Search found 15 matches
- Tue Nov 05, 2019 10:32 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Midterm
- Replies: 6
- Views: 528
Re: Midterm
In regards to the midterm, I think it is important to remember/understand the concepts of the experiments.
- Tue Nov 05, 2019 10:27 pm
- Forum: Electronegativity
- Topic: Ionization Energy
- Replies: 3
- Views: 302
Re: Ionization Energy
Since an electron is being added to an already half full orbital in oxygen, this results in electron electron repulsion, which lowers the ionisation energy. Nitrogen also has the added stability of a half filled shell of electrons in the 2p shell.
- Tue Nov 05, 2019 10:17 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Quantum Numbers Question
- Replies: 4
- Views: 513
Re: Quantum Numbers Question
The number of electrons that can occupy a given energy shell is given by the equation e−=2n^2. n represents the principal quantum number that describes the energy shell. We have n=4 which means that the number of e− is 2⋅4^2=32.
- Tue Nov 05, 2019 10:02 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Dino Nuggets 13d
- Replies: 2
- Views: 360
Re: Dino Nuggets 13d
Although Sc2+ and K have the same number of electrons, their electron configurations are different and that is why they don't have the same valence shell electron configuration.
- Tue Nov 05, 2019 9:53 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Heisenberg Problem
- Replies: 3
- Views: 378
Heisenberg Problem
An e- moving near an atomic nucleus has speed 6x10^6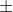 1% m/s.
1% m/s.
How do you find the uncertainty of its position?
How do you find the uncertainty of its position?
- Tue Nov 05, 2019 7:40 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: D Subshell
- Replies: 7
- Views: 748
D Subshell
What is the maximum number of electrons that the d subshell can hold? Why?
- Tue Nov 05, 2019 7:29 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3589186
Re: Post All Chemistry Jokes Here
What happened to the man stopped for having sodium chloride and a 9-volt in his car? He was booked for a salt and battery.
- Tue Nov 05, 2019 7:24 pm
- Forum: Properties of Electrons
- Topic: Midterm Practice Test #11
- Replies: 1
- Views: 381
Midterm Practice Test #11
11. a) An excited hydrogen atom undergoes an electronic transition from n=3 to n=1. Calculate the frequency of the photon emitted. 11. b) If a hydrogen electron goes from n=6 to n=4, will ∆E be negative or positive? Will the energy of the photon emitted be negative or positive? I missed the end of t...
- Tue Nov 05, 2019 7:06 pm
- Forum: Properties of Light
- Topic: 4b practice midterm
- Replies: 11
- Views: 1618
Re: 4b practice midterm
For this problem, you must use the De Broglie equation. De Broglie is typically used for an electron's wavelength with mass, while c=λν or E=hv is used for massless particles, like photons.
- Sun Oct 20, 2019 9:43 pm
- Forum: Trends in The Periodic Table
- Topic: Atomic Radius
- Replies: 7
- Views: 477
Atomic Radius
How do you find the atomic radius of an element?
- Sun Oct 06, 2019 3:39 pm
- Forum: Ideal Gases
- Topic: Reading the textbook
- Replies: 262
- Views: 149922
Re: Reading the textbook
I too get a bit intimidated by reading the textbook. However, doing so is extremely helpful. To make it easier for myself, I split my reading into different chunks and take breaks in between so I won’t be so overwhelmed by all of the material at once. Sometimes, I also take notes on my computer abou...
- Sun Oct 06, 2019 3:33 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Advice from a Medical Student [ENDORSED]
- Replies: 297
- Views: 408563
Re: Advice from a Medical Student [ENDORSED]
Wow! Thank you so much for your insight. As a currently overwhelmed STEM student, this is very helpful.
- Sun Oct 06, 2019 3:31 pm
- Forum: Student Social/Study Group
- Topic: Chemistry News
- Replies: 135
- Views: 166959
Re: Chemistry News
Chemists possibly found a fix for insoluble drugs:
https://phys.org/news/2019-10-chemists- ... drugs.html
https://phys.org/news/2019-10-chemists- ... drugs.html
- Sun Oct 06, 2019 3:25 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Rydberg equation [ENDORSED]
- Replies: 73
- Views: 9008
Re: Rydberg equation [ENDORSED]
The Rydberg formula, by definition, is a mathematical formula which predicts the wavelength of light coming from an electron moving between energy levels of an atom according to the science website, ThoughtCo.
- Sun Oct 06, 2019 3:19 pm
- Forum: Student Social/Study Group
- Topic: Final Jitters
- Replies: 457
- Views: 357371
Re: Final Jitters
I also struggle with nerves before a big exam, some of the things I do to prepare include: 1.Getting a good night's sleep before the day of! 2. Having a healthy breakfast 3. Read the material right before class so it is fresh in my mind (and obviously study lots beforehand). 4. Tell myself how I hav...

