Search found 59 matches
- Mon Jan 13, 2020 5:36 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Textbook question 5J.11
- Replies: 3
- Views: 182
Re: Textbook question 5J.11
Because it might be difficult to memorize whether the formation or breaking of bonds releases heat or absorbs heat, I always use this to remember: when you break a bond, you're ENDing it; this is an ENDothermic process (it absorbs heat).
- Mon Jan 13, 2020 5:30 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Autoprotolysis
- Replies: 6
- Views: 375
Re: Autoprotolysis
Autoprotolysis can occur between any two identical amphoteric molecules. In this reaction, one molecule will act as a Bronsted acid and the other as a Bronsted base. Water is just one example of a molecule that meets these conditions.
- Mon Jan 13, 2020 5:28 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5I.27
- Replies: 8
- Views: 440
Re: 5I.27
Because you know that Q is less than K (as found in part a of the question), you know that the reaction will continue in the direction of the products. To solve for the equilibrium composition of reactants and products, set up an ICE table. There are no coefficients in the chemical equation, so you ...
- Mon Jan 13, 2020 5:23 pm
- Forum: Ideal Gases
- Topic: R constant
- Replies: 6
- Views: 282
Re: R constant
Units of gas can be expressed in atmospheres or in bars. However, the textbook uses mostly bars to express partial pressure. I would be prepared to do some conversions between the two units though.
- Mon Jan 13, 2020 5:21 pm
- Forum: Ideal Gases
- Topic: Kc and Kp
- Replies: 5
- Views: 269
Re: Kc and Kp
I understand your confusion; in the textbook, Kc and K are assigned different values in some parts of Table 5G.2. However, this isn't something to worry about, since because when there was ambiguity involved with which values to use, the question provided the correct constant. I expect the same will...
- Sat Dec 07, 2019 10:01 pm
- Forum: Lewis Acids & Bases
- Topic: Water
- Replies: 63
- Views: 3024
Re: Water
To provide a more technical and detailed answer:
Water (H2O) is able to release a proton (H+) to form hydroxide ions. Water can also accept a proton to form hydronium (H3O+). Thus, water can act as both a Bronsted acid and a Bronsted base, making it amphoteric/amphiprotic.
Water (H2O) is able to release a proton (H+) to form hydroxide ions. Water can also accept a proton to form hydronium (H3O+). Thus, water can act as both a Bronsted acid and a Bronsted base, making it amphoteric/amphiprotic.
- Sat Dec 07, 2019 9:57 pm
- Forum: Identifying Acidic & Basic Salts
- Topic: Hw Problem 6D.11
- Replies: 4
- Views: 411
Re: Hw Problem 6D.11
A good acronym for remembering all of the strong acids is B I C P E N S
- B --> HBr (hydrobromic acid)
- I --> HI (hydroiodic acid)
- C --> HCl (hydrochloric acid)
- P E --> HClO4 (perchloric acid)
- N --> HNO3 (nitric acid)
- S --> H2SO4 (sulfuric acid)
- B --> HBr (hydrobromic acid)
- I --> HI (hydroiodic acid)
- C --> HCl (hydrochloric acid)
- P E --> HClO4 (perchloric acid)
- N --> HNO3 (nitric acid)
- S --> H2SO4 (sulfuric acid)
- Sat Dec 07, 2019 9:54 pm
- Forum: Identifying Acidic & Basic Salts
- Topic: AlCl3 & Cu(NO3)2
- Replies: 3
- Views: 436
Re: AlCl3 & Cu(NO3)2
The Al 3+ ion that dissociates when you dissolve AlCl3 is especially noteworthy: This is a small, highly charged ion. Because it has a small atomic radius and a high oxidation state, it has a strong pull on electron pairs and will form coordinate covalent bonds more easily. The Al 3+ ion will lower ...
- Sat Dec 07, 2019 9:49 pm
- Forum: Identifying Acidic & Basic Salts
- Topic: Knowing strong acids and bases
- Replies: 8
- Views: 568
Re: Knowing strong acids and bases
I don't think elements from groups 1 and 2 form oxoacids. Alkali metals and alkali earth metals form cations (which are electropositive) and are more likely to be bonded to a hydroxide group, which is basic. Generally, nonmetals will form oxoacids. Examples of these include the strong acids H2SO4 (s...
- Sat Dec 07, 2019 9:44 pm
- Forum: Identifying Acidic & Basic Salts
- Topic: Nonmetal oxides
- Replies: 2
- Views: 259
Re: Nonmetal oxides
This isn't an all-encompassing rule. Although nonpolar compounds don't dissolve in water, some nonmetal oxides will dissolve in water. Make sure you note the exception of carbon dioxide dissolving in water to create a weak carbonic acid. This is an especially important reaction to know since it also...
- Sun Dec 01, 2019 10:19 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: D-block
- Replies: 4
- Views: 395
Re: D-block
It's not that d-block elements are more electronegative. Because transition elements more easily form differing order cations, they are stronger Lewis acids and will form coordinate covalent bonds more easily.
Re: chelating
Is chelating another name for the formation of a polydentate? Or is chelating just the occurrence of a ring made by ligands?
Is there any way to tell a coordination compound is chelating through its formula name? Or do you need to do a Lewis structure sketch?
Is there any way to tell a coordination compound is chelating through its formula name? Or do you need to do a Lewis structure sketch?
- Sun Dec 01, 2019 10:14 pm
- Forum: Naming
- Topic: -bis, -tris, etc
- Replies: 5
- Views: 457
Re: -bis, -tris, etc
To clarify for anyone reading the post, polydentate suggests that there is a greek prefix to the ligand name.
For example, ethylenediamine (as mentioned above) is a bidentate; you need to use the altered suffixes.
For example, ethylenediamine (as mentioned above) is a bidentate; you need to use the altered suffixes.
- Sun Dec 01, 2019 10:10 pm
- Forum: Naming
- Topic: Water in Coordination Compounds
- Replies: 7
- Views: 605
Re: Water in Coordination Compounds
When writing the formula for the coordination compound (as opposed to simply stating what the ligand is or drawing the lewis structure), should water be written as H2O or OH2?
- Sun Dec 01, 2019 8:22 pm
- Forum: Hybridization
- Topic: 2F. 15 General Pattern?
- Replies: 2
- Views: 248
2F. 15 General Pattern?
2F. 15 reads: "Noting that the bond angle of an sp3 hybridized atom is 109.5 and that of an sp2 hybridized atom is 120, do you expect the bond angle between two hybrid orbitals to increase or decrease as the s-character of the hybrids is increased?" My first question is: what is the s-char...
- Sun Nov 24, 2019 9:31 pm
- Forum: Hybridization
- Topic: dsp^3 vs sp^3d
- Replies: 1
- Views: 123
Re: dsp^3 vs sp^3d
The order doesn’t really make a difference, but it’s a good idea to write in order of increasing sublevel. sp^3d is the preferred notation.
- Sun Nov 24, 2019 9:29 pm
- Forum: Hybridization
- Topic: sigma or pi?
- Replies: 20
- Views: 1178
Re: sigma or pi?
Just to clarify: a single bond is one sigma, a double bond is a sigma and a pi, and a triple bond is a sigma and two pi bonds.
- Sun Nov 24, 2019 9:19 pm
- Forum: Hybridization
- Topic: C2H4
- Replies: 2
- Views: 195
Re: C2H4
Each carbon has three hybridized sp2 orbitals: two bonded with an s orbital in hydrogen, and one bonded to an sp2 orbital in the opposite carbon.
- Sun Nov 24, 2019 9:17 pm
- Forum: Hybridization
- Topic: H
- Replies: 3
- Views: 262
Re: H
To add, not all orbitals in an energy level are necessarily hybridized (the hybridized orbitals are for creating sigma bonds, and then pi bonds would be unhybridized)
- Sun Nov 24, 2019 9:17 pm
- Forum: Hybridization
- Topic: H
- Replies: 3
- Views: 262
Re: H
Hybridization also provides a practical explanation for VSEPR shapes that wouldn’t be consistent with the limited view basic orbital energy levels would suggest.
- Sun Nov 17, 2019 11:56 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Radicals and molecular shape
- Replies: 5
- Views: 379
Re: Radicals and molecular shape
Do single electrons in a radical create weaker repulsion than lone pairs?
- Sun Nov 17, 2019 11:53 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Multiple central atoms
- Replies: 2
- Views: 226
Re: Multiple central atoms
As a running rule, is it safe to determine bond angles by looking at single central molecules and determine polarity by looking at the whole molecule?
- Sun Nov 17, 2019 11:31 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Repulsion Power
- Replies: 1
- Views: 214
Repulsion Power
Similarly to lone pair repulsion, do double bonds (or triple bonds) create more repulsion than single bonds?
- Sun Nov 17, 2019 11:15 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Lone vs. Bonding Pair
- Replies: 6
- Views: 388
Re: Lone vs. Bonding Pair
Lone pairs also exist closer to other bonds in the molecule; they aren’t pulled away from the central atom by another electronegative atom. Therefore, they will have more repulsion than a bond.
- Sun Nov 17, 2019 11:12 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: T-shape
- Replies: 6
- Views: 510
Re: T-shape
T-shaped and seesaw both describe molecules with 5 bonding sites. However, T-shaped molecules have two lone pairs and 3 bonds, while seesaw molecules have one lone pair and 4 bonds.
- Sun Nov 17, 2019 11:07 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Instantaneous Dipole versus Induced Dipole
- Replies: 4
- Views: 355
Re: Instantaneous Dipole versus Induced Dipole
It is possible for a dipole created by a polar molecule can induce a dipole on a nonpolar molecule.
This kind of molecular interaction is described as dipole-induced dipole.
This kind of molecular interaction is described as dipole-induced dipole.
- Sun Nov 17, 2019 11:05 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: 2E. 5 Question
- Replies: 4
- Views: 246
Re: 2E. 5 Question
The chlorite ion has chlorine at the center connected by a single bond to one oxygen and a double bond connected to the other. There are also two lone pairs on the chlorine. From here it follows that the structure would have the same angles as a tetrahedral structure, except less than 120 degrees du...
- Sun Nov 17, 2019 7:24 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSEPR Angles
- Replies: 4
- Views: 304
Re: VSEPR Angles
A followup question:
How do we determine if the bond angles are greater than or less than 109.5 degrees (or 90 degrees or 120 degrees)?
Do lone pairs repel more than bonding sites?
How do we determine if the bond angles are greater than or less than 109.5 degrees (or 90 degrees or 120 degrees)?
Do lone pairs repel more than bonding sites?
- Fri Nov 08, 2019 3:23 pm
- Forum: Coordinate Covalent Bonds
- Topic: Coordinate vs polar covalent
- Replies: 10
- Views: 2506
Re: Coordinate vs polar covalent
A coordinate covalent will occur between a Lewis acid and a Lewis base (think of NH3 or F- sharing a pair of electrons). A polar covalent bond is when one electron is shared between two atoms; polar describes the ionic character of the bond. More electrons in the electron cloud will be pulled toward...
- Fri Nov 08, 2019 3:19 pm
- Forum: Bond Lengths & Energies
- Topic: dissociation energy
- Replies: 6
- Views: 417
Re: dissociation energy
I think our material only covers the definition and patterns surrounding dissociation energy. We will calculate the dissociation energy of bonds next quarter in Chem 14B. However, I remember in high school chemistry that bond dissociation energy is measured by the change in enthalpy ( \Delta H ) as ...
- Fri Nov 08, 2019 3:10 pm
- Forum: General Science Questions
- Topic: Test 2
- Replies: 20
- Views: 856
Re: Test 2
If I want to look ahead, how far will the test cover? Does anyone know if it goes into the next focus group or the next two focus groups?
- Fri Nov 08, 2019 3:08 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Dispersion forces
- Replies: 6
- Views: 245
Re: Dispersion forces
A dispersion force is synonymous with induced dipole-induced dipole forces. To go into a deeper definition, the electrons holding together a polar molecule will shift around and create dipoles for split seconds at a time. When two nonpolar molecules interact during this shift, they will be held toge...
- Fri Nov 08, 2019 3:06 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Shape of Molecule
- Replies: 5
- Views: 315
Re: Shape of Molecule
To add on: When two oblong polar molecules have induced-dipole induced-dipole attraction, the distance between the two dipoles is much shorter in comparison to the attraction between two spherical molecules. This allows longer polar molecules to orient themselves such that they align and have strong...
- Fri Nov 01, 2019 9:44 am
- Forum: Formal Charge and Oxidation Numbers
- Topic: Formal Charges on Atoms Summed in Ions?
- Replies: 6
- Views: 225
Re: Formal Charges on Atoms Summed in Ions?
In covalent bonding with a zero charge, the formal charges will always work out to cancel each other out. For example, the least stable resonance hybrid of HClO4 has all single bonds. The formal charges on three oxygens are -1 (6 valence - (1 bonding site + 6 lone electrons), and the formal charge o...
- Fri Nov 01, 2019 9:40 am
- Forum: Ionic & Covalent Bonds
- Topic: Radicals
- Replies: 9
- Views: 635
Re: Radicals
Like Professor Lavelle said in class, radicals are also formed through a high energy catalyst. The examples of a photon hitting a water molecule in the upper atmosphere to form hydroxide or the formation of methyl during the burning of hydrocarbons show that radicals are formed under non-standard co...
- Fri Nov 01, 2019 9:34 am
- Forum: Octet Exceptions
- Topic: Why do these not have octets?
- Replies: 3
- Views: 185
Re: Why do these not have octets?
For Part C, there is a triple bond between the carbon and the oxygen, and again, each atom has one lone pair. The formal charge works out to be +1 for the oxygen and -1 for the carbon. In the same manner that the octets for Part A are fulfilled, the octets are also fulfilled for the triple bond betw...
- Fri Nov 01, 2019 9:29 am
- Forum: Octet Exceptions
- Topic: Why do these not have octets?
- Replies: 3
- Views: 185
Re: Why do these not have octets?
For Part A of this question, the nitrogen and oxygen are actually connected by a triple bond, with each atom having a lone pair of electrons. The formal charge ends up being zero on the nitrogen (5 valence - (6bonding/2 +2 lone electrons) and +1 on the oxygen (6 valence - (6bonding/2 +2 lone electro...
- Fri Nov 01, 2019 9:22 am
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Polarizability
- Replies: 3
- Views: 201
Re: Polarizability
Another way of looking at this is the equation for Coulombic potential energy: E=\frac{kq_{1}q_{2}}{r} where k is a proportional constant, q1 is the charge of the anion, q2 is the charge of the anion, and r is the distance between the two nuclei. As you can understand from this equation, the greater...
- Mon Oct 28, 2019 2:10 am
- Forum: Lewis Structures
- Topic: 2B.9
- Replies: 2
- Views: 118
Re: 2B.9
This problem uses polyatomic ions, so it's important to show the distinction between a covalently bonded molecule, and an ionically bonded molecule.
- Mon Oct 28, 2019 2:05 am
- Forum: Octet Exceptions
- Topic: expanded octet?
- Replies: 9
- Views: 476
Re: expanded octet?
Looking at the periodic table, elements with unfilled d-orbitals will be able to sustain expanded octets. Because phosphorus, sulfur, and chlorine are in Period 3, they don't have filled d-orbitals. Thus, they will accept bonding pairs.
- Mon Oct 28, 2019 2:01 am
- Forum: Resonance Structures
- Topic: Delocalization
- Replies: 6
- Views: 417
Re: Delocalization
When a molecule has two or more possible structures, it will form a resonance hybrid. This means that bonds will change around a molecule at a pace such that recorded bond length will reach an average of all resonance hybrids (with those with the least energy dominating the average). Delocalized ele...
- Mon Oct 28, 2019 1:56 am
- Forum: Lewis Structures
- Topic: how to know which elements bonds to which?
- Replies: 3
- Views: 99
Re: how to know which elements bonds to which?
The atom will bond such that formal charges for each atom are closest to zero and each octet is satisfied. The most important thing to remember is that each Lewis structure is a puzzle that doesn't have "wrong" answers, just ones that are more right than others. (Take this with a grain of ...
- Mon Oct 28, 2019 1:53 am
- Forum: Ionic & Covalent Bonds
- Topic: Anions and Cations
- Replies: 4
- Views: 251
Re: Anions and Cations
The pattern of ionization follows the number of valence electrons, as well as the trend of ionization energy across a period. As you go across a period, it will take more energy to remove an electron (because the effective nuclear charge increases). Therefore, atoms with more than four valence elect...
- Sun Oct 20, 2019 8:25 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: How are x-rays and gamma rays emitted?
- Replies: 1
- Views: 100
How are x-rays and gamma rays emitted?
Out of curiosity, how are x-rays and gamma rays emitted from atoms? The Lyman series (UV light) deals with all electrons returning to n=1, the Balmer series (visible light) deals with returns to n=2, and the Paschen series (infrared) is for returns to n=3, but there is no energy level for electrons ...
- Sun Oct 20, 2019 8:19 pm
- Forum: Properties of Light
- Topic: Homework 4
- Replies: 4
- Views: 223
Re: Homework 4
I think your TA will accept any problems you haven't submitted already. However, it's always a good idea to submit homework that relates to the lecture material.
- Sun Oct 20, 2019 8:16 pm
- Forum: Trends in The Periodic Table
- Topic: Period 5 trend
- Replies: 2
- Views: 139
Re: Period 5 trend
The 4d-orbitals also exist closer to the nucleus. I've always thought about it in terms of resisting the positive nuclear charge. If an electron is further away from the nucleus, it needs more energy to resist the attractive charge.
- Sun Oct 20, 2019 8:09 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Spin numbers
- Replies: 4
- Views: 194
Re: Spin numbers
There isn't a way to specifically find the spin of an electron short of direct observation, but you should know this: In a single orbital, as stated by the Pauli Exclusion Principle, there is only space for two electrons. One electron is spinning on an arbitrary circular path, and the other electron...
- Sun Oct 20, 2019 8:05 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Orbitals
- Replies: 4
- Views: 183
Re: Orbitals
To add on, this occurrence is called electron shielding. Because there are more electrons between the positively charged nucleus and the negatively charged electron in the d-orbital (as in the electrons in the s and p orbitals), the effective nuclear charge decreases. The shielding electrons create ...
- Sun Oct 20, 2019 8:01 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: 1E. 1
- Replies: 4
- Views: 315
Re: 1E. 1
All of these also increase for a hydrogen atom as well. I think the important part of the question is understanding why these increase in a hydrogen atom versus a lithium atom. For a, b, and c, (all properties that apply to single-electron systems), the reason for the increase is the same between hy...
- Mon Oct 14, 2019 12:25 am
- Forum: Quantum Numbers and The H-Atom
- Topic: Atomic Spectra!!
- Replies: 2
- Views: 100
Re: Atomic Spectra!!
This problem is best solved by working backward. Because you know that the light emitted is in the ultraviolet spectrum, you know the electron ended at n=1. Using the given wavelength, find the frequency with the equation: \nu =\frac{c}{\lambda } Then find the energy of the light emitted with the eq...
- Mon Oct 14, 2019 12:18 am
- Forum: DeBroglie Equation
- Topic: Objects equation is used for
- Replies: 2
- Views: 188
Re: Objects equation is used for
The equation can be used for all objects. However, objects with a higher mass (such as the baseball example from class) will not have detectable DeBroglie wavelengths.
The equation is the best fit for objects with very low masses, comparable with that of an electron.
The equation is the best fit for objects with very low masses, comparable with that of an electron.
- Sun Oct 13, 2019 5:56 pm
- Forum: Properties of Light
- Topic: 1A.9
- Replies: 5
- Views: 170
Re: 1A.9
In more detail, multiply the given frequency by Planck's constant (h=6.626 * 10^-34).
And just if anyone wants more info on frequency to wavelength, to convert from frequency to wavelength, all you have to do is divide the speed of light (c=2.998 * 10^8 m/s) by the given frequency.
:)
And just if anyone wants more info on frequency to wavelength, to convert from frequency to wavelength, all you have to do is divide the speed of light (c=2.998 * 10^8 m/s) by the given frequency.
:)
- Sun Oct 13, 2019 5:50 pm
- Forum: Properties of Light
- Topic: Finding Energy for Single Electron Systems (non-hydrogen)
- Replies: 1
- Views: 33
Finding Energy for Single Electron Systems (non-hydrogen)
We learned that the equations: E_{n}= \frac{-hR}{n^2} and \Delta E=E_{final}-E_{initial} only works for hydrogen, but I remember Professor Lavelle talking about non-hydrogen single-electron systems. Would this same equation work for an atom of Lithium with only one electron? Would the equation be mo...
- Sun Oct 13, 2019 5:40 pm
- Forum: DeBroglie Equation
- Topic: De Broglie's Equation
- Replies: 4
- Views: 249
Re: De Broglie's Equation
Can anyone explain or outline the physics-based proof for the DeBroglie Equation?
I understand that it shouldn't be used for light and that it applies to electrons (thus you shouldn't use it in relation to equations like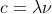 or E=h
or E=h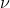 ) but I'm interested in learning more!
) but I'm interested in learning more!
I understand that it shouldn't be used for light and that it applies to electrons (thus you shouldn't use it in relation to equations like
- Sun Oct 06, 2019 10:47 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: G5
- Replies: 6
- Views: 528
Re: G5
To solve this, because the solutions are equimolar, I set up the proportion:

If you cross multiply to solve for volume, you'll get V=13.5ml
If you cross multiply to solve for volume, you'll get V=13.5ml
- Sun Oct 06, 2019 10:38 pm
- Forum: Limiting Reactant Calculations
- Topic: M19
- Replies: 3
- Views: 249
Re: M19
I broke down this kind of problem into three major steps: 1. Using the given masses, determine the moles of *ATOMS* of each element. Note that in this problem, oxygen is also on the reactant side. 2. From here, divide the number of moles of each element by the smallest value. If you end up with frac...
- Sun Oct 06, 2019 10:30 pm
- Forum: Properties of Light
- Topic: Quantum Mechanics
- Replies: 9
- Views: 373
Re: Quantum Mechanics
It looks like we're all asking questions based on the first quantum mechanics lecture, so I think this belongs here. Professor Lavelle was talking about how electric energy oscillates up and down, and magnetic oscillates orthogonal to that. Do the two components interact in any way? I've never learn...
- Sun Oct 06, 2019 10:19 pm
- Forum: Limiting Reactant Calculations
- Topic: L 35 Textbook Typo [ENDORSED]
- Replies: 4
- Views: 395
L 35 Textbook Typo [ENDORSED]
Hi conscientious student! If you're reading this, that means you're doing your homework. Keep going, you got this. :) Anyways, I just wanted to let everyone working from the looseleaf textbook that there is a typo on the question L35. The textbook reads (unbalanced equation): Fe + Br --> FeBr2 FeBr2...
- Sun Oct 06, 2019 10:08 pm
- Forum: Properties of Light
- Topic: Amplitude? [ENDORSED]
- Replies: 8
- Views: 552
Re: Amplitude? [ENDORSED]
Is there an equation that defines amplitude as a variable of wavelength and/or frequency? I discussed with my TA that amplitude changes when frequency changes to maintain the constant c (speed of light), but it would be helpful if someone could further elaborate or explain. I'm still not quite under...

