Search found 57 matches
- Sun Dec 08, 2019 11:22 pm
- Forum: Biological Examples
- Topic: porphyrin
- Replies: 3
- Views: 419
Re: porphyrin
Adding to Chris, a heme complex binds with a protein and oxygen to make a myoglobin, and four myoglobin make a hemoglobin.
- Sun Dec 08, 2019 11:15 pm
- Forum: Student Social/Study Group
- Topic: amphoteric vs. amphiprotic
- Replies: 4
- Views: 459
Re: amphoteric vs. amphiprotic
I believe amphiprotic relates to bronsted acids and bases
- Sun Dec 08, 2019 11:11 pm
- Forum: Student Social/Study Group
- Topic: heme complex
- Replies: 5
- Views: 648
Re: heme complex
I thought a heme complex had 4?
- Sun Dec 08, 2019 11:10 pm
- Forum: Lewis Acids & Bases
- Topic: Strong/Weak Acids and Bases
- Replies: 4
- Views: 503
Re: Strong/Weak Acids and Bases
Just a little trick for acids, most polyatomic ions that end in "ate", like nitrate and carbonate and sulfate, make strong acids (but not all)
- Sun Dec 08, 2019 11:05 pm
- Forum: *Titrations & Titration Calculations
- Topic: Titrations
- Replies: 2
- Views: 998
Re: Titrations
Here's a picture: 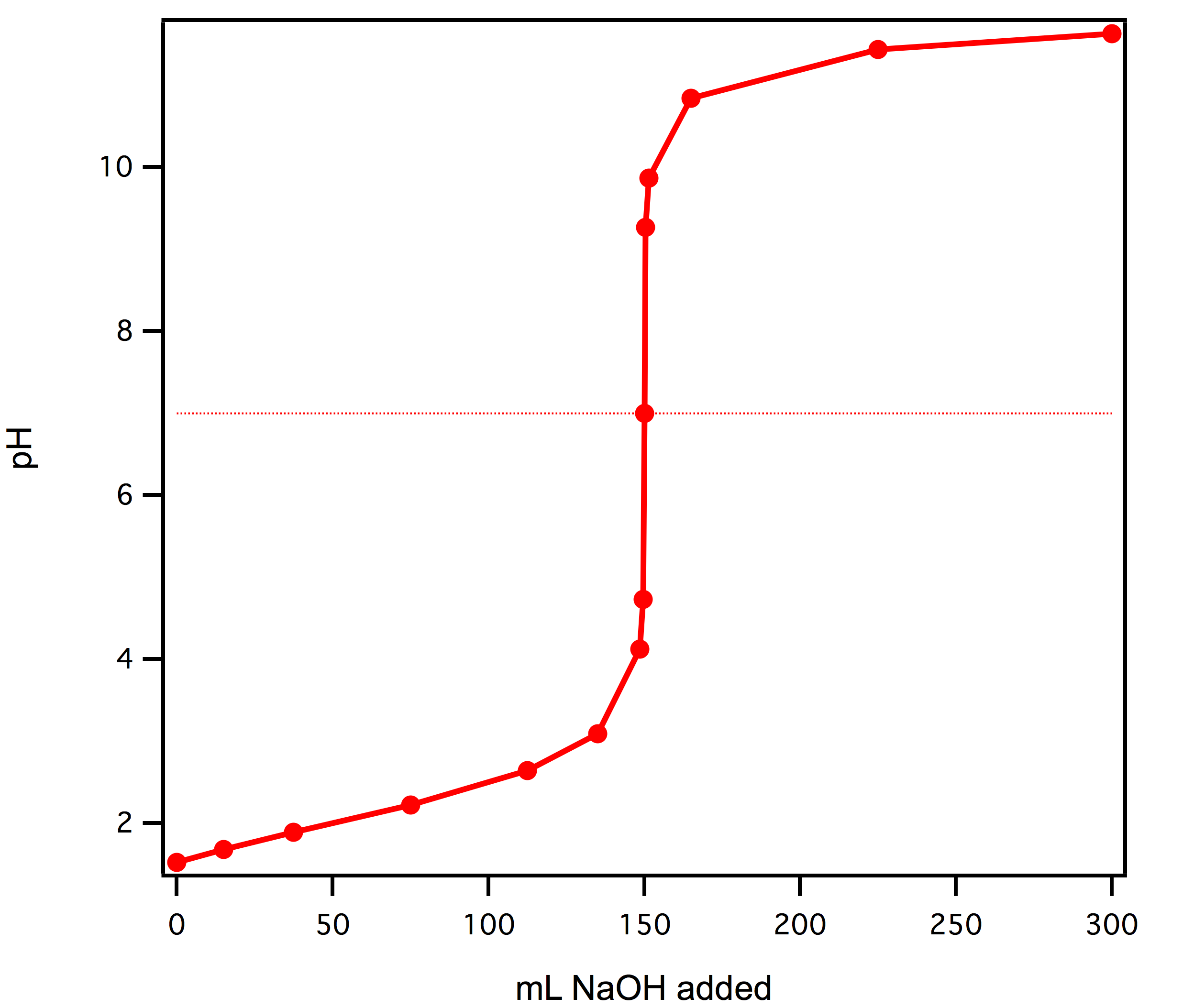

- Sun Dec 01, 2019 10:50 pm
- Forum: Hybridization
- Topic: 2F.5
- Replies: 4
- Views: 371
Re: 2F.5
a) sp
b) sp^2
c) sp^3
d) sp^3
b) sp^2
c) sp^3
d) sp^3
- Sun Dec 01, 2019 10:42 pm
- Forum: Naming
- Topic: Brackets in Chem. Formula
- Replies: 5
- Views: 360
Re: Brackets in Chem. Formula
The contents within the bracket should be the "complex", which is a species consisting of a central metal atom or ion to which a number of molecule or ions are attached by coordinate covalent bonds.
- Sun Dec 01, 2019 10:39 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Bond strength and strength of acid.
- Replies: 6
- Views: 450
Re: Bond strength and strength of acid.
The longer the bond/the weaker the bond, the stronger the acid will be.
- Sun Dec 01, 2019 10:38 pm
- Forum: Sigma & Pi Bonds
- Topic: Sigma vs. Pi
- Replies: 20
- Views: 1295
Re: Sigma vs. Pi
Sigma bonds are stronger because the two orbitals overlap, as opposed to a pi bond where the two orbitals are side by side.
- Sun Dec 01, 2019 10:36 pm
- Forum: Calculating the pH of Salt Solutions
- Topic: Sig Figs for logarithmic funcitons
- Replies: 6
- Views: 456
Re: Sig Figs for logarithmic funcitons
I'm sure the number of sig figs would be the same as the concentration that is given
- Sun Dec 01, 2019 10:30 pm
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: Acid strength relative to kA
- Replies: 2
- Views: 161
Re: Acid strength relative to kA
No, an acid with a lower pKa will have a greater Ka value because a stronger acid (high Ka value) would be more acidic (lower pKa/pH value). It would then make sense that for bases, the lower the Ka value the higher the pKa value.
- Sun Dec 01, 2019 10:18 pm
- Forum: Student Social/Study Group
- Topic: Vocabulary
- Replies: 7
- Views: 541
Re: Vocabulary
I'm sure knowing the key words in the textbook would help (ligand, Bronsted-Lowry Theory, Deprotonated, Conjugate Acid, Conjugate Base, etc.)
- Sun Nov 24, 2019 9:28 pm
- Forum: Hybridization
- Topic: strategy for hybridization
- Replies: 2
- Views: 209
Re: strategy for hybridization
I would just count the amount of bonds and from there write down the orbital that corresponds to it. For example, if there were three bonds, the hybridization would be sp^2 (there is one s orbital and 2 p for a total of 3)
- Sun Nov 24, 2019 9:23 pm
- Forum: Sigma & Pi Bonds
- Topic: Electron density and pi bonds
- Replies: 2
- Views: 210
Re: Electron density and pi bonds
A sigma bond is cylindrically symmetric with no nodal planes and contains an internuclear axis, while a pi bond has a single nodal plane and contains the internuclear axis.
- Sun Nov 24, 2019 9:19 pm
- Forum: General Science Questions
- Topic: Ligand question
- Replies: 2
- Views: 230
Re: Ligand question
A ligand is an ion or molecule attached to a metal atom by coordinate bonding.
- Sun Nov 24, 2019 9:14 pm
- Forum: Hybridization
- Topic: hybridization
- Replies: 5
- Views: 571
Re: hybridization
Hybridization is the idea that atomic orbitals fuse to form newly hybridized orbitals, which in turn, influences molecular geometry and bonding properties.
- Sun Nov 24, 2019 8:46 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Ligand
- Replies: 10
- Views: 539
Re: Ligand
A ligand is an ion or molecule attached to a metal atom by coordinate bonding.
- Sun Nov 24, 2019 8:37 pm
- Forum: Hybridization
- Topic: Counting for hybridization
- Replies: 2
- Views: 246
Re: Counting for hybridization
Yes, that's how our TA taught us to do it!
- Sun Nov 24, 2019 8:36 pm
- Forum: Lewis Acids & Bases
- Topic: Lewis acids + bases and Bronsted acids + bases
- Replies: 6
- Views: 470
Re: Lewis acids + bases and Bronsted acids + bases
A Bronsted acid is a proton donor and a Bronsted base is a proton acceptor, while a Lewis base is an electron acceptor, while a Lewis base is an electron donor. (Bronsted refers to protons while Lewis refers to electrons)
- Sun Nov 24, 2019 8:26 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Definiton
- Replies: 1
- Views: 111
Re: Definiton
A ligand is an ion or molecule attached to a metal atom by coordinate bonding.
- Sun Nov 17, 2019 6:11 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Prediction of Angles
- Replies: 4
- Views: 299
Re: Prediction of Angles
Angles are based off of the amount of areas of high electron density, as well as whether these areas are bonds or lone pairs. There is a chart you can find on google images that specifies bond angles based off of VSEPR equations. And technically the names of the shape come from the VSEPR model, so y...
- Sun Nov 17, 2019 6:05 pm
- Forum: Dipole Moments
- Topic: SCl4 Molecule
- Replies: 5
- Views: 1045
Re: SCl4 Molecule
Because there is a lone pair that acts as an area of high electron density, thus, affecting the shape of and polarity of the molecule.
- Sun Nov 17, 2019 6:03 pm
- Forum: Lewis Structures
- Topic: Noble gases
- Replies: 3
- Views: 294
Re: Noble gases
This occurs because some elements are able to have an expanded octet. This occurs when the valence shell has enough orbitals to accommodate for the additional electrons.
- Sun Nov 17, 2019 6:00 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: dipole moments
- Replies: 7
- Views: 435
Re: dipole moments
yes, a dipole moment is a measurement of the separation of two opposite electrical charges.
- Sun Nov 17, 2019 5:54 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: two central atoms
- Replies: 3
- Views: 240
Re: two central atoms
I'm pretty sure you just say that it has two different shapes (one for each central atom)
- Sun Nov 17, 2019 5:45 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: repulsion strength
- Replies: 5
- Views: 307
Re: repulsion strength
The strengths of repulsions are in the order lone pair - lone pair > lone pair - atom > atom -atom
- Sun Nov 10, 2019 7:56 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: polarizability
- Replies: 2
- Views: 158
Re: polarizability
An anion will be highly polarizable if its large, such as an iodine ion. A cation will have a high polarizing power if it is small and highly charged such as Al3+ (From the textbook).
- Sun Nov 10, 2019 7:50 pm
- Forum: Coordinate Covalent Bonds
- Topic: coordinate covalent bond
- Replies: 9
- Views: 665
Re: coordinate covalent bond
CO, because the oxygen will give a lone pair of electrons to fulfill the octet of both atoms.
- Sun Nov 10, 2019 7:47 pm
- Forum: Resonance Structures
- Topic: octet v. expanded octet
- Replies: 5
- Views: 349
Re: octet v. expanded octet
Although certain atoms can either have an octet or an expanded octet, I'm pretty sure they can only have one or the other at a given time.
- Sun Nov 10, 2019 7:38 pm
- Forum: Bond Lengths & Energies
- Topic: Resonance Bond Lengths
- Replies: 3
- Views: 165
Re: Resonance Bond Lengths
Yes, the length would be a value in between (the average) the amount of double and single bonds within that particular bond.
- Sun Nov 10, 2019 7:29 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bond Angles
- Replies: 2
- Views: 189
Re: Bond Angles
According to the VSPER theory, lone pair electrons will repel each other, therefore, pushing other atoms in specific angles.
- Sun Nov 10, 2019 7:23 pm
- Forum: Resonance Structures
- Topic: Contribution of each structure?
- Replies: 4
- Views: 437
Re: Contribution of each structure?
Wouldn't you just look at formal charges?
- Sun Nov 03, 2019 11:10 pm
- Forum: *Shrodinger Equation
- Topic: effective nuclear charge
- Replies: 6
- Views: 686
Re: effective nuclear charge
Effective nuclear charge is the net nuclear charge after taking into account the shielding caused by other electrons in the atom.
- Sun Nov 03, 2019 10:55 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Quantum Numbers
- Replies: 4
- Views: 1014
Re: Quantum Numbers
There are some energy levels that don't have certain orbitals. For example, for part b, the f block (l=3) exists within the 6th energy level (n=6), as a result, n = 3, l = 3, ml = 1, ms = -1/2 does not exist.
- Sun Nov 03, 2019 10:50 pm
- Forum: Lewis Structures
- Topic: Drawing lewis structures
- Replies: 5
- Views: 228
Re: Drawing lewis structures
Our TA said to try to draw lewis structures with as many bond with a formal charge of 0 (especially on the central element)
- Sun Nov 03, 2019 10:44 pm
- Forum: Administrative Questions and Class Announcements
- Topic: study guide
- Replies: 2
- Views: 247
Re: study guide
I don't believe so. The textbook should cover everything though.
- Sun Nov 03, 2019 10:40 pm
- Forum: Resonance Structures
- Topic: Resonance Structures
- Replies: 18
- Views: 1149
Re: Resonance Structures
Resonance structures are a set of two or more Lewis Structures that collectively describe the electronic bonding a single polyatomic species including fractional bonds and fractional charges
- Mon Oct 28, 2019 12:22 am
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3643960
Re: Post All Chemistry Jokes Here
Q: What kind of fish is made out of 2 sodium atoms?
A: 2 Na
A: 2 Na
- Mon Oct 28, 2019 12:19 am
- Forum: Trends in The Periodic Table
- Topic: Exact radii/ionization energies
- Replies: 1
- Views: 140
Re: Exact radii/ionization energies
I believe we just have to recognize trends, as opposed to memorizing actual numbers.
- Mon Oct 28, 2019 12:16 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: HW question 1D.13
- Replies: 1
- Views: 95
Re: HW question 1D.13
a) 7 values (0,1,2,3,4,5,6)
b) 5 values (-2,-1,0,1,2)
c) 3 values (-1,0,1)
d) 4 subshells: 4s, 4p, 4d, 4f
b) 5 values (-2,-1,0,1,2)
c) 3 values (-1,0,1)
d) 4 subshells: 4s, 4p, 4d, 4f
- Mon Oct 28, 2019 12:12 am
- Forum: Sigma & Pi Bonds
- Topic: Single vs. Double bonds
- Replies: 15
- Views: 1959
Re: Single vs. Double bonds
More electrons leads to shorter bonds while less electrons means less of an attraction
- Mon Oct 28, 2019 12:11 am
- Forum: Ionic & Covalent Bonds
- Topic: Anions and Cations
- Replies: 4
- Views: 251
Re: Anions and Cations
Anions refer to elements that gain electrons and cations are elements that lose electrons
- Sun Oct 20, 2019 9:43 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Balmer and Lyman Series
- Replies: 6
- Views: 655
Re: Balmer and Lyman Series
The Lyman series involves an electron that jumps to or from the ground state (n=1), and the Balmer series involves an electron that jumps to or from the n=2 energy level. The Lyman series corresponds to ultraviolet rays while I believe the Balmer series corresponds to visible rays.
- Sun Oct 20, 2019 9:38 pm
- Forum: DeBroglie Equation
- Topic: Unit Conversion
- Replies: 5
- Views: 177
Re: Unit Conversion
1 mHz = 1000000 Hz
- Sun Oct 20, 2019 9:36 pm
- Forum: General Science Questions
- Topic: Quick clarification
- Replies: 4
- Views: 319
Re: Quick clarification
It's Planck's constant (h)
- Sun Oct 20, 2019 9:33 pm
- Forum: Properties of Light
- Topic: light regions
- Replies: 4
- Views: 178
Re: light regions
My TA told us that we have to memorize it. I believe a wave that is 8.8 nm is in the x-ray region.
- Sun Oct 20, 2019 9:26 pm
- Forum: General Science Questions
- Topic: Test #1
- Replies: 6
- Views: 329
Re: Test #1
The theoretical yield is always a percentage so multiplying by 100 will always be required.
- Sun Oct 13, 2019 10:19 pm
- Forum: *Black Body Radiation
- Topic: What is the function of blackbodies?
- Replies: 4
- Views: 577
Re: What is the function of blackbodies?
A black body is a surface or object that absorbs all wavelengths of light/doesn't reflect any light. I believe Dr. Lavelle said that there aren't any objects with true blackbody characteristics, and therefore, we aren't going to learn much about them in this class.
- Sun Oct 13, 2019 10:07 pm
- Forum: Significant Figures
- Topic: sig figs and periodic tables
- Replies: 11
- Views: 711
Re: sig figs and periodic tables
The IUPAC Periodic table is the one we used on the test and I believe it goes to three our four decimal places
- Sun Oct 13, 2019 9:54 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: 1D #11
- Replies: 2
- Views: 144
Re: 1D #11
a) 1
b) 5
c) 3
d) 7
b) 5
c) 3
d) 7
- Sun Oct 13, 2019 9:45 pm
- Forum: Photoelectric Effect
- Topic: Post Assessment Help
- Replies: 2
- Views: 203
Re: Post Assessment Help
Kinetic Energy = 1/2 mv^2
- Sun Oct 13, 2019 9:36 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: 1D #19
- Replies: 2
- Views: 108
Re: 1D #19
a) 3
b) 5
c) 1
d) 7
b) 5
c) 1
d) 7
- Sun Oct 06, 2019 8:23 pm
- Forum: Balancing Chemical Reactions
- Topic: H.19
- Replies: 2
- Views: 250
Re: H.19
2 C11H18N2O5 + 17 O2  22 CO2 + 18 H2 +2 N2
22 CO2 + 18 H2 +2 N2
- Sun Oct 06, 2019 7:40 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Test 1
- Replies: 3
- Views: 258
Re: Test 1
Our TA said that all of the problems are free response, as we are essential doing very similar problems as the ones given on our homework.
- Fri Oct 04, 2019 11:32 am
- Forum: Limiting Reactant Calculations
- Topic: Study Strategies/Youtube Videos
- Replies: 14
- Views: 606
Re: Study Strategies/Youtube Videos
Besides Crash Course, Khan Academy, and Tyler DeWitt, Melissa Maribel does a good job at explaining both high school and college chemistry topics.
- Fri Oct 04, 2019 11:25 am
- Forum: Molarity, Solutions, Dilutions
- Topic: Dilution Calculation
- Replies: 5
- Views: 242
Re: Dilution Calculation
If the question wants the final answer in milliliters, then there is no need to convert anything into liters. However, if the measurement is given in liters, the you would need to convert that into milliliters by using the conversion: 1 milliliter=0.001 liters.
- Fri Oct 04, 2019 11:17 am
- Forum: Empirical & Molecular Formulas
- Topic: Order of elements in formulas
- Replies: 5
- Views: 391
Re: Order of elements in formulas
I'm not entirely sure, but I think it follows an order outlined by the Hill system. Essentially, an individual most list carbon atoms first, then hydrogen, and finally any other elements are arranged alphabetically.

