Search found 104 matches
- Thu Mar 12, 2020 10:09 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Frequency Factor
- Replies: 6
- Views: 496
Re: Frequency Factor
It's pretty much the likelihood of a molecular collision to occur. An H+ and H+ collision is way more likely to occur then an H H O since the molecules have to hit each other at a certain angle in order for the bonds to form.
- Thu Mar 12, 2020 10:04 am
- Forum: Administrative Questions and Class Announcements
- Topic: Final
- Replies: 12
- Views: 842
Re: Final
You can access the final from the website and it will be open book. However, I do not know if it will be similar to the Audio-Visual Assessments in that it will be multiple choice or free response. I expect it to be free response and fairly simple.
- Thu Mar 12, 2020 10:02 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: 7D7
- Replies: 2
- Views: 218
Re: 7D7
An Endothermic reaction requires an input of energy to get over the hump that is the Activation Energy, in order to turn Reactants into Products. It makes sense if you think about which one requires more energy. Since an endothermic reaction requires an input, but an exothermic does not, you know th...
- Thu Mar 12, 2020 9:53 am
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: kinetics vs. thermodynamics
- Replies: 23
- Views: 1302
Re: kinetics vs. thermodynamics
So Kinetics deals with how fast or slow a species react, but Thermodynamics deals with how stable they are in one state or another. That's essentially what the question is asking.
- Thu Mar 12, 2020 9:46 am
- Forum: Administrative Questions and Class Announcements
- Topic: "Open Book" Final?
- Replies: 30
- Views: 2124
Re: "Open Book" Final?
Mariah wrote:Any word on how long it will be ?
He mentioned that it was going to be pretty short and simple so I would assume no more than 1.5 hours, so half of what the actual final would have been like.
- Sun Mar 08, 2020 8:05 pm
- Forum: Student Social/Study Group
- Topic: Test 2 Homework Problems, Etc
- Replies: 6
- Views: 445
Re: Test 2 Homework Problems, Etc
The only awkward part I had when doing the homework was the way in which they set up the reactions, something my TA also addressed. Other than that its simply finding out which equation to use and what components are needed.
- Sun Mar 08, 2020 8:03 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: galvanic vs electrolytic
- Replies: 12
- Views: 909
Re: galvanic vs electrolytic
Galvanic Cell and Electrolytic Cells are essentially opposites
Galvanic Cell =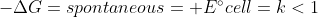
Electrolytic Cell =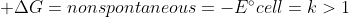
Galvanic Cell =
Electrolytic Cell =
- Sun Mar 08, 2020 8:01 pm
- Forum: Balancing Redox Reactions
- Topic: Redox Table
- Replies: 6
- Views: 429
Re: Redox Table
If you are asking whether or not to change the sign when subtracting the anode from cathode then remember this simple rule. When you are doing E(cell) = E(cathode) - E(anode), then you use the numbers given as is, and DO NOT change the sign based on whether its Oxidized or not. For example If your r...
- Sun Mar 08, 2020 7:53 pm
- Forum: Balancing Redox Reactions
- Topic: Balancing Basic Redox Reactions
- Replies: 6
- Views: 490
Re: Balancing Basic Redox Reactions
Just remember that when balacing basic solutions you balance the O with OH- and the H with H20
- Fri Mar 06, 2020 1:28 pm
- Forum: General Science Questions
- Topic: Test 2 Grades [ENDORSED]
- Replies: 23
- Views: 1639
Re: Test 2 Grades [ENDORSED]
I wouldn't be surprised if we get our tests back on Wednesday as the Ta's might have to work on the final so they get the tests over with first.
- Fri Mar 06, 2020 1:25 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Nernst
- Replies: 10
- Views: 647
Re: Nernst
Instead of having to plug in all of those constants you can remember it as something that looks like this
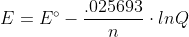
Here the RT/F is converted into .025693 to make the equation simpler.
Here the RT/F is converted into .025693 to make the equation simpler.
- Wed Mar 04, 2020 11:13 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Finding Q
- Replies: 7
- Views: 560
Finding Q
When we are given a Cell Diagram and concentrations and asked to find the Q what do we use as the Products and Reactants. Would we not use the right side (cathode, reduced) side as the Product and the (anode,oxidized) side as the Reactant?
- Sun Mar 01, 2020 2:37 pm
- Forum: First Order Reactions
- Topic: first order
- Replies: 4
- Views: 362
Re: first order
First order reactions and second order reactions are way more common than third and forth, those are very unlikely because it requires 3 or 4 separate entities to perfectly come together, which is very unlikely.
- Sun Mar 01, 2020 2:34 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Balancing react & prod in cell diagram
- Replies: 2
- Views: 224
Re: Balancing react & prod in cell diagram
When you write the cell diagram you do not include the balancing stoichiometric number. Essentially, you don't include any coefficients.
- Sun Mar 01, 2020 2:31 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: non ideal gases
- Replies: 1
- Views: 136
Re: non ideal gases
If you are referring to what we learned on Friday in class and the n in the following equation Rate = k[A]^{n} [B]^{m} then you need to do the following You essentially gather all of the data you have in terms of which experiment you are doing and compare the rates at which the equation changes. So ...
- Sun Mar 01, 2020 2:23 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: salt bridge
- Replies: 11
- Views: 689
Re: salt bridge
So [censored] electrons flow from the anode to the cathode, essentially leaves a more positive charge on the left side (anode) compared to the cathode. The salt bridge neutralizes that charge by allowing electrons to migrate back to the left via a sort of concentration gradient where solutes will al...
- Sun Mar 01, 2020 2:20 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Galvanic Cells
- Replies: 6
- Views: 444
Re: Galvanic Cells
So essentially there are 2 sets of correlations, if that's a way to put it, but you can remember it as the following.
Galvanic Cell = -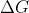 = Spontaneous = + E knot = K > 1
= Spontaneous = + E knot = K > 1
Electrolytic Cell =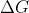 = non spontaneous = - E knot = K < 1
= non spontaneous = - E knot = K < 1
Galvanic Cell = -
Electrolytic Cell =
- Sun Mar 01, 2020 2:16 pm
- Forum: Balancing Redox Reactions
- Topic: H+ or H2O
- Replies: 9
- Views: 638
Re: H+ or H2O
One of the rules our TA taught us regarding this was that it's based off what type of reaction you have.
If it's acidic, balance O with water, then balance H with protons
If it's basic, balance O with OH-, and H with H20
If it's acidic, balance O with water, then balance H with protons
If it's basic, balance O with OH-, and H with H20
- Thu Feb 27, 2020 10:19 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gas constant R
- Replies: 5
- Views: 354
Re: Gas constant R
Essentially look at what units you have and what units you need to cancel out so that it makes sense for Gibbs Free Energy. Gibbs Free Energy should be kj.mol^-1
- Thu Feb 27, 2020 10:15 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagrams
- Replies: 2
- Views: 249
Re: Cell Diagrams
Within a battery, electrons are pumped away from the anode and into the cathode. I believe the same can be said for electrolytes as they become ions when dissolved in water.
- Thu Feb 27, 2020 10:13 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Electrolyte
- Replies: 4
- Views: 299
Re: Electrolyte
An electrolyte is something that dissolves into ions when placed in a solution such as water.
- Thu Feb 27, 2020 10:12 pm
- Forum: Administrative Questions and Class Announcements
- Topic: UA sessions
- Replies: 5
- Views: 339
Re: UA sessions
I'm sure if you have a quick question regarding the topic the UA's will be more than happy to help.
- Thu Feb 27, 2020 10:11 pm
- Forum: General Science Questions
- Topic: Test 2
- Replies: 16
- Views: 1010
Re: Test 2
Although it covers the second page of Outline 4 and all of Outline 5, many of the concepts in Outline 4 will be needed for Outline 5 as well as determining concentrations.
- Sun Feb 16, 2020 8:36 pm
- Forum: Calculating Work of Expansion
- Topic: Work without volume
- Replies: 5
- Views: 405
Re: Work without volume
I don't know if this really helps, but if we are given the Internal Energy, q, or things that can be used to find q such as 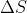 and t, then we can find q using
and t, then we can find q using 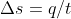 . Once you find the q, you can plug it into
. Once you find the q, you can plug it into 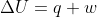 to get w.
to get w.
- Sun Feb 16, 2020 8:33 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Bond Enthalpies with Molecules
- Replies: 4
- Views: 302
Re: Bond Enthalpies with Molecules
The type of bond has a constant enthalpy which will always be provided were we to have to solve for it. My advice for calculating these problems is to draw it out first and write out how many of what type of bond you have. Once you have that, you can cancel out duplicates as this will make the calcu...
- Sun Feb 16, 2020 8:31 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: memorize
- Replies: 14
- Views: 868
Re: memorize
I believe that every constant we may ever need will be provided for us on every test and exam. However, some equations may not be and we may have to derive them.
- Sun Feb 16, 2020 8:28 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Spontaneous
- Replies: 13
- Views: 826
Re: Spontaneous
When Delta G is negative, the reaction is exothermic. This means that it releases heat and does not require an input of energy; hence, it is spontaneous. An exothermic reaction indicates that an input of energy is required. This makes it a non-spontaneous reaction as it needs something else (not spo...
- Sun Feb 16, 2020 8:25 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Meaning of q=-w
- Replies: 14
- Views: 2375
Re: Meaning of q=-w
If the Internal Energy of the System is equal to 0, then q must = - w so that they cancel out and you're left with 0.
- Sun Feb 09, 2020 1:05 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Higher the heat capacity
- Replies: 4
- Views: 139
Re: Higher the heat capacity
It is the heat required to change 1 mole of a substance, by 1 Degree Celsius
- Sun Feb 09, 2020 1:04 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Spontaneous Reaction
- Replies: 8
- Views: 401
Re: Spontaneous Reaction
By 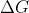 being Negative, we know that this is an exothermic reaction. As it is an exothermic reaction, we don't need and input of energy for it to proceed. As a result, the reaction is favorable and thus, spontaneous.
being Negative, we know that this is an exothermic reaction. As it is an exothermic reaction, we don't need and input of energy for it to proceed. As a result, the reaction is favorable and thus, spontaneous.
- Sun Feb 09, 2020 1:02 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Calculating Bond Enthalpies
- Replies: 5
- Views: 201
Re: Calculating Bond Enthalpies
Ya, I would simply draw out the Lewis Structures then compare the bonds. If one structure has 2 C-C bonds and the other has 1 C-C bond, then you can cancel 1 from each side to make the calculations easier.
- Sun Feb 09, 2020 12:59 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Finding whether exothermic or Endothermic
- Replies: 4
- Views: 186
Re: Finding whether exothermic or Endothermic
One of the ways you can tell if an equation is exothermic or endothermic is using Gibbs Free Energy.
If the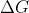 is negative then it is exothermic, if it is positive then it is endothermic.
is negative then it is exothermic, if it is positive then it is endothermic.
If the
- Sun Feb 09, 2020 12:57 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: change in entropy
- Replies: 3
- Views: 282
Re: change in entropy
I don't know the first part to your question, but I do know the equation used to calculate enthalpy.
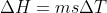
The Delta H = Change in Enthalpy
The m = mass
The s = heat
and the Delta T = the temperature change
The Delta H = Change in Enthalpy
The m = mass
The s = heat
and the Delta T = the temperature change
- Sun Feb 02, 2020 4:13 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Isolated vs Closed [ENDORSED]
- Replies: 34
- Views: 4337
Re: Isolated vs Closed [ENDORSED]
Open System ( matter & energy can exchange with surroundings) Beaker of Water Water can evaporate & Beaker does not insulate Closed System( energy can exchange with surroundings) Sealed beaker of water Beaker does not insulate Isolated System ( nothing exchanged with surroundings) Combustion...
- Sun Feb 02, 2020 4:11 pm
- Forum: Phase Changes & Related Calculations
- Topic: phase change from liquid to vapor
- Replies: 8
- Views: 370
Re: phase change from liquid to vapor
The process of steaming converting back into water as it touches your skin releases a lot of heat as a result of the phase change. That transition emits heat which can be seen as heat on top of the pre-existing 100 degrees.
- Sun Feb 02, 2020 4:05 pm
- Forum: Calculating Work of Expansion
- Topic: Work positive or negative
- Replies: 5
- Views: 207
Re: Work positive or negative
Can we think of work as either exothermic and endothermic. The work can be in the form of heat so would that be a valid statement.
- Fri Jan 31, 2020 1:14 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Test 1 # 4
- Replies: 10
- Views: 390
Test 1 # 4
Can someone please explain how to do #4 on Test # 1. The questions states
There is an unknown quantity of Xenon in a 25 L container at .50 atm and 27 C. How many grams of Xenon are present?
There is an unknown quantity of Xenon in a 25 L container at .50 atm and 27 C. How many grams of Xenon are present?
- Fri Jan 31, 2020 1:11 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Midterm
- Replies: 8
- Views: 384
Re: Midterm
Based off last years midterm I would say most of the content will revolve around the new topics. As we have already been tested on Chemical Equillibrium and Acids and Bases expect more Thermodynamics and Thermochemistry. However, I believe that there will be 1 or 2 questions covering the entire sect...
- Mon Jan 27, 2020 3:13 pm
- Forum: Phase Changes & Related Calculations
- Topic: State Property
- Replies: 6
- Views: 206
Re: State Property
State property is like the distance between UCLA and downtown. The state property distance will be different from the actual route to take their as you can't go through buildings when driving.
- Mon Jan 27, 2020 3:09 pm
- Forum: Student Social/Study Group
- Topic: midterm/final
- Replies: 12
- Views: 2253
Re: midterm/final
The midterms/final are similar in the sense that the questions are sandwiched between an equation sheet and a periodic table. However, the midterms are harder overall as the questions require you master the subject or you will get the question wrong, as well as the follow up questions regarding the ...
- Mon Jan 27, 2020 3:06 pm
- Forum: Phase Changes & Related Calculations
- Topic: ∆H
- Replies: 17
- Views: 681
Re: ∆H
∆H tells us the change in temperature so I don't know what else could tell us if its an endothermic or exothermic reaction.
- Mon Jan 27, 2020 3:03 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3668721
Re: Post All Chemistry Jokes Here
What should you do if noone laughs at your Chemistry joke.
Keep telling them until you get a reaction
Keep telling them until you get a reaction
- Tue Jan 21, 2020 6:25 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Chemical Equilibrium Part 4 Question 13
- Replies: 3
- Views: 194
Chemical Equilibrium Part 4 Question 13
I've been doing the following problem and I feel like I am doing it right, but the results say it is wrong. I would assume that i is neither since the moles are equal on both sides. The pressure increases with the volume decreasing so the chemical reaction would favor the side with less moles but th...
- Sun Jan 19, 2020 7:02 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Buffer
- Replies: 6
- Views: 364
Re: Buffer
It's just an aqueous solution of a weak acid and a conjugate base that helps keep the pH at a constant value.
- Sun Jan 19, 2020 7:01 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Molar concentration of acids & bases
- Replies: 8
- Views: 409
Re: Molar concentration of acids & bases
Just remember that pH = -log[H30+] and pOH= - log [OH-] so try not to get them confused. If only one is given, then subtract the pH from 14 to get the other.
- Sun Jan 19, 2020 6:50 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Approximation
- Replies: 6
- Views: 387
Re: Approximation
I doubt there's any science behind it being 5%. Scientists are lazy so they just choose a good number to go by and stick with that. The 5% is just something for us to follow as to whether or not to use the quadratic formula.
- Sun Jan 19, 2020 6:44 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Concentrations
- Replies: 12
- Views: 422
Re: Concentrations
Temperature is the only thing that affects the constant because even if the concentration changes, the equation will do its best to mitigate the change. Le Chatelier's Principle pretty much explains this.
- Sun Jan 19, 2020 6:41 pm
- Forum: Phase Changes & Related Calculations
- Topic: lecture on 1/17
- Replies: 3
- Views: 273
Re: lecture on 1/17
He just gave us examples of calculating the pH of a weak acid and its salt ( to make a buffer) as well as just calculating the pH of a salt solution.
- Sat Jan 11, 2020 7:54 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Difference between K and Q
- Replies: 6
- Views: 282
Re: Difference between K and Q
In addition to what everyone else has said regarding Q being used at any point in the equation, it can alos help us solve problems as to which way the reaction is heading. If Q < K, pre equilibrium, then reaction will proceed forward ( too much reactant , or not enough product) Greater Denominator I...
- Sat Jan 11, 2020 7:51 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Adding inert gas [ENDORSED]
- Replies: 9
- Views: 307
Re: Adding inert gas [ENDORSED]
Will helium always be the inert gas we would add, or what other gases could also work? I'm fairly certain that any inert gas can be added but Helium is the most ideal as it is the least reactive. Helium exists as a single atom so the van der Waals forces are even weaker. This makes it less reactive...
- Sat Jan 11, 2020 7:47 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Simplifying cubic equations
- Replies: 3
- Views: 142
Re: Simplifying cubic equations
The professor said that we won't be dealing with cubic expressions when it comes to finding the concentrations. He did however state that the quadratic formula would not work so you would have to find it by hand or via a calculator.
- Sat Jan 11, 2020 7:38 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: ICE box
- Replies: 9
- Views: 311
Re: ICE box
It can only be the positive number as there is no such thing as a negative concentration
- Sat Dec 07, 2019 5:37 pm
- Forum: Administrative Questions and Class Announcements
- Topic: MARSHMALLOW- FINAL REVIEW SESSION [ENDORSED]
- Replies: 115
- Views: 13321
Re: MARSHMALLOW- FINAL REVIEW SESSION [ENDORSED]
When HCl and CaO are accidentally mixed in the flask, they will react to form salt and water making the following equation: 2HCl + CaO ---> CaCl2 + H2O. Knowing this, find the limiting reactant that was poured into the the flask. (Its CaO) so that means that HCl will be in excess in the solution. U...
- Sat Dec 07, 2019 5:21 pm
- Forum: Administrative Questions and Class Announcements
- Topic: MARSHMALLOW- FINAL REVIEW SESSION [ENDORSED]
- Replies: 115
- Views: 13321
Re: MARSHMALLOW- FINAL REVIEW SESSION [ENDORSED]
Can someone please explain how to do #34. I know that you find the moles then use the excess moles and find the pH using that, but my math doesn't add up. When HCl and CaO are accidentally mixed in the flask, they will react to form salt and water making the following equation: 2HCl + CaO ---> CaCl...
- Sat Dec 07, 2019 5:04 pm
- Forum: Administrative Questions and Class Announcements
- Topic: MARSHMALLOW- FINAL REVIEW SESSION [ENDORSED]
- Replies: 115
- Views: 13321
Re: MARSHMALLOW- FINAL REVIEW SESSION [ENDORSED]
Can someone please explain how to do #34. I know that you find the moles then use the excess moles and find the pH using that, but my math doesn't add up.
- Sat Dec 07, 2019 2:28 pm
- Forum: Identifying Acidic & Basic Salts
- Topic: 6.21
- Replies: 1
- Views: 213
Re: 6.21
It's amphiprotic because it can act as both an acid and base. The nitrogen atoms with lone pairs can accept two protons. It can also accept a proton to form a conjugate base.
- Sat Dec 07, 2019 2:25 pm
- Forum: Biological Examples
- Topic: Hemoglobin and Myoglobin
- Replies: 2
- Views: 149
Re: Hemoglobin and Myoglobin
There are many differences but I think the major difference is that Hemoglobin binds with 4 oxygen molecules while Myoglobin binds with 1.
- Sat Dec 07, 2019 2:18 pm
- Forum: Properties of Electrons
- Topic: Mass of an electron
- Replies: 9
- Views: 778
Re: Mass of an electron
I'm fairly certain that almost all of the equations and constants will be provided for the final.
- Sat Dec 07, 2019 2:18 pm
- Forum: Administrative Questions and Class Announcements
- Topic: MARSHMALLOW- FINAL REVIEW SESSION [ENDORSED]
- Replies: 115
- Views: 13321
Re: MARSHMALLOW- FINAL REVIEW SESSION [ENDORSED]
Does anyone know where the answers to the practice are since its well over the time they were supposed to be posted?
- Sun Dec 01, 2019 4:55 pm
- Forum: Student Social/Study Group
- Topic: readings
- Replies: 13
- Views: 950
Re: readings
The readings are there to get a better understanding of what the professor lectures in class. If there are things you don't get in class, look to the textbook to get a better idea.
- Sun Dec 01, 2019 4:53 pm
- Forum: Lewis Acids & Bases
- Topic: bronsted vs lewis
- Replies: 9
- Views: 585
Re: bronsted vs lewis
I believe the main difference is that bronsted deals with proton donors/acceptors, while Lewis deals with electron donors/acceptors.
- Sun Dec 01, 2019 4:51 pm
- Forum: Naming
- Topic: Roman numerals
- Replies: 6
- Views: 505
Re: Roman numerals
The roman numeral is simply the oxidation number of the central atom that makes the chemical compound neutral
- Sun Dec 01, 2019 4:49 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Oxidation Number
- Replies: 9
- Views: 778
Re: Oxidation Number
The oxidation number is vital to the name as it tells us what the oxidation of the central atom must be to make the compound neutral.
- Sun Nov 24, 2019 4:20 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Ligand
- Replies: 1
- Views: 160
Re: Ligand
Ligands bond to the central metal atom or ion. The ligands act as the Lewis Bases(donors) and the central atom acts as the Lewis Acid (receiver).
- Sun Nov 24, 2019 4:16 pm
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: Drawing sigma & Pi bonds
- Replies: 7
- Views: 640
Re: Drawing sigma & Pi bonds
Sigma and Pi bonds have their own shapes. A sigma bond is more like an oval, while a Pi bond looks like a single macaroni of Kraft Mac n Cheese
- Sun Nov 24, 2019 4:14 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Coordination Number
- Replies: 3
- Views: 257
Re: Coordination Number
By knowing the coordination number we can then determine the name of the coordination complex.
- Sun Nov 24, 2019 4:10 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Ligand
- Replies: 4
- Views: 321
Re: Ligand
The number of ligands are also represented by the X number in the AXE method.
- Sun Nov 24, 2019 4:08 pm
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: AXE
- Replies: 6
- Views: 562
Re: AXE
The X represents the Ligands and the E represents the lone pairs.
- Sat Nov 16, 2019 5:11 pm
- Forum: Student Social/Study Group
- Topic: Test 2
- Replies: 7
- Views: 501
Re: Test 2
Is test 2 just on sections 2E and 2F, or am I forgetting something?
- Sat Nov 16, 2019 5:01 pm
- Forum: Lewis Structures
- Topic: Balanced Lewis Structures
- Replies: 6
- Views: 452
Re: Balanced Lewis Structures
The drawing won't ever be quite balanced as it's a 2 dimensional version of the the shape. What matters more is the formal charge of the Lewis structure.
- Sat Nov 16, 2019 4:58 pm
- Forum: Dipole Moments
- Topic: Hydrogen bonds
- Replies: 17
- Views: 824
Re: Hydrogen bonds
They can only form with Oxygen, Nitrogen, and Fluorine as this is the greatest difference in electronegativity between them and Hydrogen.
- Sat Nov 16, 2019 4:56 pm
- Forum: Dipole Moments
- Topic: Shape of Molecule/Strength of Interactions
- Replies: 4
- Views: 314
Re: Shape of Molecule/Strength of Interactions
It's all based on the surface area and how much surface the atoms can cling on to.
- Sat Nov 16, 2019 4:56 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Polarizability
- Replies: 5
- Views: 500
Re: Polarizability
If the difference in electronegativity for the atoms in a bond is greater than 0.4, then it's polar. If the difference in electronegativity is less than 0.4, then it's non polar.
- Sat Nov 16, 2019 4:53 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: strongest intermolecular forces
- Replies: 7
- Views: 773
Re: strongest intermolecular forces
From what we've learned, Van Der Waals are actually the weakest. That is then followed by Dipole-Dipole which is the 2nd strongest and Hydrogen Bonds which are the strongest. You can look at it like this
Van Der Waals < Dipole-Dipole < Hydrogen Bonds
Van Der Waals < Dipole-Dipole < Hydrogen Bonds
- Sat Nov 09, 2019 10:55 pm
- Forum: Bond Lengths & Energies
- Topic: how to determine the energy of a bond
- Replies: 6
- Views: 569
Re: how to determine the energy of a bond
Bond Energy can be found by subtracting the sum of all the bonds formed from the sum of all bonds broken.
- Sat Nov 09, 2019 10:52 pm
- Forum: Resonance Structures
- Topic: Resonance Structures
- Replies: 18
- Views: 1155
Re: Resonance Structures
Resonance structures are simply alternate Lewis structures for a given ion or molecule.
- Sat Nov 09, 2019 10:45 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Formal Charge
- Replies: 9
- Views: 376
Re: Formal Charge
A formal charge of 0 simply states that a Lewis Structure is at its lowest energy level. Some structures may not have a formal charge of 0, but the goal is to draw a structure so that it is at its lowest formal charge/ more stable.
- Sat Nov 09, 2019 10:40 pm
- Forum: Dipole Moments
- Topic: Van Der Waals
- Replies: 5
- Views: 232
Re: Van Der Waals
Van Der Waals - the weakest interaction and is present in all compounds, something like Iodine and Argong
Dipole-Dipole - stronger, between more positively charged element and negative. An example being Carbon and Oxygen
Hydrogen Bond- strongest, essentially a super bond between H and C,N,O,F
Dipole-Dipole - stronger, between more positively charged element and negative. An example being Carbon and Oxygen
Hydrogen Bond- strongest, essentially a super bond between H and C,N,O,F
- Sat Nov 09, 2019 10:37 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Liquid and Solid formation
- Replies: 2
- Views: 109
Re: Liquid and Solid formation
The balance between the kinetic energies of the molecules and the inter molecular attractions determines whether something will be a solid, liquid, or gas .The attractive forces between the molecules increase as you go down a group so that explains the change.
- Sun Nov 03, 2019 2:37 pm
- Forum: General Science Questions
- Topic: Study Tips
- Replies: 13
- Views: 935
Re: Study Tips
I would do the online modules and go through the problems assigned for homework. I believe all of the questions will be taken from there, so it's almost like an exact study guide.
- Sun Nov 03, 2019 2:35 pm
- Forum: Lewis Structures
- Topic: Names
- Replies: 4
- Views: 273
Re: Names
I doubt it. Unless it is something we learned in class or from our homework. I believe all of the questions will be taken from the homeworks and Online Modules so it will not be something we have never seen before.
- Sun Nov 03, 2019 2:33 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: electron configuration
- Replies: 4
- Views: 208
Re: electron configuration
The electrons are removed from the right most sections of the electron configuration. Electrons are typically removed from the valence shells, which are the highest in s and p orbitals.
- Sun Nov 03, 2019 2:30 pm
- Forum: Dipole Moments
- Topic: Dipole moments
- Replies: 5
- Views: 322
Re: Dipole moments
The dipole moment also tells us about the charge. The bigger the difference in electronegativity, the larger the charge and vice versa.
- Sun Nov 03, 2019 2:27 pm
- Forum: Bond Lengths & Energies
- Topic: Strength of Bonds
- Replies: 16
- Views: 686
Re: Strength of Bonds
Shorter Bonds are stronger since the & less reactive, so triple bonds would be the strongest. Longer bonds are weaker and are more likely to be involved in a reaction, so single bonds are the weakest.
- Sat Oct 26, 2019 11:11 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Lowering Formal Charge
- Replies: 6
- Views: 698
Re: Lowering Formal Charge
A more stable Lewis structure will have a formal charge of 0. That is why we try to draw the Lewis Structure in different ways to see if we can get the formal charge to 0. In the example provided of (S04^2-), the first Lewis Structure we drew had a final formal charge of (-1); whereas, the second on...
- Sat Oct 26, 2019 11:07 pm
- Forum: Ionic & Covalent Bonds
- Topic: Ionic and Covalent Bonds
- Replies: 5
- Views: 268
Re: Ionic and Covalent Bonds
Ionic compounds transfer dots (electrons) from metal to nonmetal atom.
Non metals do not form cations because their ionization energies are too high sot hey share electrons to form covalent bonds.
Non metals do not form cations because their ionization energies are too high sot hey share electrons to form covalent bonds.
- Sat Oct 26, 2019 11:05 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Shorthand Notation
- Replies: 4
- Views: 263
Re: Shorthand Notation
My TA let us write the short form of the electron configuration so I believe this is something one of the mods can clarify. Either way its good to know how to do the whole thing.
- Sat Oct 26, 2019 11:02 pm
- Forum: Lewis Structures
- Topic: Drawing the Lewis Structure
- Replies: 2
- Views: 117
Re: Drawing the Lewis Structure
The best way to draw a Lewis Structure is by putting the element with the ionization energy in the middle. This is because all the other atoms will be placed around. You then want to make sure the dots and bonds drawn correlates to the valence electrons calculated from the elements. Also, make sure ...
- Sat Oct 26, 2019 10:59 pm
- Forum: Bond Lengths & Energies
- Topic: Bond Lengths
- Replies: 4
- Views: 225
Re: Bond Lengths
Double bonds and triple bonds have more electrons so they exert a stronger attraction force. This pulls the atoms closer together, creating shorter bond lengths.
- Sat Oct 19, 2019 7:44 pm
- Forum: Empirical & Molecular Formulas
- Topic: Molecular to Empirical Formula
- Replies: 10
- Views: 996
Re: Molecular to Empirical Formula
The Empirical formula is needed to calculate the molecular formula but cannot be done so without the molar mass. Once you have the molar masses of both the Empirical formula and the Molecular formula you divide the two to get the ration. If E(molar mass)/M(molar mass)= 2, then multiply the Empirical...
- Sat Oct 19, 2019 7:41 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Orbitals in relation to arrows
- Replies: 10
- Views: 679
Re: Orbitals in relation to arrows
Is it possible to have more arrows pointing in one direction than another. If so, what does that entail?
- Sat Oct 19, 2019 7:38 pm
- Forum: Photoelectric Effect
- Topic: Work Funtion
- Replies: 2
- Views: 125
Re: Work Funtion
Both the Work Function and the Threshold Energy function refer to the energy required to eject the electron. They are fundamentally the same when it comes down to calculating. The work function refers to the energy that needs to be put in and the Threshold Energy function refers to the frequency req...
- Sat Oct 19, 2019 7:34 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Rydberg equation [ENDORSED]
- Replies: 73
- Views: 9184
Re: Rydberg equation [ENDORSED]
Are all of these equations meant to be memorized, or will they be provided on tests?
- Sat Oct 19, 2019 7:30 pm
- Forum: Limiting Reactant Calculations
- Topic: Showing Work for Limiting Reactant Calculations on Tests [ENDORSED]
- Replies: 68
- Views: 7104
Re: Showing Work for Limiting Reactant Calculations on Tests [ENDORSED]
I would show all work. I had points taken off my first test for not showing work in some parts. You don't have to show everything step by step, but as long as it shows your calculations you should be fine. If you feel you should have gotten more points for the work you showed you can always talk wit...
- Tue Oct 08, 2019 12:34 pm
- Forum: Limiting Reactant Calculations
- Topic: Showing Work for Limiting Reactant Calculations on Tests [ENDORSED]
- Replies: 68
- Views: 7104
Re: Showing Work for Limiting Reactant Calculations on Tests [ENDORSED]
From what I recall, if you get the answer correct, then you are given full credit as long as some work is shown. However, I would still show work in case I get the problem wrong and my work shown will be worth some partial credit.
- Tue Oct 08, 2019 12:33 pm
- Forum: Balancing Chemical Reactions
- Topic: Fractions
- Replies: 34
- Views: 1445
Re: Fractions
I would personally refrain from keeping fractions in the formula because of how easy of a fix it is. All you really have to do is multiply everything by the denominator to remove the fraction. It's an easy fix for something you won't have to worry about later.
- Tue Oct 08, 2019 12:31 pm
- Forum: Empirical & Molecular Formulas
- Topic: Limiting Reactants
- Replies: 4
- Views: 197
Re: Limiting Reactants
Maybe, but if that's the case then it shouldn't be much harder than finding how much product we can make. We would simply find it by using the remaining moles or even by subtracting what we have from the total
- Tue Oct 08, 2019 12:29 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Word Problem Efficiency
- Replies: 7
- Views: 450
Re: Word Problem Efficiency
The professor went over this in class and the way he taught us seems to be working fine for me. The steps are as follows: Give yourself time, be mindful What is the question? What concepts do I know? What model or equation do I know? What is known? What is unknown? Can I express the unknown with res...
- Tue Oct 08, 2019 12:27 pm
- Forum: Significant Figures
- Topic: How many significant figures are in 7.00 x 10^2?
- Replies: 25
- Views: 3623
Re: How many significant figures are in 7.00 x 10^2?
Sig Figs have to do with any number that isn't a 0 or a 0 between two numbers.
So for example:
72345 has 5 Sig figs
72300 has 3 Sig figs
72032 has 5 sig figs
So in your case there are 3 sig figs.
So for example:
72345 has 5 Sig figs
72300 has 3 Sig figs
72032 has 5 sig figs
So in your case there are 3 sig figs.
- Thu Oct 03, 2019 8:13 pm
- Forum: Properties of Light
- Topic: EM Spectrum
- Replies: 4
- Views: 263
Re: EM Spectrum
I'd assume he 'll provide us with a sort of image/ reference. Just how it's ridiculous to have us memorize the periodic table, so he provides us with one on tests. We are learning about the topic and how to find what it is we are looking for, not memorize something that has already been established.

