- Replies: 16
- Views: 865
Re: Gibbs Free energy concept
The definition given in class is that Gibbs free energy is the energy given off that’s now available (“free”) to do work. Basically, if deltaG is negative, that means it gives off energy that is now “free” to do work, which results in a spontaneous reaction. The important thing to know is that when ...
- Fri Feb 14, 2020 9:35 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: midterm question// Concentration ratio [ENDORSED]
- Replies: 9
- Views: 724
Re: midterm question// Concentration ratio [ENDORSED]
What I did was I found Ka and used the pH given to find the concentration of H+. Since the equation for Ka is [H+][A-]/[HA], you can find the ratio of conjugate base to acid by dividing Ka by the concentration of H+.
- Sun Feb 09, 2020 11:27 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: State Functions (Pizza Rolls 3H)
- Replies: 1
- Views: 169
State Functions (Pizza Rolls 3H)
I don't know if this is the right section to post this question under, but I know state functions is a topic in thermodynamics and this was the closest category I could find in asking this question. In Lyndon's review session, he mentioned that the answer to 3H "Which of the following are not s...
- Sun Feb 09, 2020 11:20 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Midterm Review #12b
- Replies: 4
- Views: 291
Re: Midterm Review #12b
For this problem, you have 3 mini reactions. The first is the rise in temperature of the reactants. The second is the change from reactants to products at the higher temperature. The third is the decrease in temperature of the products. In other words, the three steps are 1) the enthalpy change of t...
- Sun Feb 09, 2020 11:16 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Isothermal
- Replies: 9
- Views: 540
Re: Isothermal
Not necessarily. Isothermal means that there is no temperature change (aka deltaT = 0) whereas reversibility has to do with entropy and if total entropy is 0 (aka in a reversible system) or if entropy of the surroundings is 0 (aka in an irreversible system).
- Sun Feb 09, 2020 11:12 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Negative entropies
- Replies: 3
- Views: 172
Re: Negative entropies
We cannot have negative entropies because entropy is defined as the energy needed to break a bond, which is always positive (aka it always requires energy to break a bond)
- Sun Feb 09, 2020 11:09 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: gas constant
- Replies: 3
- Views: 132
Re: gas constant
If you have L.atm and want to get to joules, you wouldn't use a gas constant, but you would multiply by 101.325 J.L^-1.atm^-1 to get the L.atm to cancel out with only J left
- Tue Feb 04, 2020 9:18 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 4E.9
- Replies: 6
- Views: 248
Re: 4E.9
Benzene is more stable and less reactive than would be predicted from its Kekulé structures. Use the data in Table 4E.3 to calculate the lowering in molar energy when resonance is allowed between the Kekulé structures of benzene. When we calculate the molar energy without resonance, we get 2880 kJ,...
- Sun Feb 02, 2020 2:31 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Delta U = Q
- Replies: 8
- Views: 212
Re: Delta U = Q
Adding to this, you know work = 0 when 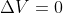 because
because 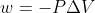 so 0 for
so 0 for 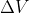 (aka there is no change in volume, aka the volume is constant) makes the entire work equation 0
(aka there is no change in volume, aka the volume is constant) makes the entire work equation 0
- Sun Feb 02, 2020 2:27 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: pressure v. volume
- Replies: 3
- Views: 133
Re: pressure v. volume
For equations with constant pressure, you can assume that 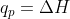 , and for equations with constant volume,
, and for equations with constant volume, 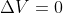 so w = 0 (since
so w = 0 (since 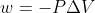 )
)
- Sun Feb 02, 2020 2:20 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: energy transfered
- Replies: 2
- Views: 98
Re: energy transfered
You can think of \Delta{U} = q + w where q = heat and w = work. Since you know q_{p} (heat with constant pressure) can also be considered \Delta{H} , you now have \Delta{U} = \Delta{H} + w . To make \Delta{U} = \Delta{H} , you have to make work equal 0. Since w = -P\Delta{V} , work equals 0 when \De...
- Sun Feb 02, 2020 2:12 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Extensive vs. intensive property
- Replies: 3
- Views: 204
Re: Extensive vs. intensive property
Another example is the difference between specific heat capacity and heat capacity. Specific heat capacity is the heat required to raise the temperature of an object per mole. Since the "per mole" is there, regardless of how many moles are added, the heat required per mole is the same, the...
- Sun Jan 26, 2020 4:07 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Lewis Structures Method 2
- Replies: 6
- Views: 178
Re: Lewis Structures Method 2
It also provides context on why we add and subtract bond enthalpies to calculate the overall change in enthalpy. When we draw Lewis structure, we show that we know that it's because of the bonds breaking and forming that we get changes in enthalpy.
- Sun Jan 26, 2020 3:59 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Enthalpies
- Replies: 3
- Views: 139
Re: Enthalpies
Standard enthalpy of formation (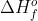 ) is the standard reaction enthalpy (
) is the standard reaction enthalpy (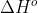 ) to form one mole of the substance, which explains why the units for
) to form one mole of the substance, which explains why the units for 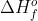 is kJ/mol
is kJ/mol
- Sun Jan 26, 2020 3:54 pm
- Forum: Phase Changes & Related Calculations
- Topic: Standard State
- Replies: 2
- Views: 193
Re: Standard State
However, if they give you the standard state of the entire reaction (i.e. if they said that 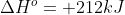 ) you don't have to worry about calculating each individual element's standard state, because they've shortened your work for you by giving you the overall standard state.
) you don't have to worry about calculating each individual element's standard state, because they've shortened your work for you by giving you the overall standard state.
- Sun Jan 26, 2020 3:22 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: hess's law
- Replies: 5
- Views: 254
Re: hess's law
Because enthalpy is a state function, meaning it depends on the physical state of the molecule, enthalpy changes are additive.
- Tue Jan 21, 2020 11:24 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Where is the Practice WS?
- Replies: 3
- Views: 148
- Tue Jan 21, 2020 9:51 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Where is the Practice WS?
- Replies: 3
- Views: 148
Where is the Practice WS?
Hi!
Where can we find the practice test ws?
Where can we find the practice test ws?
- Sat Jan 18, 2020 6:32 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6D13 part d
- Replies: 2
- Views: 108
6D13 part d
We are given .20 M of C6H5NH2 which has a Kb of 4.3 x 10^-10 and we have to find the pH. The first way I tried it was finding Ka by 1.0x10^-14/4.3x10^-10 and then finding the concentration of H30+ and then calculating pH from it with -log[H3O+]. However, this got me the wrong answer. The second way ...
- Sat Jan 18, 2020 4:21 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Acids and Bases Ice Box
- Replies: 5
- Views: 171
Re: Acids and Bases Ice Box
If one of the products in the reaction is H3O+, use Ka because the original compound given was an acid.
If one of the products in the reaction is OH-, use Kb because the original compound given was a base.
If one of the products in the reaction is OH-, use Kb because the original compound given was a base.
- Sat Jan 18, 2020 4:17 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Test 1
- Replies: 6
- Views: 340
Re: Test 1
I believe so. It's Topic 1 and 2 on the syllabus.
- Sat Jan 18, 2020 4:16 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Pressure
- Replies: 3
- Views: 132
Re: Pressure
Yes, increasing the pressure by decreasing the volume makes the side of the reaction (products or reactants) with less moles of gas favorable. The reason is that the concentrations of everything changes by the amount of volume lowered (aka if V halves, the concentration would double) which alters Q,...
- Sat Jan 18, 2020 4:12 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Exercise 6A.19
- Replies: 4
- Views: 175
Re: Exercise 6A.19
I also got 3.2 x 10^-15 M
- Sat Jan 18, 2020 4:10 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: pKa versus pH
- Replies: 3
- Views: 136
pKa versus pH
I understand the difference between pKa and pH in terms of the formula, but I don't really understand how they differ conceptually. Adding to this, I don't understand why when pKa > pH, it's acidic and when pKa < pH, it's basic. Does it have something to do with the conjugate base? It has to be righ...
- Fri Jan 10, 2020 1:16 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Calculating eq with percentages of reaction
- Replies: 3
- Views: 88
Re: Calculating eq with percentages of reaction
Adding to this, for example, if you were asked to find K and you were given that N2 had an initial concentration of .1 mol and 60% of it turned into NH3 at equilibrium, you know that there is a final concentration of .06 mol NH3. And by doing ICE calculations, you can calculate the equilibrium molar...
- Fri Jan 10, 2020 1:13 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Why Q would be greater than K
- Replies: 5
- Views: 145
Re: Why Q would be greater than K
I think Lavelle mentioned this in lecture, but yeah, in an experiment, Q wouldn't be greater than K, however, if you were taking a sample of a river let’s say, you don’t know the exact step in the reaction that your sample is in, so there could be more products than reactants because of you writing ...
- Fri Jan 10, 2020 11:29 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: 5.61b
- Replies: 3
- Views: 202
Re: 5.61b
I think that since you’re compressing the entire system, both the pressure of the reactants and products are increasing by the same amount, so in a sense, the ratios aren’t changing so there is little/no effect on the system.
- Fri Jan 10, 2020 11:21 am
- Forum: Administrative Questions and Class Announcements
- Topic: Peer Learning
- Replies: 4
- Views: 239
Re: Peer Learning
They’re extremely helpful for learning material as most Undergraduate Assistants (UAs) make their own practice problems for you to do and worksheets. They’re also available to go to for almost any time of day and any day, so if you ever have free time, you can see which ones are available (which can...
- Fri Jan 10, 2020 11:07 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: K for Heterogeneous Equilibria
- Replies: 4
- Views: 241
Re: K for Heterogeneous Equilibria
Most of the time, if you’re dealing with aqueous solutions, you use Kc. Also, a lot of time, the problems just have K without the subscript c or p, so if you want to be safe, you can just solve for K.
- Fri Jan 10, 2020 11:04 am
- Forum: Administrative Questions and Class Announcements
- Topic: Required number of responses for chem community
- Replies: 7
- Views: 376
- Tue Dec 03, 2019 11:33 pm
- Forum: Biological Examples
- Topic: hemoglobin and myoglobin
- Replies: 3
- Views: 440
Re: hemoglobin and myoglobin
Yes, but according to what I've found online, I think the main difference is that hemoglobin has 4 heme complexes
- Tue Dec 03, 2019 11:30 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: 6C17 - strength of bases
- Replies: 4
- Views: 252
Re: 6C17 - strength of bases
As a follow up question, what are the general rules for determining the strengths of bases? For acids, it's through bond length and electronegativity, but I don't think Lavelle explicitly mentioned the criteria for bases.
Thanks!
Thanks!
- Tue Dec 03, 2019 11:29 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Hw 6C19
- Replies: 3
- Views: 209
Hw 6C19
The question asks about which acid is stronger in a pair of acids. My question is about part d where it asks which is stronger, HClO4 or H3PO4. How can we figure this out? My original thought process was that H3PO4 would be stronger because it's polyprotic, but the answer key says it's HClO4. Thanks!
- Mon Dec 02, 2019 11:42 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3590498
Re: Post All Chemistry Jokes Here
Original meme content
- Mon Dec 02, 2019 2:00 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Test 2 Hydrogen Bonding Question
- Replies: 3
- Views: 174
Re: Test 2 Hydrogen Bonding Question
Nevermind, I understand it now
- Sun Dec 01, 2019 5:59 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Test 2 Hydrogen Bonding Question
- Replies: 3
- Views: 174
Re: Test 2 Hydrogen Bonding Question
So in terms of IMFs, more specifically H-bonds, we're taking into consideration the possible bonds between the H atom and the F, O, or N atom. When a compound has a F, O, or N atom, it is automatically a hydrogen bonding site (it's lone pairs of electrons each count as 1 site). Regardless if an H a...
- Sun Dec 01, 2019 9:07 am
- Forum: Amphoteric Compounds
- Topic: 6A.17
- Replies: 3
- Views: 220
Re: 6A.17
Nikki Razal 4E wrote:How would you know that Bi2O3 is amphoteric?
Since Bi is one of the amphoteric elements (which you can tell because it’s near the metalloids), that means Bi2O3 is amphoteric.
- Sun Dec 01, 2019 9:04 am
- Forum: Biological Examples
- Topic: Vitamin B12
- Replies: 3
- Views: 181
Re: Vitamin B12
B12 helps make DNA, helps make the myelin in neurons, and helps in the production of red blood cells.
- Sun Dec 01, 2019 8:49 am
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Acid Strength
- Replies: 3
- Views: 248
Re: Acid Strength
Similarly, if there are atoms with more electronegativity, they result in a stronger acid because the more electronegative atoms are “better at pulling” the electron density away from the oxygens and spread more evenly across the molecule.
- Thu Nov 28, 2019 12:16 am
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Test 2 Hydrogen Bonding Question
- Replies: 3
- Views: 174
Test 2 Hydrogen Bonding Question
On test 2, there was a question (7a) about how many hydrogen bonds can form in paraxanthine. According to my TA, the correct answer should be 11 H-bonds, but I don’t understand why. I thought H-bonds were between H and N, O, F so shouldn’t we only count the areas where there are lone pairs on NOF or...
- Sun Nov 24, 2019 11:24 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: 2F.3
- Replies: 3
- Views: 138
Re: 2F.3
(a) 2 sigma bonds (b) 2 sigma and 2 pi bonds You can tell based on the lewis structure - single bonds are one sigma bond, double bonds are one sigma and one pi bond, and triple bonds are one sigma and two pi bonds. Note that there are 2 ways to draw SO2, one with 2 double bonds linking S with the 2...
- Sun Nov 24, 2019 11:23 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: 2F.3
- Replies: 3
- Views: 138
Re: 2F.3
There are 2 sigma bonds in H2S which are the single bonds between sulfur and the two hydrogens There are 2 sigma bonds and 1 pi bond in SO2 because there is a double bond connecting the sulfur to one oxygen (double bond = 1 sigma and 1 pi bond) and a single bond connecting the sulfur to the other ox...
- Sat Nov 23, 2019 10:18 pm
- Forum: Hybridization
- Topic: Hybridization Notation
- Replies: 5
- Views: 395
Re: Hybridization Notation
I don't think it matters too much, but in general, I think it's preferred to write the hybridizations in the order of s, p, d, then f, so for your example, it would be sp^3d
- Sat Nov 23, 2019 10:13 pm
- Forum: Naming
- Topic: Toolbox 9C.1
- Replies: 2
- Views: 121
Re: Toolbox 9C.1
Adding to this, Lavelle mentioned in class that the -ate adds an emphasis that it is an anion because normally, the compounds are cations
- Fri Nov 22, 2019 12:22 pm
- Forum: Octet Exceptions
- Topic: Homework 2E.19d
- Replies: 3
- Views: 231
Re: Homework 2E.19d
Yes, SnCl2 is not a radical, but you are correct in that it doesn't fill the octet, so Sn has a single bond with the 2 Cl and has only 1 lone pair of electrons
- Fri Nov 22, 2019 12:12 pm
- Forum: Dipole Moments
- Topic: Polarity with non-polar bonds
- Replies: 3
- Views: 329
Re: Polarity with non-polar bonds
Adding to this, make sure to recognize that just because a Lewis structure might look like it's symmetrical, you have to take into account the VSEPR shape to see if the molecule truly is symmetrical (i.e. tetrahedrals such as CH2Cl2 in a Lewis structure looks symmetrical, but in VSEPR, it's not)
- Fri Nov 22, 2019 12:07 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: AXE Format
- Replies: 34
- Views: 1244
Re: AXE Format
And along the same thread, if there is no E, you don't have to write E with a subscript of 0, you can just write AX4 or whatever
- Sun Nov 17, 2019 10:05 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Lone Pairs
- Replies: 2
- Views: 234
Re: Lone Pairs
Lone pairs do influence the bond angles and contribute to molecular shape (i.e. H2O is a bent shape instead of linear because of the 2 lone pairs) but when we are asked to find bond angles or draw dipole moments, we focus on the bonded atoms and not the lone pairs. And though we know that H2O has te...
- Sun Nov 17, 2019 9:59 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Week 8 HW
- Replies: 4
- Views: 311
Re: Week 8 HW
3F and 2E
2F is about sigma and pi bonds so we'll probably be covering that tomorrow.
2F is about sigma and pi bonds so we'll probably be covering that tomorrow.
- Fri Nov 15, 2019 12:45 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Interaction potential energy
- Replies: 7
- Views: 424
Re: Interaction potential energy
After browsing chem community further, I think I have a better grasp on IPE. Since IPE is the amount of energy released when two atoms interact (which is always negative because it always /takes/ energy for atoms to interact) so when two atoms are more naturally attractive (aka more polarizable), th...
- Fri Nov 15, 2019 12:41 pm
- Forum: Dipole Moments
- Topic: Interaction Potential Energy Equation?
- Replies: 2
- Views: 214
Re: Interaction Potential Energy Equation?
The two symbols are alpha1 and alpha2 which refer to the polarizability of each individual atom that is interacting with one another.
- Fri Nov 15, 2019 12:32 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: nonpolar molecules
- Replies: 5
- Views: 292
Re: nonpolar molecules
While London dispersion forces are the go-to for nonpolar molecules, make sure to also check for dipole-induced dipole intermolecular forces which is the interaction of a polar molecule and a nonpolar molecule.
- Fri Nov 15, 2019 12:23 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Interaction potential energy
- Replies: 7
- Views: 424
Re: Interaction potential energy
In lecture, Lavelle said that "increasing size or molar mass results in stronger attractive interactions" so that means IPE gets closer to 0 because the denominator becomes larger, right? So I think it means that values closer to 0 are more attracted to each other. Saying this, I'm not 100...
- Fri Nov 15, 2019 12:15 pm
- Forum: Bond Lengths & Energies
- Topic: Calculating Radius Length?
- Replies: 1
- Views: 148
Re: Calculating Radius Length?
I think that if there was a question with interaction potential energy, they would have to give you the radius and charges in order for you to calculate the IPE, or they would ask more conceptually and ask you given two IPEs, which sets of molecules have the greater radius which you don't need to ac...
- Fri Nov 08, 2019 1:41 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Interaction potential energy
- Replies: 7
- Views: 424
Re: Interaction potential energy
I think this is what happens. When alpha increases, that means the polarizability increases, and since polarizability depends on the size of the atom (and how many e- there are), r increases too so the denominator "grows" faster than the alphas in the numerator so even though the numerator...
- Fri Nov 08, 2019 1:27 pm
- Forum: Dipole Moments
- Topic: Dipole moments
- Replies: 5
- Views: 426
Re: Dipole moments
And when molecules with dipole moments are around other molecules with dipole moments, they can automatically arrange themselves so that their opposite dipoles are closer to each other (induced-dipoles) which is a type of attractive force that explains some of the patterns of how molecules arrange t...
- Fri Nov 08, 2019 12:40 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Interaction Potential Energy
- Replies: 3
- Views: 226
Re: Interaction Potential Energy
Also, in context, this means that if the radius between the atoms increases, the denominator gets larger, meaning that the interaction potential energy gets less negative (so it has stronger attractive interactions).
- Fri Nov 08, 2019 12:34 pm
- Forum: Ionic & Covalent Bonds
- Topic: Ionization of Nitrogen vs Oxygen
- Replies: 11
- Views: 1641
Re: Ionization of Nitrogen vs Oxygen
You're correct in that IE increases from left to right, but Nitrogen has a higher IE because it has a half-filled p subshell which is more stable than Oxygen's 4 e- p subshell, so it actually requires more energy to take away that electron from Nitrogen than Oxygen
- Fri Nov 08, 2019 12:12 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Interaction potential energy
- Replies: 7
- Views: 424
Re: Interaction potential energy
Since there's a negative sign in front of the equation, that means that when r increases, the denominator increases meaning it gets closer to 0, which means that Ep actually increases (from more negative to less negative).
- Fri Nov 08, 2019 12:08 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Electron Distortion
- Replies: 2
- Views: 200
Re: Electron Distortion
The more polarizable an atom is, the more likely the atom's electron cloud can be distorted. Meaning that when an atom has a higher polarizability, the electrons are more likely to be attracted to the other atom (aka the original atom is more likely to lose or share it's electrons).
- Fri Nov 01, 2019 12:38 pm
- Forum: Octet Exceptions
- Topic: Expanded Valence Shells
- Replies: 2
- Views: 87
Re: Expanded Valence Shells
I don't know that there is a certain "max" amount of bonds those elements can have, but some of the most common molecules are PCl5 and SF6 which you can see has the same amount of bonds as they have valence electrons initially (aka before bonding). I think you just have to be aware it's po...
- Fri Nov 01, 2019 12:26 pm
- Forum: Coordinate Covalent Bonds
- Topic: Distinguishing a coordinate covalent bond
- Replies: 5
- Views: 265
Re: Distinguishing a coordinate covalent bond
All atoms have some covalent character, but the atoms can have more ionic character (aka less covalent character) if there is a greater difference in electronegativity between the atoms. This also means that atoms with more covalent character (aka less ionic character) has a smaller difference in el...
- Fri Nov 01, 2019 12:18 pm
- Forum: Ionic & Covalent Bonds
- Topic: How does one find a most likely charge for ions for a given element?
- Replies: 6
- Views: 516
Re: How does one find a most likely charge for ions for a given element?
In addition, if the element is in the d blocks, they are more likely to lose their "s" electrons first in order to fill a full or half shell of "d" electrons.
- Tue Oct 29, 2019 9:36 am
- Forum: Dipole Moments
- Topic: How to draw the dipole moment
- Replies: 2
- Views: 204
How to draw the dipole moment
The book says to draw the dipole arrow with the head pointing to the positive side and the tail at the negative side. However, it does acknowledge that it can be reversed. What does Dr. Lavelle prefer?
- Fri Oct 25, 2019 12:30 pm
- Forum: Lewis Structures
- Topic: Formal Charges
- Replies: 4
- Views: 174
Re: Formal Charges
It's used as a tool to draw Lewis Structures in their most stable form. To do this, you manipulate L and S until FC = 0 (as best as possible).
- Fri Oct 25, 2019 12:16 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Electron Configurations (p-orbital)
- Replies: 5
- Views: 227
Re: Electron Configurations (p-orbital)
I agree with everyone else that you don't have to write out the px, py, and pz, but for example, if the question asks you how many unpaired electrons are there for an element, writing px, py, and pz out can be helpful.
- Fri Oct 25, 2019 12:11 pm
- Forum: Lewis Structures
- Topic: Central Atom
- Replies: 7
- Views: 987
Re: Central Atom
Also, in general, if there is a problem that asks you to draw the Lewis Structure for a molecule, the element in the middle of the molecular formula normally actually IS the element in the middle. Of course, this doesn't work all the time, and that's where checking electronegativity is important, bu...
- Fri Oct 25, 2019 12:06 pm
- Forum: Trends in The Periodic Table
- Topic: Electron Affinty
- Replies: 5
- Views: 225
Re: Electron Affinty
Electron affinity has the same periodic trend as ionization energy, so if you remember IE, you can remember EA
- Wed Oct 16, 2019 4:24 pm
- Forum: Trends in The Periodic Table
- Topic: Ionic radii
- Replies: 11
- Views: 369
Re: Ionic radii
As the atoms go down a group, there are more electron levels added and the valence electrons (the outer shell of electrons) gets farther away from the nucleus, which not only increases the radius because there are more electron levels in between, but the attraction that binds the electrons and nucle...
- Wed Oct 16, 2019 4:22 pm
- Forum: Trends in The Periodic Table
- Topic: Atomic radii
- Replies: 9
- Views: 530
Re: Atomic radii
As there are more valence electrons (aka from left to right on the periodic table), the attraction between the valence electrons and the nucleus grows stronger, so the electrons gravitate closer, making the radius smaller
- Wed Oct 16, 2019 1:41 pm
- Forum: General Science Questions
- Topic: Salt Water: Mixture or Compound?
- Replies: 4
- Views: 666
Re: Salt Water: Mixture or Compound?
I agree that salt water is a mixture, because you can separate the two (salt and water) from each other simply by boiling away the water. The salt crystals from the salt water don't chemically combine/react with the water molecules, so it wouldn't be a compound

