Search found 106 matches
- Thu Mar 12, 2020 11:23 am
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Kelvin or Celsius?
- Replies: 86
- Views: 5658
Re: Kelvin or Celsius?
Kelvin, just make sure all the units cancel
- Thu Mar 12, 2020 11:19 am
- Forum: Administrative Questions and Class Announcements
- Topic: Step Up Sessions
- Replies: 71
- Views: 6981
Re: Step Up Sessions
Jade Hinds 2B wrote:due to classes now being cancelled, will step-up/review sessions presume as scheduled or are those all cancelled as well?
I'm pretty sure all sessions will be cancelled
- Thu Mar 12, 2020 11:18 am
- Forum: Administrative Questions and Class Announcements
- Topic: Excellence in Chemistry Award!
- Replies: 27
- Views: 10008
Re: Excellence in Chemistry Award!
Congrats!!!
- Thu Mar 12, 2020 11:17 am
- Forum: Administrative Questions and Class Announcements
- Topic: Final
- Replies: 12
- Views: 828
Re: Final
I think Lavelle or the ta's will send out more information about it soon
- Thu Mar 12, 2020 11:15 am
- Forum: Administrative Questions and Class Announcements
- Topic: Take Home Final
- Replies: 16
- Views: 1033
Re: Take Home Final
For my math take home final, he is going to upload a pdf of the exam, have us print it out and complete it and then upload it to ccle. He's giving us 24 hours to do so but Idk if we'll get that much time for Chem.
- Mon Mar 02, 2020 8:38 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Salt bridges
- Replies: 11
- Views: 776
Re: Salt bridges
Without a salt bridge, the cell would stop working soon because the cathode would be too negative and would repulse the electrons which would stop working. If this were to occur, would the cathode begin releasing electrons back to the anode since it repulses the electrons? I think so. I'm pretty su...
- Mon Mar 02, 2020 8:37 pm
- Forum: Van't Hoff Equation
- Topic: 5G.21
- Replies: 4
- Views: 525
Re: 5G.21
I have tried part A several times but I keep coming up with K=1.096. How does the book manage to get 1.0 x 10^80. Does it have something to do with the 2 in front of H20 in the balanced equation? The only Standard Gibbs free energy of formation given by the appendix is -228.57 for H2O I believe. Ma...
- Sat Feb 29, 2020 6:26 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: spontaneity
- Replies: 39
- Views: 1929
Re: spontaneity
gibbs free energy!!
- Sat Feb 29, 2020 6:26 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Salt bridges
- Replies: 11
- Views: 776
Re: Salt bridges
Without a salt bridge, the cell would stop working soon because the cathode would be too negative and would repulse the electrons which would stop working.
- Sat Feb 29, 2020 6:24 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Electrolysis
- Replies: 3
- Views: 284
Re: Electrolysis
I think that if there's no salt bridge, the cathode will become more negative and the anode will become more positive since electrons move from the anode to the cathode. However, the presence of a salt bridge prevents the buildup of charge.
- Tue Feb 25, 2020 11:34 am
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3591762
Re: Post All Chemistry Jokes Here
Chemistry jokes are sodium funny, I slapped my neon this one!
- Tue Feb 25, 2020 11:30 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Test 2
- Replies: 6
- Views: 414
Re: Test 2
The test will be on the second page of outline 4 and all of outline 5! In terms of lecture, it covers material from the friday after the midterm until Monday's (2/24) lecture.
- Tue Feb 25, 2020 11:28 am
- Forum: Balancing Redox Reactions
- Topic: reducing agent
- Replies: 5
- Views: 352
Re: reducing agent
Reducing agent: gets oxidized so it gains electrons
Oxidizing agent: gets reduced so it loses electrons
Oxidizing agent: gets reduced so it loses electrons
- Thu Feb 20, 2020 11:46 am
- Forum: Balancing Redox Reactions
- Topic: oxidation number
- Replies: 3
- Views: 339
Re: oxidation number
The oxidation number is the number of electrons that an element gains or loses when it makes a bond.
- Thu Feb 20, 2020 11:41 am
- Forum: Balancing Redox Reactions
- Topic: Reduction vs. oxidation
- Replies: 29
- Views: 1195
Re: Reduction vs. oxidation
LEO the lion goes GER. LEO: lose electrons oxidation GER: gain electrons reduction.
The idea is that when an element's oxidation number (number of electrons) goes down, it's reduced. So gaining electrons will reduce the oxidation number.
The idea is that when an element's oxidation number (number of electrons) goes down, it's reduced. So gaining electrons will reduce the oxidation number.
- Thu Feb 20, 2020 11:39 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cathode to the Right Rule
- Replies: 6
- Views: 477
Re: Cathode to the Right Rule
The cathode should always be listed on the right I think it's the standard.
- Thu Feb 20, 2020 11:32 am
- Forum: Lewis Acids & Bases
- Topic: Midterm Question 3B
- Replies: 3
- Views: 502
Re: Midterm Question 3B
I just knew from AP Chem that neutralization reactions are exothermic. So for this question, I just determined which reaction had the most moles of reaction in the smallest amount of liquid. The more liquid there is, the more heat that can be absorbed without increasing the temperature much. In othe...
- Tue Feb 18, 2020 11:37 am
- Forum: Administrative Questions and Class Announcements
- Topic: Test 2
- Replies: 10
- Views: 666
Test 2
What sections of the book are going to be covered in test 2? I know Dr. Lavelle didn't get to all of outline 4. So does the material start with 5G?
- Sun Feb 16, 2020 12:25 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: memorize
- Replies: 14
- Views: 859
Re: memorize
No, they give us any needed entropy values but you should know how entropy will change in general. ex. entropy increases if a liquid becomes a gas
- Sun Feb 16, 2020 12:23 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Delta E
- Replies: 11
- Views: 811
- Sun Feb 16, 2020 12:22 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Midterm Curve
- Replies: 45
- Views: 2259
Re: Midterm Curve
He doesn't curve individual tests :( but the overall grade is adjusted at the end of the quarter. There's no hope til the end of the quarter :(((
- Sun Feb 16, 2020 12:21 pm
- Forum: Balancing Redox Reactions
- Topic: oxidation vs reduction
- Replies: 19
- Views: 1022
Re: oxidation vs reduction
LEO the lion goes GER. LEO: lose electrons oxidation GER: gain electrons reduction.
The idea is that when an element's oxidation number (number of electrons) goes down, it's reduced. So gaining electrons will reduce the oxidation number.
The idea is that when an element's oxidation number (number of electrons) goes down, it's reduced. So gaining electrons will reduce the oxidation number.
- Tue Feb 11, 2020 4:48 pm
- Forum: Phase Changes & Related Calculations
- Topic: 4B.5
- Replies: 7
- Views: 480
Re: 4B.5
Matthew ILG 1L wrote:Will the conversion of 1atm=760 Torr be given to us at all? I have yet to find that on the equations sheet from test one. I could be looking in all the wrong places though.
It's on the constants and formula sheet
- Mon Feb 03, 2020 10:44 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Shift Change Rules
- Replies: 5
- Views: 323
Re: Shift Change Rules
You should know how an equilibrium shifts when concentrations of species are changed, temperature is changed, or when pressure is changed
- Mon Feb 03, 2020 10:44 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: enthalpy
- Replies: 7
- Views: 237
Re: enthalpy
Enthalpy is equal to the heat of a system if there is no expansion work.
- Mon Feb 03, 2020 10:43 am
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: enthalpy
- Replies: 7
- Views: 248
Re: enthalpy
Enthalpy is the amount of heat absorbed or released by a reaction if there is no expansion work
- Mon Feb 03, 2020 10:41 am
- Forum: Calculating Work of Expansion
- Topic: Calculus on The Midterm
- Replies: 8
- Views: 468
Calculus on The Midterm
Will the midterm have problems where we use derivatives and integrals? In what problems would these be used.
- Mon Feb 03, 2020 10:38 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: First Law
- Replies: 5
- Views: 345
Re: First Law
Internal energy is the sum of potential and kinetic energy.
- Tue Jan 28, 2020 6:02 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: How to do 4A.13
- Replies: 1
- Views: 168
How to do 4A.13
A constant-volume calorimeter was calibrated by carrying out a reaction known to release 3.50 kJ of heat in 0.200 L of solution in the calorimeter (q 5 23.50 kJ), resulting in a temperature rise of 7.32 8C. In a subsequent experiment, 100.0 mL of 0.200 m HBr(aq) and 100.0 mL of 0.200 m KOH(aq) were ...
- Mon Jan 27, 2020 10:18 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: "Breaking bonds is always endothermic"
- Replies: 6
- Views: 994
Re: "Breaking bonds is always endothermic"
When the phosphate bond of ATP is broken, a small input of energy is required. However, the formation of new bonds (between OH and the last P in ADP) releases lots of energy so the net energy change is an increase in energy.
- Mon Jan 27, 2020 10:14 am
- Forum: Phase Changes & Related Calculations
- Topic: Steam vs water
- Replies: 5
- Views: 204
Re: Steam vs water
Steam, despite being at the same temperature as water, has more energy because during a phase change, a substance can absorb energy without increasing in temperature. This excess energy is transferred to your skin and causes burns
- Mon Jan 27, 2020 10:12 am
- Forum: Phase Changes & Related Calculations
- Topic: Heat capacities
- Replies: 6
- Views: 280
Re: Heat capacities
Heat capacities will generally be given on tests.
- Mon Jan 27, 2020 10:11 am
- Forum: Phase Changes & Related Calculations
- Topic: Heat capacities
- Replies: 6
- Views: 280
Re: Heat capacities
Heat capacities will generally be given on tests.
- Mon Jan 27, 2020 10:10 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: autoprotolysis
- Replies: 7
- Views: 299
Re: autoprotolysis
Autoprotolysis is when H+ ions are transferred from 2 molecules of the same species. When you write out the chemical equation for this transfer, you can write a K equation for it.
- Mon Jan 27, 2020 10:10 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: autoprotolysis
- Replies: 7
- Views: 299
Re: autoprotolysis
Autoprotolysis is when H+ ions are transferred from 2 molecules of the same species. When you write out the chemical equation for this transfer, you can write a K equation for it.
- Mon Jan 27, 2020 10:08 am
- Forum: General Science Questions
- Topic: Fall 2019 final
- Replies: 7
- Views: 233
Re: Fall 2019 final
Lavelle said week 3 but I've heard that they will be available for most of this quarter
- Sun Jan 26, 2020 4:52 pm
- Forum: Phase Changes & Related Calculations
- Topic: phase changes
- Replies: 6
- Views: 265
Re: phase changes
not all phase changes are exothermic, some are endothermic
- Sun Jan 26, 2020 4:51 pm
- Forum: Phase Changes & Related Calculations
- Topic: Phase changes: endothermic vs exothermic
- Replies: 12
- Views: 1248
Re: Phase changes: endothermic vs exothermic
yes, the reverse of endothermic reactions are exothermic and vice versa
- Sun Jan 26, 2020 4:45 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Heat Capacity
- Replies: 7
- Views: 400
Re: Heat Capacity
High heat capacity means that more energy is taken to increase or decrease the overall temperature.
- Sun Jan 26, 2020 4:44 pm
- Forum: Phase Changes & Related Calculations
- Topic: State property
- Replies: 6
- Views: 299
Re: State property
A state property doesn't depend on the path taken to get to that state but just the state itself.
- Sun Jan 26, 2020 4:44 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Lewis Structures Method 2
- Replies: 6
- Views: 178
Re: Lewis Structures Method 2
Although it's not absolutely necessary, it can be helpful.
- Sat Jan 18, 2020 10:05 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Equilibrium Constant Units
- Replies: 4
- Views: 505
Re: Equilibrium Constant Units
The reason why is because when you say you're using concentrations of partial pressures, you're actually using activities of each species. I think when you calculate the activity of each species, the units cancel out.
- Sat Jan 18, 2020 10:03 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Decreasing pressure
- Replies: 4
- Views: 168
Re: Decreasing pressure
If pressure is increased, volume decreases. If volume decreases the reaction proceeds to the side with less moles of gas.
If pressure is decreased, volume increases. If volume increases the reaction proceeds to the side with more moles of gas.
If pressure is decreased, volume increases. If volume increases the reaction proceeds to the side with more moles of gas.
- Fri Jan 17, 2020 3:45 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5 percent rule
- Replies: 10
- Views: 533
Re: 5 percent rule
The 5% rule is just a way of making sure that when you remove the x from the bottom of the fraction, you aren't affecting the answer. The idea is if x is less than 5% of the number its being subtracted from, it makes such a little difference that it is mathematically ok to assume x is 0 relative to ...
- Fri Jan 17, 2020 3:42 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kc & Kp
- Replies: 12
- Views: 327
Re: Kc & Kp
gabbymaraziti wrote:Can we just use K and not specify Kc or Kp? Or would we be marked down?
I feel like they won't care on the test. But it can't hurt to take a look at the units and add the C or P.
- Fri Jan 17, 2020 3:35 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Temperature Change
- Replies: 9
- Views: 592
Re: Temperature Change
If the products require energy to be formed and the temperature increases, the forward reaction will be favored because the system will want to decrease the temperature. To do so, the reactants will react and absorb excess heat (because the forward reaction is endothermic). On the other hand, if the...
- Wed Jan 08, 2020 11:18 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Q vs K
- Replies: 13
- Views: 496
Re: Q vs K
Although Q and K are calculated the same way, the difference is the state of the reaction at the time when this is calculated: Q can be any specific point of time while K must be when the reaction is at equilibrium.
- Wed Jan 08, 2020 11:14 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: No Solvent Concentration in the Calculating Equilibrium Constant
- Replies: 4
- Views: 288
Re: No Solvent Concentration in the Calculating Equilibrium Constant
The activity level for both solids and liquids are 1
- Wed Jan 08, 2020 11:12 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Pressure changes to equilibrium equations
- Replies: 5
- Views: 259
Re: Pressure changes to equilibrium equations
When a mixture is compressed, there is less volume for gasses to exist so the system will shift towards the side with fewer moles of gas. In other words, gasses take up less space so when there is less space, the reaction will go more towards whatever side has fewer moles of gas.
- Wed Jan 08, 2020 11:10 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: How to make ICE box
- Replies: 17
- Views: 1259
Re: How to make ICE box
Allow the change to be x (ie. on the reactants side, the change would be -2x if the stoichiometric coefficient is 2). Add the change to the initial value to get the equilibrium expression. Plug in the expressions into a K equation and if the value of K is given, algebra can be used to solve for x ak...
- Wed Jan 08, 2020 11:02 am
- Forum: Ideal Gases
- Topic: Equilibrium
- Replies: 7
- Views: 230
Re: Equilibrium
Although Q and K are calculated the same way, the difference is the state of the reaction at the time when this is calculated: Q can be any specific point of time while K must be when the reaction is at equilibrium.
- Sun Dec 08, 2019 5:57 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bent vs linear
- Replies: 56
- Views: 4271
Re: Bent vs linear
If there are lone pairs, the molecule is probably bent
- Sun Dec 08, 2019 5:56 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: n, l ,ml, ms
- Replies: 13
- Views: 1507
Re: n, l ,ml, ms
Spin up is +1/2
Spin down is -1/2
Spin down is -1/2
- Sun Dec 08, 2019 5:56 pm
- Forum: Hybridization
- Topic: lone pairs in hybridization
- Replies: 6
- Views: 564
Re: lone pairs in hybridization
You use the number of electron densities (lone pairs and bonding pairs) to determine the number of orbitals required.
- Sun Dec 08, 2019 5:54 pm
- Forum: Air Pollution & Acid Rain
- Topic: Respiratory Acidosis
- Replies: 4
- Views: 611
Re: Respiratory Acidosis
Just a note on this topic: blood usually has a ph of 7.35-7.45 so technically even 7 is acidic
- Sat Dec 07, 2019 11:51 pm
- Forum: Air Pollution & Acid Rain
- Topic: Carbon Dioxide and respiratory acidosis
- Replies: 5
- Views: 1232
Re: Carbon Dioxide and respiratory acidosis
Chantel_4B wrote:Does the CO2 make the body more acidic when it interacts with water? Or is it when it interacts with something else? Or by itself?
CO2 reacts with water to form carbonic acid (H2CO3) which is a weak acid that increases the acidity of blood
- Fri Nov 29, 2019 12:13 am
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Inorganic vs organic
- Replies: 5
- Views: 342
Re: Inorganic vs organic
If it contains carbon it is organic ! Actually there are a few molecules that have carbon but are inorganic: CO2, CO, carbonates, carbides, cyanides, cyanates, and thiocyanates. But most bigger molecules that contain carbon are organic so its a good rule of thumb but just keep in mind that that isn...
- Thu Nov 28, 2019 11:56 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Acid Strength
- Replies: 6
- Views: 428
Re: Acid Strength
These are the strong acids:
HCl, HBr, HI,
HNO3, H2SO4, HClO4, HClO3
These are the strong bases:
LiOH, NaOH, KOH, RbOH, CsOH
Ca(OH)2, Sr(OH)2, Ba(OH)2
HCl, HBr, HI,
HNO3, H2SO4, HClO4, HClO3
These are the strong bases:
LiOH, NaOH, KOH, RbOH, CsOH
Ca(OH)2, Sr(OH)2, Ba(OH)2
- Thu Nov 28, 2019 11:48 pm
- Forum: Lewis Acids & Bases
- Topic: Lewis v Bronsted acids/bases
- Replies: 2
- Views: 226
Re: Lewis v Bronsted acids/bases
A Lewis acid and base accept and donate electrons respectively. Make sure you know the difference between that and Bronsted-Lowry acid and base which donate and accept protons respectively.
- Thu Nov 28, 2019 11:47 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Bronsted Acid/Base
- Replies: 4
- Views: 280
Re: Bronsted Acid/Base
A Lewis acid and base accept and donate electrons respectively. Make sure you know the difference between that and Bronsted-Lowry acid and base which donate and accept protons respectively.
- Thu Nov 28, 2019 11:46 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Lewis Acid/Base
- Replies: 5
- Views: 242
Re: Lewis Acid/Base
A Lewis acid and base accept and donate electrons respectively. Make sure you know the difference between that and Bronsted-Lowry acid and base which donate and accept protons respectively.
- Thu Nov 21, 2019 3:29 pm
- Forum: Hybridization
- Topic: 2F.1
- Replies: 3
- Views: 184
Re: 2F.1
I think its asking where the orbitals are pointing. For example, sp2 orbitals are pointing towards the corners of an equilateral triangle and form bond angles of 120.
- Thu Nov 21, 2019 3:23 pm
- Forum: Sigma & Pi Bonds
- Topic: Pi bonding
- Replies: 3
- Views: 227
Re: Pi bonding
Because in biology, the form of a molecule is vital for its function. The fact that pi bonds don't allow for rotation locks the molecule into a certain shape at double and triple bonds which allow molecules to do its function. Also, the fact that pi bonds don't allow for rotation leads to cis and tr...
- Wed Nov 20, 2019 2:41 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bond Angles in Square Pyramidal and T-Shaped
- Replies: 4
- Views: 290
Re: Bond Angles in Square Pyramidal and T-Shaped
The bond angles in a square pyramidal are slightly less than 90 degrees because of the extra repulsion of the lone pair. The electron geometry is a octahedral so all the bond angles are the same (the term equatorial atom is used for trigonal bipyramidal). For T-shape, the bond angles will always be ...
- Tue Nov 19, 2019 8:57 pm
- Forum: General Science Questions
- Topic: Test 2 Review
- Replies: 8
- Views: 643
Re: Test 2 Review
What do we need to know for "lone pairs and the reason for their locations"? I'm not sure what this is referring to I think its talking about molecules with the formula AX4E because the electron geometry is trigonal bipyramidal and the lone pair can be on either the axial or the equatoria...
- Tue Nov 19, 2019 8:25 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bond Angles
- Replies: 3
- Views: 1194
Re: Bond Angles
Also for the specific formulas you're asking about, there's only 2 atoms so they can only form 180 degree angles. Just cuz any two points can make a straight line.
- Tue Nov 19, 2019 8:22 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Dipole-Dipole
- Replies: 5
- Views: 307
Re: Dipole-Dipole
If the two dipole moments cancel out (ex. CO2), the molecule is considered non-polar and doesn't have dipole-dipole interactions.
- Thu Nov 14, 2019 7:18 pm
- Forum: Naming
- Topic: Naming Compounds (general question)
- Replies: 5
- Views: 470
Re: Naming Compounds (general question)
I doubt it since we didn't really go over it in class/discussion
- Thu Nov 14, 2019 7:18 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Homework 2E.1
- Replies: 4
- Views: 175
Re: Homework 2E.1
You figure that out by drawing the Lewis structure. You should follow all the rules that we learned the past few weeks (most atoms must have complete octets, you want the lowest possible formal charge, etc)
- Thu Nov 14, 2019 7:13 pm
- Forum: Trends in The Periodic Table
- Topic: Question
- Replies: 17
- Views: 1405
Re: Question
Ionization energy is the energy needed to remove electrons from atoms. Electronegativity is how much an atom attracts electrons.
- Thu Nov 14, 2019 7:07 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Atom size vs. boiling point
- Replies: 4
- Views: 577
Re: Atom size vs. boiling point
A bigger atomic radii increases the polarizability of the molecule which increases the amount of induced dipole- induced dipole forces in a body of that molecule. More intermolecular forces will lead to a higher boiling point. In short, bigger atomic size leads to a higher boiling point.
- Thu Nov 14, 2019 7:05 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Quick Question
- Replies: 4
- Views: 435
Re: Quick Question
For a 4p electron, n=4 and l=1. A 4d electron would be l=2.
- Thu Nov 14, 2019 6:57 pm
- Forum: Lewis Structures
- Topic: Balanced Lewis Structures
- Replies: 6
- Views: 447
Re: Balanced Lewis Structures
It doesn't really matter how you draw lewis structures (in the sense that it doesn't matter if a certain atom is attached horizontally or vertically to the central atom). Lewis structures are mainly just meant to show which atoms are bonded to which, the number of bonds between two atoms, the number...
- Wed Nov 13, 2019 11:28 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: 3F.1 Part a
- Replies: 4
- Views: 393
Re: 3F.1 Part a
The first step is to draw the lewis structure https://img1.daumcdn.net/thumb/R800x0/?scode=mtistory2&fname=https%3A%2F%2Ft1.daumcdn.net%2Fcfile%2Ftistory%2F2506B63D57609A5621 Because there are H, O and N, this molecule forms hydrogen bonds. Also, every molecule has london forces.
- Wed Nov 13, 2019 2:47 pm
- Forum: Bronsted Acids & Bases
- Topic: Autoprotolysis
- Replies: 1
- Views: 212
Re: Autoprotolysis
Autoprotolysis is when a proton (H+) is transferred from 1 molecule to another molecule that is identical to the first. For example: 2 H2O --> H3O + +OH - in that reaction, a proton is transferred from 1 water to another, making 1 a bronsted base and the other a bronsted acid. The autoprotolysis con...
- Wed Nov 06, 2019 12:26 pm
- Forum: General Science Questions
- Topic: Midterm
- Replies: 2
- Views: 221
Midterm
Will the midterm test on people/experiments? (like Thomson or Millikan)
- Tue Nov 05, 2019 7:39 pm
- Forum: General Science Questions
- Topic: Line Structures on the Midterm?
- Replies: 1
- Views: 136
Re: Line Structures on the Midterm?
I really doubt you need to know line structures tbh.
- Tue Nov 05, 2019 9:51 am
- Forum: Lewis Structures
- Topic: 2c.17
- Replies: 1
- Views: 125
Re: 2c.17
Stated another way, the question is just asking which structure is more stable and thus more common. So you should check the structure to make sure that all the elements with expanded/incomplete octets are correct, that formal charges are as low as possible, that the number of valence electrons is c...
- Tue Nov 05, 2019 9:39 am
- Forum: Bond Lengths & Energies
- Topic: Finding the Length of the Bond/Is this on the midterm? [ENDORSED]
- Replies: 1
- Views: 284
Re: Finding the Length of the Bond/Is this on the midterm? [ENDORSED]
The figure shows a periodic table with the common bond lengths of some elements. So for CF4, each bond is a single bond between C and F (draw the lewis structure to know that). You just go to the table, find the values for a single bond C and for F, and add the numbers together. The length of a sing...
- Tue Nov 05, 2019 9:31 am
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Heisenberg Uncertainty in Position
- Replies: 4
- Views: 402
Re: Heisenberg Uncertainty in Position
Yes because the total possible uncertainty in position is +5 AND -5. So if the position is 10m +/-5m. The position can be anywhere from 5m to 15 m so the uncertainty is 10 m. But you do have to be careful because if the position of a thrown ball is 3m to the left +/- 5m, the uncertainty is not 10 m ...
- Tue Nov 05, 2019 7:56 am
- Forum: Ionic & Covalent Bonds
- Topic: 2D.3
- Replies: 3
- Views: 266
Re: 2D.3
If you find the difference in electronegativity between Be and Br, it's 1.39. Generally, if the difference is less than about 1.5, it will show covalent character.
- Wed Oct 30, 2019 3:11 am
- Forum: Octet Exceptions
- Topic: List of Octet exceptions
- Replies: 6
- Views: 325
Re: List of Octet exceptions
I know for sure that H, Li, Be, and B generally form a duplet instead of an octet. Also, once the elements pass the 3p block, it can have an expanded valence electron shell and form more than an octet.
- Wed Oct 30, 2019 3:08 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Hw problem 2A.15
- Replies: 1
- Views: 146
Re: Hw problem 2A.15
Elements are generally likely to try and form an octet with the valence electrons (exceptions include H, Li, Be, and B who want to create a duplet). So S needs 2 electrons to form a full valence shell so the most likely ion is S 2- . Te is also 2 electrons so it will form Te 2- . Rb is 1 electron aw...
- Wed Oct 30, 2019 2:54 am
- Forum: Ionic & Covalent Bonds
- Topic: Homework for Week 5
- Replies: 8
- Views: 334
Re: Homework for Week 5
I genuinely don't think the TA cares what questions you do as long as they are somewhat current. I say do it on whatever you want to review.
- Wed Oct 30, 2019 2:53 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: d block before s block
- Replies: 3
- Views: 217
Re: d block before s block
Some people write the d-block before the s-block because when an element is losing electrons, it'll lose them from the s-block first. However, my TA said that it's more common for people to write the s-block before the d-block because when electrons are being placed into an element, electrons are ad...
- Wed Oct 30, 2019 2:50 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Question 1E 1
- Replies: 3
- Views: 218
Re: Question 1E 1
The answer at the back of the book says that it wouldn't matter if the element is H or Li.
- Wed Oct 23, 2019 1:03 am
- Forum: Photoelectric Effect
- Topic: Work Function?
- Replies: 7
- Views: 537
Re: Work Function?
The work function is used to calculate the energy required to remove an electron from a metal.
- Wed Oct 23, 2019 12:55 am
- Forum: Photoelectric Effect
- Topic: post module assignment Q 19
- Replies: 4
- Views: 176
Re: post module assignment Q 19
Yes, the answer is D. The first three are all variations of the energy change between a photon and the electron/metal.
- Wed Oct 23, 2019 12:51 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: predicting the type of orbital
- Replies: 2
- Views: 153
Re: predicting the type of orbital
You remove an electron from the highest energy level (furthest from the nucleus). You can figure out the relative energy levels of orbitals using a periodic table and moving across each row from left to right.
- Wed Oct 23, 2019 12:44 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: s, p, d, f orbitals
- Replies: 15
- Views: 776
Re: s, p, d, f orbitals
This picture shows the energy levels of all the orbitals:
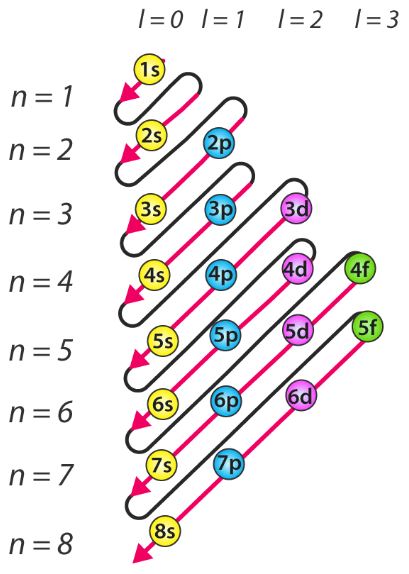

- Wed Oct 23, 2019 12:43 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Electron Configurations
- Replies: 13
- Views: 2965
Re: Electron Configurations
The easiest way is to use the periodic table but you can also use this chat:


- Sat Oct 19, 2019 4:22 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Lobes
- Replies: 2
- Views: 126
Re: Lobes
A lobe is a section of an orbital bordered by one or more orbital nodes.
- Sat Oct 19, 2019 4:14 pm
- Forum: Properties of Electrons
- Topic: Balmar and Lyman series
- Replies: 2
- Views: 148
Re: Balmar and Lyman series
N(1) is the final energy level and N(2) is the initial energy level.
- Sat Oct 19, 2019 2:54 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Writing Electron Configurations
- Replies: 7
- Views: 361
Re: Writing Electron Configurations
I think in general you don't include the x, y, z. I know for sure that you don't use it for d orbitals.
- Sat Oct 19, 2019 2:50 pm
- Forum: General Science Questions
- Topic: Midterm
- Replies: 12
- Views: 499
Midterm
When and what time is the midterm? (Chem 14a)
- Sat Oct 19, 2019 2:37 pm
- Forum: Photoelectric Effect
- Topic: One photon one atom interaction
- Replies: 14
- Views: 593
Re: One photon one atom interaction
After the energy of the photon surpasses the minimum energy, increasing the frequency of the photon would not increase the number of electrons ejected because each photon interacts with 1 electron, not multiple. A higher frequency correlates to a higher kinetic energy of the ejected electron. Increa...
- Sun Oct 13, 2019 11:15 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Best Way To Study?
- Replies: 56
- Views: 3505
Re: Best Way To Study?
I study best by doing all the problems in the syllabus (or at least run through them so I know how to do them). I also suggest going through the book or watching the modules and taking the most important notes (like a 1 page cram sheet).
- Sun Oct 13, 2019 11:00 pm
- Forum: DeBroglie Equation
- Topic: Using the De Broglie equation
- Replies: 1
- Views: 113
Re: Using the De Broglie equation
If the particle has a de Broglie wavelength of less than 10-15, it doesn't have detectable wavelike properties.
- Sun Oct 13, 2019 10:57 pm
- Forum: *Shrodinger Equation
- Topic: Schrodinger's Equation
- Replies: 8
- Views: 505
Re: Schrodinger's Equation
Schrodinger's equation: a very complicated equation that describes the wave function of an atom and thus created quantum mechanics. De Broglie's wave equation: tells you the wavelength given off by any moving particle with momentum (mass and velocity). Heisenberg's indeterminacy equation: says that ...
- Thu Oct 10, 2019 4:36 pm
- Forum: Properties of Electrons
- Topic: Understanding Balmer & Lyman Series
- Replies: 3
- Views: 194
Re: Understanding Balmer & Lyman Series
As the others said, the Lyman series is for electrons that end at n=1 and the Balmer series is for electrons that end at n=2. But the main reason why these two regions are highlighted is just because they involve the 2 biggest "jumps" or energy differences. There are other series for other...

