Search found 106 matches
- Sat Mar 14, 2020 10:44 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Slow step
- Replies: 3
- Views: 285
Re: Slow step
Your slow step would be the one that determines the rate, so you can compare the rate laws of the steps to whichever rate law is experimentally determined for the complete reaction.
- Sat Mar 14, 2020 10:37 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: E vs Eo
- Replies: 6
- Views: 534
Re: E vs Eo
Eº is the standard cell potential which in this case refers to a cell under standard conditions (25ºC, 1atm, 1mol/L). E doesn't have these conditions, so you'd be calculating the cell potential for any differences in temperature or concentration, as the equation suggests.
- Sat Mar 14, 2020 10:05 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagram
- Replies: 11
- Views: 753
Re: Cell Diagram
Platinum is the most common electrode that you'd use if you need a solid electrode for a cell, so I'd assume that you can use it anytime you need to.
- Sat Mar 14, 2020 10:02 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: intermediates
- Replies: 8
- Views: 593
Re: intermediates
Intermediates are any species that are initially formed at some step in the reaction and then are consumed entirely in the reaction process. So, they won't be in the final products but they will be apparent if you write out all of the steps of the reaction.
- Sat Mar 14, 2020 9:58 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Catalyst's effect on overall reaction
- Replies: 7
- Views: 486
Re: Catalyst's effect on overall reaction
If the slow step is the rate determining step, then in order for a catalyst to speed up the reaction it would need to lower the activation energy of that slowest step. Changing the activation energy of the faster steps wouldn't do anything for the overall reaction because the rate is still determine...
- Sun Mar 08, 2020 1:37 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: activation energy/ energy barrier
- Replies: 6
- Views: 464
Re: activation energy/ energy barrier
A reaction needs to reach the activation energy in order for the reactants to convert to products. You can calculate it numerically with the Arrhenius equation (which shows that it depends on the rate constant k and the temperature of the reaction).
- Sun Mar 08, 2020 1:27 pm
- Forum: General Rate Laws
- Topic: Rate law
- Replies: 3
- Views: 334
Re: Rate law
I think that you should probably specify if there are multiple rates being dealt with (ie an equilibrium reaction with forward or reverse rates, or multiple reactions occurring) just to be clear as to what reactants are being included in which rate laws.
- Sun Mar 08, 2020 1:16 pm
- Forum: Zero Order Reactions
- Topic: Rearranging First Order Integrated Rate Law
- Replies: 1
- Views: 218
Re: Rearranging First Order Integrated Rate Law
If you look at the initial and final equations, the numerator and the denominator of the ln of concentrations is flipped. That's where the negative sign went (since -ln(x/y) = ln(y) - ln(x) = ln(x/y) ).
- Sun Mar 08, 2020 1:03 pm
- Forum: General Rate Laws
- Topic: slow step
- Replies: 4
- Views: 352
Re: slow step
If you are given the experimentally determined rate law or something like that, you can find an intermediate step that matches that rate law, making that the slowest step.
- Sun Mar 08, 2020 12:53 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: concentration cells
- Replies: 5
- Views: 423
Re: concentration cells
In concentration cells, the same half reaction is occurring but in opposite directions. For the lower concentration, the half reaction would be increasing the amount of the species in solution, while the half reaction for the higher concentration would decreased the amount. The transfer of electrons...
- Sat Feb 29, 2020 7:08 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell diagram order
- Replies: 4
- Views: 366
Re: Cell diagram order
You might want to put it so that the reactants are first and the products are second for the half reaction, just so that it is clear what is being oxidized and reduced in the reactions.
- Sat Feb 29, 2020 7:06 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs Free Energy Relation to Q
- Replies: 1
- Views: 170
Re: Gibbs Free Energy Relation to Q
The Gibbs free energy at any particular stage in the reaction is based on the concentrations of the reactants at that time. For finding the Gibbs free energy at a time, it is the difference of the molar Gibbs free energy of the products and reactants times the number of moles at the time. So, the Q ...
- Sat Feb 29, 2020 5:41 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Concentration Cells and Nernst Equation
- Replies: 4
- Views: 341
Re: Concentration Cells and Nernst Equation
You could use the Nernst equation for concentration cells since you could find the reaction quotient Q and then determine the cell potential using the equation. You can also use it for any cells that aren't at the standard potential (with different concentrations of species affecting the cell potent...
- Sat Feb 29, 2020 5:32 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Salt bridges
- Replies: 11
- Views: 780
Re: Salt bridges
As the redox reaction continues in a cell, the electrons will move from the anode to the cathode. If the salt bridge weren't there, eventually the reaction would stop as one half reaction gets increasingly positive and the other gets increasingly negative. With the salt bridge, the ion flow countera...
- Sat Feb 29, 2020 5:22 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: electrochemical series
- Replies: 1
- Views: 129
Re: electrochemical series
The electrochemical series organizes certain ions and compounds based on how well it is able to oxidize or reduce other species. The oxidizing ability of species on the left of half reactions increases as you go up (standard potential gets higher), while the reducing ability of species on the right ...
- Sat Feb 22, 2020 5:48 pm
- Forum: Balancing Redox Reactions
- Topic: Redox in Acidic/Basic Solutions
- Replies: 3
- Views: 272
Re: Redox in Acidic/Basic Solutions
In acidic solutions, you have an excess of H+ which can be used along with H2O to balance H and O in the half reactions. In basic solutions, you have excess OH- which you would use to balance the half reactions. This is necessary to make sure that the charges and elements are balanced in the redox r...
- Sat Feb 22, 2020 5:43 pm
- Forum: Balancing Redox Reactions
- Topic: Cell Diagrams
- Replies: 5
- Views: 314
Re: Cell Diagrams
Platinum is by far the most common electrode so it can be used if there aren't any solids in the problem that could conduct e-. In the problem in lecture for example, the side with Cu(s) didn't need Pt as an electrode because Cu was the electrode and is a conductive solid. However, the side with Fe3...
- Sat Feb 22, 2020 5:36 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Nernst Equation and Le Chatelier's Principle
- Replies: 3
- Views: 559
Re: Nernst Equation and Le Chatelier's Principle
Basically, the cell potential of a given reaction is dependent on the concentrations on the species in the reaction. At equilibrium in a reaction, the cell potential is zero because the reaction no longer does any work. The Nernst equation gives a quantitative answer as to how different concentratio...
- Sat Feb 22, 2020 5:32 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Electrochemical Cells
- Replies: 2
- Views: 161
Re: Electrochemical Cells
Both voltage and electric potential are measured in volts, but voltage is the difference in the electric potentials across two points.
- Sat Feb 22, 2020 5:28 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Standard Cell Potentials
- Replies: 4
- Views: 302
Re: Standard Cell Potentials
By convention in writing out cell diagrams, the anode is typically on the left side and the cathode is typically on the right side. So, the standard cell potential would be the potential of the cathode minus the potential of the anode. You can tell if the left or right side is the cathode or anode b...
- Sun Feb 16, 2020 5:25 pm
- Forum: Van't Hoff Equation
- Topic: When to use Van't Hoff equation
- Replies: 2
- Views: 180
Re: When to use Van't Hoff equation
The changes in 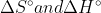 are generally negligible with a change in temperature so we just assume that they are constant when deriving (delta G, on the other hand, does change significantly with temperature changes).
are generally negligible with a change in temperature so we just assume that they are constant when deriving (delta G, on the other hand, does change significantly with temperature changes).
- Sun Feb 16, 2020 5:12 pm
- Forum: Balancing Redox Reactions
- Topic: Half reactions
- Replies: 7
- Views: 526
Re: Half reactions
Half reactions separate the oxidation and the reduction reactions that occur in a redox reaction. You can use it to find out which elements are oxidized and reduced, and also balance redox reactions (since the number of electrons transferred should cancel out in the two half reactions combined.
- Sun Feb 16, 2020 5:08 pm
- Forum: Balancing Redox Reactions
- Topic: Oxidation Reduction
- Replies: 3
- Views: 272
Re: Oxidation Reduction
Generally, oxygen will have an oxidation number of -2 and hydrogen will have an oxidation number of +1 across different compounds. However, there are a few exceptions to this (for example, H2O2 where oxygen has an oxidation number of -1.)
- Sun Feb 16, 2020 5:04 pm
- Forum: Van't Hoff Equation
- Topic: K in Van't Hoff
- Replies: 3
- Views: 251
Re: K in Van't Hoff
The Van't Hoff equation gives us a quantitative means of relating the change in temperature to the change in the equilibrium constant (and we can find the new equilibrium constants for a reaction at different temperatures).
- Sun Feb 16, 2020 5:01 pm
- Forum: Balancing Redox Reactions
- Topic: Electrons in Compounds
- Replies: 1
- Views: 95
Re: Electrons in Compounds
If you want to find the oxidation states of molecules, you can base it knowing that certain groups of elements tend to have certain oxidation states (Group 1 elements typically have +1, Group 2 have +2, halogens have -1, etc.) with a few exceptions. You can use those to find the oxidation numbers fo...
- Sat Feb 08, 2020 3:56 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs free energy of ice and liquid water
- Replies: 3
- Views: 451
Re: Gibbs free energy of ice and liquid water
I think they mean that there is a point where the Gibbs free energy of ice and Gibbs free energy of water are equal, so there is no change in it and it is at equilibrium. There is a graph in the section that has a diagram of temperature vs molar Gibbs free energy where the liquid and solid states in...
- Sat Feb 08, 2020 3:51 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Using Cv,m and Cp,m
- Replies: 2
- Views: 116
Re: Using Cv,m and Cp,m
You would used Cv,m and Cp,m if you want to find Cv or Cp in calculating q (using q=C T). The values using R to calculate C are ways of estimating the heat capacities of certain molecules if you don't know it, but I don't really think he went over that.
T). The values using R to calculate C are ways of estimating the heat capacities of certain molecules if you don't know it, but I don't really think he went over that.
- Sat Feb 08, 2020 3:45 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Units for heat of reaction
- Replies: 9
- Views: 297
Re: Units for heat of reaction
805097738 wrote:do you have to explicitly state per mole or is that implied already
You should explicitly state it per mole in the case of standard reaction enthalpy or standard formation enthalpy because those are definitionally for one mole of a compound. I guess it's mostly for the consistency of units, though.
- Sat Feb 08, 2020 3:37 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Calculating bond enthalpies
- Replies: 4
- Views: 142
Re: Calculating bond enthalpies
The number that you multiply by depends on the number of bonds broken or formed. For example, if there are 2 C-C bonds broken in the reaction, then take the bond enthalpy for a C-C bond and multiply it by 2. You should probably know how to do it because certain problems might only give you the bond ...
- Sat Feb 08, 2020 3:32 pm
- Forum: Calculating Work of Expansion
- Topic: Integral for work
- Replies: 4
- Views: 170
Re: Integral for work
When you have a reversible system, you would want to use the equation that you get from the integral because the changes in volume are infinitesimal. So, use w = -nRTln(V2/V1) for a reversible system, which you can derive from the integral.
- Sun Feb 02, 2020 5:11 pm
- Forum: Phase Changes & Related Calculations
- Topic: Liquid/Steam
- Replies: 3
- Views: 195
Re: Liquid/Steam
Basically, during phase changes the temperature of a substance remains constant despite increasing amounts of energy (he showed us that phase diagram in lecture.) So, water at 100 degrees Celsius has much less energy in it than water vapor at 100 degrees Celsius because the vapor has additional ener...
- Sun Feb 02, 2020 5:03 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Enthalpy units
- Replies: 5
- Views: 308
Re: Enthalpy units
Enthalpy for a reaction would typically be given in joules or kilojoules. For molar enthalpy, standard reaction enthalpy or standard formation enthalpy, it would be given in J/mol or kJ/mol.
- Sun Feb 02, 2020 5:00 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Three Methods
- Replies: 3
- Views: 200
Re: Three Methods
I think that depending on whichever method (I'm assuming for finding delta H) they'll give you the specific info that you need in the problem or just outright tell you. For example, if you need to use Hess's law then the problem will give some equations that you can use to find the enthalpy in the o...
- Sun Feb 02, 2020 4:56 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Types of Systems
- Replies: 3
- Views: 1200
Re: Types of Systems
I think it will either be given or at least obvious if the system or any energy transfer described in the problem. Just remember that open systems allow matter and energy to transfer with surroundings, closed systems only allow energy, and isolated systems don't allow any transfer with surroundings....
- Sun Feb 02, 2020 4:52 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Degeneracy
- Replies: 1
- Views: 123
Re: Degeneracy
Boltzmann's formula in particular is a means of calculating statistical entropy based on W (the amount of different microstates with the same total energy.) The entropy S calculated there is equivalent to the thermodynamic definition of entropy. I think that if using the Boltzmann equation would get...
- Sat Jan 25, 2020 4:54 pm
- Forum: Ideal Gases
- Topic: R Constant
- Replies: 26
- Views: 1226
Re: R Constant
Just make sure that you choose the right R constant depending on the units for pressure and volume, or convert all the units so that you can 0.082 L*atm*mol^-1*K^-1.
- Sat Jan 25, 2020 4:32 pm
- Forum: Phase Changes & Related Calculations
- Topic: Calculating Bond Enthalpies
- Replies: 4
- Views: 138
Re: Calculating Bond Enthalpies
He drew out the Lewis structures mainly to figure out what bonds were broken and what bonds were formed during the reaction. You want to add the bond enthalpies of the reactant bonds that are broken and subtract the bond enthalpies of the product bonds that are formed (accounting for bond enthalpies...
- Sat Jan 25, 2020 4:28 pm
- Forum: Phase Changes & Related Calculations
- Topic: State Property
- Replies: 6
- Views: 325
Re: State Property
Heat isn't a state function because the amount of energy transferred as heat depends on how the change in energy occurs. On the other hand, enthalpy is a state function because no matter how the energy change occurs, it only depends on the state of the system.
- Sat Jan 25, 2020 4:23 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Decreasing volume
- Replies: 5
- Views: 204
Re: Decreasing volume
The reaction would shift in a way that wants to minimize the resulting increase in pressure, so that reaction would shift to the side with fewer gas molecules because it would decrease the pressure of the system.
- Sat Jan 25, 2020 4:16 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Standard enthalpy
- Replies: 2
- Views: 55
Re: Standard enthalpy
The standard enthalpy of formation is the enthalpy change that comes from forming one mole of a compound from the elements in its standard states. The standard reaction enthalpy is the change that comes from having all products and reactants in their standard states. You can find the standard enthal...
- Sun Jan 19, 2020 3:31 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Concentrations
- Replies: 12
- Views: 423
Re: Concentrations
Concentration and pressure only affects Q and not K; the reaction will shift accordingly to return to a ratio of reactants and products equal to K. Temperature actually changes K because of the changes in energy that are involved in the reaction.
- Sun Jan 19, 2020 3:08 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: autoprotolysis
- Replies: 5
- Views: 154
Re: autoprotolysis
Could someone explain how autoprotolysis of water leads to Kw? need help on this too; also, does anyone know how much we need to know about the topic of autoprotolysis for the test? In the autoprotolysis reaction 2H2O(l) \rightleftharpoons H3O+(aq) + OH-(aq), we get an equilibrium constant Kw = [H3...
- Sun Jan 19, 2020 3:01 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kc from K
- Replies: 3
- Views: 119
Re: Kc from K
I think the textbook is using K to refer to Kp, so the K value uses partial pressures. There's an equation to convert Kp to Kc in section 5.H, but I don't think we're expected to know it since he didn't go over it in lecture. Basically it's derived using the Ideal Gas Law to substitute in the K equa...
- Sun Jan 19, 2020 2:54 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Change in K when reaction is endothermic?
- Replies: 2
- Views: 63
Re: Change in K when reaction is endothermic?
When the reaction is endothermic, heat energy is absorbed from surroundings. Because of this, K would increase with increasing the temperature since mean that there is greater heat energy to absorb. This would mean that the reaction would shift towards the products.
- Sun Jan 19, 2020 2:47 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: approximation
- Replies: 4
- Views: 132
Re: approximation
When you have a very small value of x it is essentially negligible when compared to the initial concentration, so we can approximate to make the calculations much simpler as compared to using a quadratic equation.
- Sat Jan 11, 2020 4:50 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: ICE box
- Replies: 9
- Views: 311
Re: ICE box
The acceptable answers would be any that has it so that the equilibrium concentrations or partial pressures of the reactants or products are positive.
- Sat Jan 11, 2020 4:40 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Table 5G.2
- Replies: 5
- Views: 155
Re: Table 5G.2
In the table I think that K is just referring to Kp based on the fact that all of the equations only have gases. So, use "K" in the table if you are solving using partial pressure and use "Kc" in the table if using concentrations.
- Sat Jan 11, 2020 4:24 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Significance of principle
- Replies: 6
- Views: 347
Re: Significance of principle
Le Chatelier's principle is useful in describing how a chemical reaction shifts in response to changes in conditions. Changes in concentration of reactants or products, pressure, and temperature would result in shifts in the equilibrium, which is consistent with Le Chatelier's principle.
- Sat Jan 11, 2020 3:50 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: finding change in concentration in ICE tables
- Replies: 3
- Views: 249
Re: finding change in concentration in ICE tables
You'd use the tables if you have an unknown change in the concentrations (represented by X). In the equation you said, the initial concentrations of each of the reactants would be changed by -X, while each of the products would be changed by +X. You would find the final equilibrium concentrations in...
- Sat Jan 11, 2020 3:20 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Decreasing pressure by increasing volume [ENDORSED]
- Replies: 4
- Views: 156
Re: Decreasing pressure by increasing volume [ENDORSED]
Decreasing the pressure would cause the reaction to shift towards whichever side has more moles. Because of the pressure decrease and the volume increase, the concentrations would decrease resulting in the shift.
- Sat Dec 07, 2019 4:38 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: NH3
- Replies: 1
- Views: 76
Re: NH3
NH3 has one lone pair if you draw out its Lewis structure, meaning that it can form one coordinate covalent bond with a central metal.
- Sat Dec 07, 2019 3:46 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Polydentate
- Replies: 1
- Views: 111
Re: Polydentate
If you're drawing it out, the nitrogen with one H bonded together would be central and bonded to two carbons (you can pretty much figure out the order of the chain based on the order of the formula, especially if its written out at NH2CH2CH2NHCH2CH2NH2). The formal charge would be zero for everythin...
- Sat Dec 07, 2019 3:30 pm
- Forum: Lewis Acids & Bases
- Topic: Strong bases
- Replies: 2
- Views: 214
Re: Strong bases
The strong bases that I think we have to know are the Group 1 and 2 hydroxides and oxides.
- Sat Dec 07, 2019 3:18 pm
- Forum: Conjugate Acids & Bases
- Topic: How was this equation derived?? Marshmallow #34
- Replies: 1
- Views: 358
Re: How was this equation derived?? Marshmallow #34
The reaction between HCl and CaO would be a neutralization reaction between an acid and base that results in the formation of a salt and water. The balanced reaction would be 2HCl + CaO -> CaCl2 + H2O. Basically once you have the equation you want to find out how much of either HCl or CaO is leftove...
- Sat Dec 07, 2019 3:06 pm
- Forum: Dipole Moments
- Topic: CCl2H2
- Replies: 2
- Views: 648
Re: CCl2H2
The molecular shape for CCl2H2 would be tetrahedral using VSEPR, so the dipoles wouldn't actually cancel each other out and there would be a net dipole.
- Sun Dec 01, 2019 11:31 pm
- Forum: Amphoteric Compounds
- Topic: Amphoteric compounds
- Replies: 1
- Views: 92
Re: Amphoteric compounds
Amphoteric compounds are compounds that can react both as acids and bases and have both acidic and basic character. Amphoteric oxides are certain oxide compounds that have those characteristics, and the metals that form them are in a diagonal line just left of the metalloids in the periodic table (F...
- Sun Dec 01, 2019 11:24 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Deprotonated Acids
- Replies: 2
- Views: 112
Re: Deprotonated Acids
The calculations are more complicated and involve chemical equilibrium which is something that I don't think gets covered until 14B, so I think you just need to know the basics for now.
- Sun Dec 01, 2019 11:15 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Strong Acids
- Replies: 3
- Views: 187
Re: Strong Acids
If you look at the equation for chemical equilibrium, a larger Ka value would result if there is a greater amount of product than reactant at equilibrium (and so there would be a lower pKa value because pKa is -log Ka). Since strong acids almost entirely dissociate, there would be a much greater con...
- Sun Dec 01, 2019 11:11 pm
- Forum: Conjugate Acids & Bases
- Topic: conjugate acids/bases
- Replies: 1
- Views: 97
Re: conjugate acids/bases
Generally the conjugate base of an acid is the species remaining after removing a proton, and the conjugate acid of a base is the species after accepting a proton.
- Sun Dec 01, 2019 11:07 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Shapes and Corners
- Replies: 2
- Views: 96
Re: Shapes and Corners
It's possible for there to be odd numbers of ligands, though rarer. The textbook says that the most common coordination numbers for the central metal atom are 6 and 4, so those are probably the ones that you would see the most as examples.
- Sun Dec 01, 2019 11:01 pm
- Forum: Naming
- Topic: Order of Ligands
- Replies: 4
- Views: 298
Re: Order of Ligands
You want to always name the ligands in alphabetical order based on the root (not the prefix), regardless of the formula order.
- Sat Nov 23, 2019 9:46 pm
- Forum: Bronsted Acids & Bases
- Topic: Bronsted Acids vs Lewis acids
- Replies: 4
- Views: 167
Re: Bronsted Acids vs Lewis acids
I think that a Bronsted definition of acids means that acids require a hydrogen (because a proton H+ needs to be released), whereas a Lewis acid means that some compounds without hydrogen can still be acids in certain reactions.
- Sat Nov 23, 2019 9:16 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Polydenate vs Chelate
- Replies: 1
- Views: 182
Re: Polydenate vs Chelate
A polydenate is essentially a ligand that has multiple bonds with the central metal atom in coordinate complexes. Polydenates are able to form chelates, which are a type of coordinate complex that has a ring-like structure.
- Sat Nov 23, 2019 9:03 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Denticity
- Replies: 2
- Views: 144
Re: Denticity
The denticity refers to the number of lone pairs of a ligand that are bonding to the central metal atom. For example, a monodentate ligand would have one available lone pair to make a coordinate complex with a metal.
- Wed Nov 20, 2019 1:29 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Why is CH2Cl2 polar?
- Replies: 12
- Views: 793
Re: Why is CH2Cl2 polar?
You should think of this molecule in the 3d form. In a tetrahedral, no matter where you put the atoms the Chlorine atoms will always be next to each other. I can't Draw a picture on here, but just look one up it might be helpful. The lewis structure looks like u can orient the chlorines to be oppos...
- Wed Nov 20, 2019 1:26 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Ligands
- Replies: 4
- Views: 225
Re: Ligands
I think that by definition ligands are those that bind to the central atom of the coordination complex, so they can't be the central atom.
- Fri Nov 15, 2019 1:39 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Atom size vs. boiling point
- Replies: 4
- Views: 589
Re: Atom size vs. boiling point
The strength of the Van Der Waal's forces in the atom increases because of the greater number of electrons, which would increase the polarizability and thus the strength of IMFs.
- Fri Nov 15, 2019 1:32 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Determining Lone Pair Location
- Replies: 3
- Views: 170
Re: Determining Lone Pair Location
It mainly depends on the VSEPR structure. Since lone pair-lone pair repulsion forces are the strongest, you want the lone pairs to be the farthest away from each other and have the greatest bond angle in considering the position of each of the electron density regions (bonds and lone pairs).
- Fri Nov 15, 2019 1:21 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: How do we determine bond angles?
- Replies: 4
- Views: 253
Re: How do we determine bond angles?
You can generally predict the bond angle based on the VSEPR molecular structure (eg a tetrahedral molecule would have bond angles of 109.5 degrees). If a central atom has lone pairs, using VSEPR you can approximate the bond angle just knowing that the lone pair-atom repulsions are greater than atom-...
- Fri Nov 15, 2019 1:10 pm
- Forum: Hybridization
- Topic: Relationship btw arrangement and hybrid orbitals
- Replies: 2
- Views: 247
Re: Relationship btw arrangement and hybrid orbitals
If you mean arrangement as in VSEPR, then it relates to hybridization because the hybridization is according to the number of bonds (and thus electron density regions which affects molecular shape).
- Fri Nov 15, 2019 1:00 pm
- Forum: Dipole Moments
- Topic: Shape of Molecules affect boiling point?
- Replies: 7
- Views: 914
Re: Shape of Molecules affect boiling point?
Rod shaped molecules have a larger surface area that are close to each other, meaning that there is less distance between molecules and stronger interaction of Van Der Waal's forces. The stronger intermolecular forces causes an increase in the boiling point for rod-shaped molecules as compared to sp...
- Mon Nov 11, 2019 8:00 pm
- Forum: Resonance Structures
- Topic: What is a Resonance "Structure"
- Replies: 12
- Views: 1036
Re: What is a Resonance "Structure"
Resonance structures are just all of the possible Lewis structures that work for the atom when it has resonance, representing that the bonding of the resonance hybrid is an average of the resonance structure.
- Mon Nov 11, 2019 7:47 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: melting points
- Replies: 6
- Views: 314
Re: melting points
905416023 wrote:So hydrogen bonds make the molecule harder to break apart? So then they have a higher boiling point?
Yes, the strength of hydrogen bonds causes the molecules to have a greater intermolecular attraction which increases their melting and boiling points.
- Mon Nov 11, 2019 7:37 pm
- Forum: Dipole Moments
- Topic: Dipole-Dipole Forces
- Replies: 2
- Views: 202
Re: Dipole-Dipole Forces
I think it would be a lower energy orientation because the molecule would have the positive and negative ends of adjacent atoms would be near each other, since it would be favorable to be attracted and have the molecules stay in that orientation longer.
- Wed Nov 06, 2019 11:09 am
- Forum: Octet Exceptions
- Topic: When to use Expanded Octet?
- Replies: 2
- Views: 212
Re: When to use Expanded Octet?
If you want to find the most stable Lewis structure, you would want to minimize formal charge and then use expanded octets if possible. Remember that expanded octets only apply for atoms in the 3rd period and after because they have d-orbitals available in their valence shell.
- Wed Nov 06, 2019 10:59 am
- Forum: Photoelectric Effect
- Topic: Intensity in Photoelectric Effect
- Replies: 6
- Views: 479
Re: Intensity in Photoelectric Effect
For the photoelectric effect, if the energy of the photons is not enough to pass the threshold energy, then electrons won't be ejected regardless of intensity. This is important because it goes against the wave model since electrons wouldn't be ejected regardless of intensity, since a wave model wou...
- Fri Nov 01, 2019 1:35 pm
- Forum: Coordinate Covalent Bonds
- Topic: Distinguishing a coordinate covalent bond
- Replies: 5
- Views: 265
Re: Distinguishing a coordinate covalent bond
In looking at a coordinate covalent bond specifically, where two shared electrons are coming from the same atom, it's probably best to look at the Lewis structure and look at how the bond formed in the first place from reaction (where by looking at the valence shells you can see if a single atom is ...
- Fri Nov 01, 2019 1:20 pm
- Forum: Bond Lengths & Energies
- Topic: Lone electron pairs weakening bonds
- Replies: 4
- Views: 171
Re: Lone electron pairs weakening bonds
The lone pair electrons of fluorine would be interacting with each other because of the small size of fluorine atoms, causing a repelling force that would weaken the overall bond. That's why fluorine has a fairly small dissociation energy because of these lone pair electron interactions between the ...
- Fri Nov 01, 2019 1:14 pm
- Forum: Ionic & Covalent Bonds
- Topic: Anions and Cations
- Replies: 9
- Views: 462
Re: Anions and Cations
Anions would be larger because the extra electrons would cause greater electron-electron repulsion in the atom and would decrease the effective nuclear charge. Cations would be the opposite, since less electrons means less electron-electron repulsion.
- Fri Nov 01, 2019 1:06 pm
- Forum: Bond Lengths & Energies
- Topic: bond lengths
- Replies: 10
- Views: 586
Re: bond lengths
The more Bonds means a stronger pull so consequently you also get a smaller length because of the increased attraction. So the smaller the bond, the stronger it is? A stronger bond will typically be shorter and have a larger dissociation energy because there are more electrons acting in the bond, m...
- Fri Nov 01, 2019 1:01 pm
- Forum: Octet Exceptions
- Topic: Expanded Valence Shells
- Replies: 2
- Views: 95
Re: Expanded Valence Shells
I think that if you're looking at the amount of expanded octet bonds or lone pairs for those elements you would want to consider formal charge. For example, PCl5 would have phosphorus with 5 single bonds, and phosphorus has 5 valence electrons, so the P atom would have a formal charge of 0.
- Thu Oct 24, 2019 6:42 pm
- Forum: Lewis Structures
- Topic: Double Bonds and Electron Numbers
- Replies: 4
- Views: 221
Re: Double Bonds and Electron Numbers
when do you know to turn into a double bond instead of leaving the electrons on the other side of the element when drawing a lewis structure? It would depend on the total number of electrons you have to work with (valence electrons of every atom, adjusting for net charge if there is any). Otherwise...
- Thu Oct 24, 2019 6:33 pm
- Forum: Trends in The Periodic Table
- Topic: Homework Question 1F.19
- Replies: 5
- Views: 292
Re: Homework Question 1F.19
The lower ionization energy means that the s-block metals are very likely to lose their valence electrons. This tendency makes them highly reactive.
- Thu Oct 24, 2019 6:10 pm
- Forum: Lewis Structures
- Topic: Ion lewis structure
- Replies: 9
- Views: 388
Re: Ion lewis structure
As long as you follow the octet rule (besides any exceptions), then it wouldn't matter where the additional electron is since you'll have satisfied the octet rule for every atom. For your example of BrO-, just make sure that you have the right amount of electrons. So, count out the valence electrons...
- Thu Oct 24, 2019 6:05 pm
- Forum: Trends in The Periodic Table
- Topic: Calculated vs Observed Value
- Replies: 6
- Views: 551
Re: Calculated vs Observed Value
Electronegativity is a value that is calculated from an formula. In the textbook section 2D.1, it mentions that the values were first calculated with an expression that determines the differences in electronegativity between bonds by using dissociation energies of that bond. All of the other periodi...
- Thu Oct 24, 2019 5:52 pm
- Forum: Resonance Structures
- Topic: Different Resonance Structures
- Replies: 4
- Views: 162
Re: Different Resonance Structures
If a Lewis structure has resonance it doesn't mean that there are actually different structures, but it means that the actual structure has bonds that are intermediate in properties. For example, Dr. Lavelle used nitrate as an example in class, which has 2 N-O single bonds and 1 N-O double bond. In ...
- Fri Oct 18, 2019 8:11 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Orbitals in relation to arrows
- Replies: 10
- Views: 679
Re: Orbitals in relation to arrows
The Pauli exclusion principle says that there can be no more than 2 e- per orbital; that's why the arrows are grouped in pairs with opposite spins. If you're filling out diagrams like that, you'd start by adding electrons with a maximum of 2 e- for each orbital, and adding up spin electrons first if...
- Fri Oct 18, 2019 12:36 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Spin State
- Replies: 17
- Views: 432
Re: Spin State
The total magnetic spin for the shell would add up to zero if it was full. Because of the Pauli exclusion principle (in that no two electrons can have the same exact quantum number configurations), an atom would need paired electrons with a positive and negative spin value in an full orbital.
- Fri Oct 18, 2019 12:22 am
- Forum: Quantum Numbers and The H-Atom
- Topic: Quantum Numbers
- Replies: 3
- Views: 157
Re: Quantum Numbers
You basically just have to familiarize yourself with the conditions for the values of each quantum number. So, n = 1,2,3,...; l = 0,1,2,...,n-1; ml = l, l-1, l-2,..., -l; and ms = +1/2 or -1/2. 2,0,0 should theoretically be possible values for n, l, and ml.
- Fri Oct 18, 2019 12:13 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Wave Function
- Replies: 2
- Views: 107
Re: Wave Function
The wave function is a way that we can model the wave-like properties of atomic particles. The orbital is the same thing, as a math function that represents the wave function nature of electrons in atoms, where orbitals can be made into a three-dimensional model for the space where certain electrons...
- Thu Oct 17, 2019 11:52 pm
- Forum: Photoelectric Effect
- Topic: Quick Question
- Replies: 3
- Views: 139
Re: Quick Question
Ionization energy refers to the minimum energy needed to remove an electron in its gas phase specifically, whereas the threshold energy is seen in the photoelectric effect (typically seen in solid metals).
- Thu Oct 10, 2019 10:14 pm
- Forum: Properties of Light
- Topic: HW 1A11
- Replies: 1
- Views: 172
Re: HW 1A11
Each of the spectral line groupings ends with the electron at a certain energy level. For example, the Lyman series is a reduction to an energy level of n=1 (ground state), so the spectral lines result from transitions from n \geq 2 to n=1. The Balmer series is a reduction to an energy level of n=2,...
- Thu Oct 10, 2019 9:58 pm
- Forum: Photoelectric Effect
- Topic: Photoelectric Effect
- Replies: 3
- Views: 177
Re: Photoelectric Effect
In the case of atomic spectra, the electron is not ejected and is still attached; a photon is emitted because of the transition from the higher to lower energy states for the electrons, and the atomic spectra lines are showing that. That's why the spectral lines correspond with the specific energy l...
- Thu Oct 10, 2019 9:36 pm
- Forum: Einstein Equation
- Topic: Energy of photons to Moles
- Replies: 2
- Views: 230
Re: Energy of photons to Moles
For a mole of photons, it would just be the energy of a single photon (so, using a formula like E=hv) multiplied by Avogadro's number.
- Thu Oct 10, 2019 12:23 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Large Objects and Heisenberg Uncertainty
- Replies: 1
- Views: 102
Re: Large Objects and Heisenberg Uncertainty
The uncertainty principle definitely isn't noticeable and has no real practical effect on a larger object because the relative uncertainty is negligible compared to the macroscopic object; the principle mainly applies to subatomic particles where measurements have to be precise. Additionally, it wou...
- Thu Oct 10, 2019 12:04 pm
- Forum: Properties of Light
- Topic: Hydrogen Atoms for White Light
- Replies: 3
- Views: 99
Re: Hydrogen Atoms for White Light
It's true that hydrogen is the simplest to analyze because it only has a single electron. The substitution for other elements refers to the fact that any element could theoretically be substituted as long as it only has a single electron (for example, He+ or Li2+). Otherwise, the interactions betwee...
- Sun Oct 06, 2019 5:16 pm
- Forum: Limiting Reactant Calculations
- Topic: How to determine limiting reactants
- Replies: 3
- Views: 160
Re: How to determine limiting reactants
Another way would be to compare the moles of reactants that you are given to the molar ratios of the stoichiometric coefficients. For each reactant, divide the moles by the stoichiometric coefficient of the reactant, and whichever is the least is the limiting reactant :)
- Sat Oct 05, 2019 12:27 am
- Forum: Balancing Chemical Reactions
- Topic: H 7 a.
- Replies: 3
- Views: 160
Re: H 7 a.
For H7a, the unbalanced equation would be Ca(s) + H2O(l) -> H2(g) + Ca(OH)2(aq). Generally for balancing equations you would just go through each element step-by-step (starting with the least occurring element) and make sure that they are all balanced individually. Ca is already balanced, so we can ...
- Fri Oct 04, 2019 5:49 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Use of Avogadro's number
- Replies: 3
- Views: 206
Re: Use of Avogadro's number
Avogadro's constant would be used if you want to find the number of molecules or atoms of something, since one mole of a substance has 6.0221*10^23 molecules of that substance. For example, if you are asked to find the number of individual atoms of an element in some amount of moles, then you would ...
- Fri Oct 04, 2019 5:36 pm
- Forum: SI Units, Unit Conversions
- Topic: Fundamental E.1
- Replies: 5
- Views: 193
Re: Fundamental E.1
pm is picometers, and 1 pm = 10^-12 m. So, 1.73*10^26 pm (10^-12 m/ 1 pm) = 1.73 * 10^14 m

