Search found 100 matches
- Fri Mar 13, 2020 6:09 pm
- Forum: Van't Hoff Equation
- Topic: When to use
- Replies: 15
- Views: 1063
Re: When to use
It relates the change in temperature to the change in reaction constant.
- Fri Mar 13, 2020 6:08 pm
- Forum: Calculating Work of Expansion
- Topic: Positive or negative work
- Replies: 15
- Views: 2174
Re: Positive or negative work
work is positive when it is done on the system. When the system does work, the work is negative.
- Fri Mar 13, 2020 6:04 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Relationship between work, cell potential, and delta G
- Replies: 6
- Views: 685
Re: Relationship between work, cell potential, and delta G
When delta G is negative, there is energy free to do work. Cell potential wants to go down. IF cell potential goes down there is a release of energy free to do work.
- Fri Mar 13, 2020 6:02 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Reduction Potential
- Replies: 4
- Views: 326
Re: Reduction Potential
the anode is more negative and the cathode is more positive.
- Fri Mar 13, 2020 6:02 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Identification
- Replies: 8
- Views: 544
Re: Identification
Generally all diatomic elements being themselves in their natural state like gaseous hydrogen or graphite for carbon.
- Fri Mar 13, 2020 5:56 pm
- Forum: Second Order Reactions
- Topic: Termolecular
- Replies: 43
- Views: 2383
Re: Termolecular
It is when three molecules collide in order to form a product.
- Fri Mar 13, 2020 5:55 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Slow Step Mechanism
- Replies: 7
- Views: 505
Re: Slow Step Mechanism
It entirely depends on the reaction itself.
- Fri Mar 13, 2020 5:54 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Identifying First and Second Order
- Replies: 3
- Views: 217
Re: Identifying First and Second Order
If you plot ln of reactant with time and it is a straight line it is first order. If you plot 1/R vs time and it is a straight line it is second order.
- Fri Mar 13, 2020 5:51 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Arrhenius Equation
- Replies: 8
- Views: 588
Re: Arrhenius Equation
The equation helps us relate temperature, reaction constant, and the activation energy. IF plotting lnk to 1/T the slope is -Ea/R
- Tue Mar 03, 2020 9:04 am
- Forum: Second Order Reactions
- Topic: rate
- Replies: 3
- Views: 573
Re: rate
if we change a second order equations reactant by a factor, its rate will change by the factor^2
- Tue Mar 03, 2020 9:03 am
- Forum: General Rate Laws
- Topic: Homework 7A.17
- Replies: 2
- Views: 329
Re: Homework 7A.17
you can look at the the factor of how much the rate and the reactant are changing then deduce which order the reactant is.
- Tue Mar 03, 2020 9:01 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: lnQ vs logQ
- Replies: 5
- Views: 381
Re: lnQ vs logQ
It is ok to use either but remember that the those two are not the same thing.
- Tue Mar 03, 2020 9:00 am
- Forum: First Order Reactions
- Topic: First order plot
- Replies: 3
- Views: 277
Re: First order plot
it is used to derive k as it is the negative slope of the equation.
- Tue Mar 03, 2020 8:59 am
- Forum: Zero Order Reactions
- Topic: Difference
- Replies: 5
- Views: 438
Re: Difference
No matter how we change the concentration of the zero order reactant, the reaction rate will not change. For a first order equation, the rate will linearly change when we change the reactant. For a second order when we change the reactant, it will change by the square of the reactant change.
- Tue Mar 03, 2020 8:55 am
- Forum: General Rate Laws
- Topic: class notes
- Replies: 2
- Views: 260
Re: class notes
A slow step is the series of reactions use to convert the initial reactants to the final products.
- Thu Feb 27, 2020 9:44 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Eo as an intensive property
- Replies: 9
- Views: 648
Re: Eo as an intensive property
The property does not change no matter how much of it occurs. Therefore if we must balance a reaction we can't change 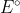
- Thu Feb 27, 2020 9:43 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Work
- Replies: 4
- Views: 363
Re: Work
The potential difference is the maximum amount of Gibbs Free energy, the energy available to do work.
- Thu Feb 27, 2020 9:39 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: delta g
- Replies: 3
- Views: 326
Re: delta g
Because there is no more free energy to do work when the reaction is at equilibrium because the reaction is at the most stable point.
- Thu Feb 27, 2020 9:38 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: E cell
- Replies: 2
- Views: 211
Re: E cell
We use )
- Thu Feb 27, 2020 9:36 am
- Forum: Balancing Redox Reactions
- Topic: Cell Diagrams
- Replies: 3
- Views: 232
Re: Cell Diagrams
Since the left side is the anode, the thing on the left must be oxidized.
- Mon Feb 17, 2020 10:06 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: G vs G knot
- Replies: 15
- Views: 1739
Re: G vs G knot
Regular G is for any temperature and for any pressure
- Mon Feb 17, 2020 10:03 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: sign of delta G
- Replies: 9
- Views: 4435
Re: sign of delta G
When delta G is negative it becomes spontaneous and the reaction will favor the products if delta G is positive the reaction will favor the reactants
- Mon Feb 17, 2020 10:02 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: importance of -RTlnk
- Replies: 7
- Views: 477
Re: importance of -RTlnk
K is the equilibrium constant where the equation will not change if K=Q then the standard change in gibbs free energy is zero. so we get the equation from ) to G=-RTlnK
to G=-RTlnK
- Mon Feb 17, 2020 9:59 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Exothermic rxns being spontaneous
- Replies: 5
- Views: 463
Re: Exothermic rxns being spontaneous
Spontaneity means that the change in gibbs free energy is negative. Most exothermic reactions have a decrease in enthalpy meaning an increase in heat to the surroundings as well as an increase in entropy
- Mon Feb 17, 2020 9:57 pm
- Forum: Van't Hoff Equation
- Topic: Entropy in Van't Hoff Equation
- Replies: 3
- Views: 236
Re: Entropy in Van't Hoff Equation
We are assuming that both the initial and final entropy will go up the same amount so that the difference in the entropy will stay the same.
- Mon Feb 10, 2020 11:46 pm
- Forum: Calculating Work of Expansion
- Topic: Reversible Reactions
- Replies: 2
- Views: 183
Re: Reversible Reactions
Since it uses all of the heat going into the system and converts it to work. The heat is not converted to the temperature of a system.
- Mon Feb 10, 2020 11:45 pm
- Forum: Calculating Work of Expansion
- Topic: calculating work of a reversible reaction
- Replies: 5
- Views: 402
Re: calculating work of a reversible reaction
work of a system will always be negative because we use the pressure external to calculate the work. The system will lose energy since it is doing work
- Mon Feb 10, 2020 11:42 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: equation conversions
- Replies: 1
- Views: 149
Re: equation conversions
We start out with the equation
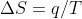
we will then have the equation
)
since for an isothermal reaction we have
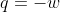
Then we can sub in that for entropy and we will have the equation
)
we will then have the equation
since for an isothermal reaction we have
Then we can sub in that for entropy and we will have the equation
- Mon Feb 10, 2020 11:35 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: q vs delta H
- Replies: 2
- Views: 112
Re: q vs delta H
They are satisfied under conditions of standard pressure.
- Mon Feb 10, 2020 11:34 pm
- Forum: Phase Changes & Related Calculations
- Topic: Work (w)
- Replies: 8
- Views: 373
Re: Work (w)
Work is not a state property because it depends on the path taken. If you walk a really roundabout path and a path straight from point a to point b you will spend a different amount of energy and do a different amount of work.
- Sun Feb 02, 2020 10:32 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Negative Enthalpy
- Replies: 3
- Views: 108
Re: Negative Enthalpy
They do not necessarily happen by themselves since enthalpy is a state function. It depends on the intermediate energy when going from the initial enthalpy to the final enthalpy.
- Sun Feb 02, 2020 10:30 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Work and Heat
- Replies: 2
- Views: 73
Re: Work and Heat
work and heat depend on how the path was taken from the initial value to final value.
- Sun Feb 02, 2020 10:29 pm
- Forum: Calculating Work of Expansion
- Topic: Force
- Replies: 3
- Views: 162
Re: Force
force is pressure times area since pressure is the amount of force in a given area.
- Sun Feb 02, 2020 10:27 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Adiabatic Wall
- Replies: 3
- Views: 199
Re: Adiabatic Wall
Adiabatic means that energy cannot be absorbed or ejected as heat so the system must do work or work must be done on a system in order to absorb or release energy.
- Sun Feb 02, 2020 10:23 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Steam burns
- Replies: 8
- Views: 299
Re: Steam burns
Because of the energy to convert boiling water to steam is a lot. So when steam condenses on your skin, all the energy expelled to form the bonds between water will get sent to your skin causing extreme burns.
- Sun Jan 26, 2020 10:29 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Water Phase Change
- Replies: 6
- Views: 190
Re: Water Phase Change
There needs to be energy required to break the hydrogen bonds in between the water molecules.
- Sun Jan 26, 2020 10:28 pm
- Forum: Phase Changes & Related Calculations
- Topic: Phase Changes
- Replies: 7
- Views: 243
Re: Phase Changes
To accommodate for the phase change of the material, add the reaction enthalpy of the phase change onto the reaction.
- Sun Jan 26, 2020 10:26 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 3 methods
- Replies: 5
- Views: 139
Re: 3 methods
It would depend on the information the question gives you. It also depends on what you are looking for. If you are looking for an accurate enthalpy, you should avoid using bond enthalpies because it is the least accurate.
- Sun Jan 26, 2020 10:25 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Hess's Law
- Replies: 9
- Views: 447
Re: Hess's Law
Hess's law states that enthalpies can be additive. You can add the total reaction enthalpies.
- Sun Jan 26, 2020 10:19 pm
- Forum: Phase Changes & Related Calculations
- Topic: Enthalpy
- Replies: 3
- Views: 161
Re: Enthalpy
Enthalpy is a state because it does matter how it gets to the final value.
- Mon Jan 20, 2020 6:24 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: definition of a buffer
- Replies: 8
- Views: 437
Re: definition of a buffer
A buffer has a weak acid/base with its conjugate. The conjugate can take/give a proton in solution, so it can resist a change in pH.
- Mon Jan 20, 2020 6:23 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Endothermic vs Exothermic
- Replies: 7
- Views: 321
Re: Endothermic vs Exothermic
A reaction is generally endothermic if involves the breaking of bonds which causes the absorption of heat. A reaction is generally exothermic when the product forms bonds.
- Mon Jan 20, 2020 6:20 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: q vs k
- Replies: 62
- Views: 2684
Re: q vs k
The q value will eventually reach k given a long period of time, because that's where the reaction occurs at equilibrium.
- Mon Jan 20, 2020 6:19 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Difference in PH between strong and weak acids
- Replies: 11
- Views: 561
Re: Difference in PH between strong and weak acids
A weak acid does not give up it's protons as easily as a strong acid. Therefore, there is a less concentration of hydronium ions and therefore a lower pH
- Mon Jan 20, 2020 6:18 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Endothermic Reactions
- Replies: 4
- Views: 237
Re: Endothermic Reactions
In an endothermic reaction, heat can be used to break bonds. Therefore, if you increase the heat (which could be noted as a reactant) by Le Chatelier's principle, the formation of products increases.
- Tue Jan 14, 2020 9:14 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Changes in pressure
- Replies: 4
- Views: 259
Re: Changes in pressure
If we change the volume so that the pressure increases, the concentration of the products and reactants changes, so more reactions happen to keep it at the equilibrium constant.
- Tue Jan 14, 2020 9:12 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Le Chatelier's Principle
- Replies: 5
- Views: 448
Re: Le Chatelier's Principle
It applies if the physical parameters are produced. For example, if a reaction produces heat, more reactions will be favored to form reactants if we add heat.
- Tue Jan 14, 2020 9:11 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: equilibrium constants and inputs
- Replies: 4
- Views: 167
Re: equilibrium constants and inputs
Solids cannot have a concentration. Liquids are not included in the equation since their concentration essentially does not change so we leave it out.
- Tue Jan 14, 2020 9:09 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Weak Bases and Acids
- Replies: 2
- Views: 86
Re: Weak Bases and Acids
All weak acids or bases have ka or kb values. Strong acids are when the compounds totally dissociate, so they would have to have remarkably high k values in order to be called strong acids or bases.
- Tue Jan 14, 2020 9:08 am
- Forum: Ideal Gases
- Topic: Partial Pressure
- Replies: 8
- Views: 324
Re: Partial Pressure
It is called partial pressure because in a mixture of gases, each individual gas contributes to the overall pressure.
- Sat Dec 07, 2019 4:44 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Weak Acids
- Replies: 4
- Views: 246
Re: Weak Acids
Weak acids generally don't give off their H+ ions as easily as strong acids. It will generally give you the pKa value of an acid since every proton of a weak acid doesn't dissociate.
- Sat Dec 07, 2019 4:42 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Ligand polydentate
- Replies: 4
- Views: 417
Re: Ligand polydentate
It depends on how many lone pairs the ligand has. For example, en has two nitrogen with lone pairs and is a bidentate.
- Sat Dec 07, 2019 4:39 pm
- Forum: Naming
- Topic: Biological Functions
- Replies: 2
- Views: 171
Re: Biological Functions
Myoglobin contains a naturally occurring coordination compound where the central TM cation is Fe. The Fe binds to oxygen in order to transport it around the body.
- Sat Dec 07, 2019 4:36 pm
- Forum: Polyprotic Acids & Bases
- Topic: polyprotic acids and bases
- Replies: 2
- Views: 1827
Re: polyprotic acids and bases
It is polyprotic if it can recieve or give more than one H+
- Sat Dec 07, 2019 4:30 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Periodic Trends
- Replies: 8
- Views: 690
Re: Periodic Trends
polarizability depends on size of an atom, because the electron cloud can distort more easily. polarizing power: the smaller the cation, the more powerful it is.
- Sat Dec 07, 2019 4:29 pm
- Forum: Naming
- Topic: transition metal suffix
- Replies: 2
- Views: 278
Re: transition metal suffix
Only if the complex is negatively will the metal end with -ate. Furthermore some metals have special names. For iron for example, you would say ferrate instead of ironate.
- Sat Dec 07, 2019 12:47 pm
- Forum: Polyprotic Acids & Bases
- Topic: polyprotic acids
- Replies: 5
- Views: 406
Re: polyprotic acids
Because it is easier for the first hydrogen to be dissociated from the acid. The atom holds on to the second and the third protons more tightly than the first.
- Sat Dec 07, 2019 12:46 pm
- Forum: Photoelectric Effect
- Topic: Module Question
- Replies: 1
- Views: 214
Re: Module Question
So to remove electrons from 1 mol of sodium it is 150.6 KJ.mol^-1. So to find the energy needed for one sodium atom, you just need to divide the number by the number of atoms in a mol.
- Sat Dec 07, 2019 12:43 pm
- Forum: Identifying Acidic & Basic Salts
- Topic: J.17
- Replies: 2
- Views: 233
Re: J.17
Lavelle has stated in class that the 1st and 2nd group metals do not affect pH so they do not have to be written down in the proton transfer.
- Sat Dec 07, 2019 12:40 pm
- Forum: Calculating the pH of Salt Solutions
- Topic: [OH-] & pOH
- Replies: 3
- Views: 287
Re: [OH-] & pOH
The p in p[OH-] stands for -log base 10. You can look at a graph of y=p(x). When the inputted value increases, the function decreases. Likewise the value of When the concentration of OH- decreases, the function p[OH-] increases. This is true for [H+] as well. https://dr282zn36sxxg.cloudfront.net/dat...
- Sat Nov 30, 2019 1:14 pm
- Forum: Bond Lengths & Energies
- Topic: Melting Points
- Replies: 8
- Views: 765
Re: Melting Points
The things that affect intermolecular bonds are the types of bonds i.e. hydrogen and dipole-dipole bonds and the polarizability of the molecule where higher polarizability will mean greater bond strength.
- Sat Nov 30, 2019 1:11 pm
- Forum: Electronegativity
- Topic: Electronegativity
- Replies: 16
- Views: 1070
Re: Electronegativity
The higher the electronegativity, the shorter the bond, the harder it is to break the bond.
- Sat Nov 30, 2019 1:09 pm
- Forum: Air Pollution & Acid Rain
- Topic: Clean Coal vs Dirty Coal?
- Replies: 16
- Views: 1479
Re: Clean Coal vs Dirty Coal?
Dirty coal has more sulfur in it. So when it burns it releases more SO2 than clean coal
- Sat Nov 30, 2019 1:08 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: polydentate and shape
- Replies: 3
- Views: 250
Re: polydentate and shape
Polydentate shapes are like rings since they have multiple bonding sites.
- Sat Nov 30, 2019 1:07 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Ligands
- Replies: 6
- Views: 453
Re: Ligands
Since ligands donate an electron pair, they are considered lewis bases.
- Sat Nov 23, 2019 6:04 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: [Fe(CN)6]4-
- Replies: 5
- Views: 481
Re: [Fe(CN)6]4-
CN binds to the Fe ion. The Fe ion is the central TM. By definition CN is a ligand. The charge is -4 because the Fe ion is +2. Since there are 6 CN- it becomes:
+2-6=-4
+2-6=-4
- Sat Nov 23, 2019 6:01 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: How to tell?
- Replies: 11
- Views: 953
Re: How to tell?
Acids typically donate a proton. For example, with HCl aqueous it beomes H+ and Cl-.
- Sat Nov 23, 2019 5:57 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Denticity
- Replies: 2
- Views: 143
Re: Denticity
Basically it refers to how many bonding sites the ligand has, like the ligand that forms the ring structure for example.
- Sat Nov 23, 2019 5:54 pm
- Forum: Biological Examples
- Topic: Myoglobin
- Replies: 3
- Views: 214
Re: Myoglobin
Many transition metals like to form octahedrals. The iron in the complex is bonded to 5 nitrogens and in this case the O2 binds with the iron ion in the complex.
- Sat Nov 23, 2019 5:49 pm
- Forum: Biological Examples
- Topic: Cisplatin
- Replies: 2
- Views: 125
Re: Cisplatin
If you look at the cisplatin there are two Cl- ions on the same side. These Cl- ions fall out and cisplatin binds to the nitrogen in two diferent guanine base pairs. This essentially stops the enzyme from taking apart the DNA, so the cell cannot divide.
- Sun Nov 17, 2019 12:08 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Angles less than 109.5 degrees
- Replies: 5
- Views: 740
Re: Angles less than 109.5 degrees
It is because of the arrangement of electron densities. Since in a trigonal planar arrangement there are three regions of electron density therefore the angle has to be <120, but in a tetrahedral arrangement there are four regions of electron density therefore the angle has to be <109.5.
- Sun Nov 17, 2019 12:05 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Polar Molecules
- Replies: 4
- Views: 238
Re: Polar Molecules
I am not sure what you mean but if dipoles are on the same side (or adjacent to each other) will not mean that they will not cancel out. For example BF3 where the shape is a trigonal planar. There are two fluorides on the bottom side of the molecule and one fluoride on the top, yet it is non-polar.
- Sun Nov 17, 2019 11:47 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Van Der Waals Interaction
- Replies: 11
- Views: 638
Re: Van Der Waals Interaction
Since every molecule has electrons, every molecule will experience these forces. A van der waal force or induced dipole - induced dipole will be created because some discrepancy in electron density will create induced dipoles.
- Sun Nov 17, 2019 11:40 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSEPR Model
- Replies: 5
- Views: 166
Re: VSEPR Model
Arrangement is the arrangement of electrons around the central atom. Shape is how those electrons repel each other and form the shape of the molecule.
- Sun Nov 17, 2019 11:39 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bent vs angular
- Replies: 1
- Views: 106
Re: Bent vs angular
Bent and angular are pretty much the same thing. You must look out however that bent or angular can have different bond angles. An arrangement of a tetrahedral and a shape of bent (>109.5) is different from an arrangement of a trigonal planar and the shape of bent (>120).
- Sun Nov 10, 2019 1:01 pm
- Forum: Bond Lengths & Energies
- Topic: Shape of Molecules and bond strength
- Replies: 5
- Views: 173
Re: Shape of Molecules and bond strength
The shape affects it since a longer molecule has more surface area to interact with the other molecule than say a spherical molecule resulting in a stronger bond.
- Sun Nov 10, 2019 12:59 pm
- Forum: Bond Lengths & Energies
- Topic: polarizability
- Replies: 9
- Views: 329
Re: polarizability
The size of the molecule generally affects polarizability. When polarizability of molecules increase the energy of bonds between molecules increase resulting in higher melting and boiling points.
- Sun Nov 10, 2019 12:53 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: 3F. 15
- Replies: 2
- Views: 195
Re: 3F. 15
AsF5 has more electrons. Therefore has more polarizability, which means a higher boiling point.
- Sun Nov 10, 2019 12:51 pm
- Forum: Dipole Moments
- Topic: Boiling Point
- Replies: 11
- Views: 730
Re: Boiling Point
Boiling point increases as a result of a higher intermolecular bond. For example water, which has hydrogen bonding, has stronger bonds than ethanol, which is nonpolar. This is shown by water's higher boiling point.
- Sun Nov 10, 2019 12:48 pm
- Forum: Bond Lengths & Energies
- Topic: Strength of Interactions
- Replies: 3
- Views: 193
Re: Strength of Interactions
It is dependent on the polarizability of the two molecules (whether it has more e-). The distance of the bonds also matters, but it is mainly dependent on polarizability.
- Sat Nov 02, 2019 1:09 pm
- Forum: Electronegativity
- Topic: Electronegativity on Test
- Replies: 7
- Views: 262
Re: Electronegativity on Test
I think you need to know the trend of electronegativity where it is opposite to the size of an atom (where it increases across a period and up a group)
- Sat Nov 02, 2019 1:07 pm
- Forum: Octet Exceptions
- Topic: Octet Exception
- Replies: 5
- Views: 282
Re: Octet Exception
B and Al are also exceptions to this rule, as they must get 5 e- to get to an octet. However, since that is too many electrons they usually get 3 more e-
- Sat Nov 02, 2019 1:06 pm
- Forum: Octet Exceptions
- Topic: 2C5a Help
- Replies: 2
- Views: 188
Re: 2C5a Help
I think is because of formal charges, Cl and O are double bonded which means that for Cl to have a formal charge of zero, the leftover radical e- must bind to Cl
- Sat Nov 02, 2019 1:02 pm
- Forum: Lewis Structures
- Topic: central atom
- Replies: 16
- Views: 2230
Re: central atom
Usually if its the only atom: e.g. CH4, then C, the unique atom, would go into the center. But in general, it is the one with the least electronegativity.
- Sat Nov 02, 2019 1:00 pm
- Forum: Ionic & Covalent Bonds
- Topic: Covalent character in ionic bonds
- Replies: 3
- Views: 162
Re: Covalent character in ionic bonds
Yes, because the cation is able to pull the shared electron more towards itself. This is used to explain why some salts cannot dissolve as easily in water than other salts.
- Sun Oct 27, 2019 11:49 am
- Forum: Ionic & Covalent Bonds
- Topic: P, Cl, and S octet tule exceptions
- Replies: 4
- Views: 276
Re: P, Cl, and S octet tule exceptions
Their n=3 therefore they have access to the d-block (l=2) allowing them to place electrons into the d-block.
- Sun Oct 27, 2019 11:47 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Assigning Orbitals to Elements
- Replies: 4
- Views: 208
Re: Assigning Orbitals to Elements
You just have to know that an electron must be in each orbital before there are any paired electrons.
- Sun Oct 27, 2019 11:45 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Ionization Energy
- Replies: 4
- Views: 263
Re: Ionization Energy
It is harder to remove the second electron since the positive charge on the nucleus has a greater force on the electrons since there are less electrons. This is also why cations get smaller.
- Sun Oct 27, 2019 11:42 am
- Forum: Trends in The Periodic Table
- Topic: atomic Radii
- Replies: 11
- Views: 442
Re: atomic Radii
You have to take into the account the charge of the nucleus as well. For example, Fluorine has more electrons than oxygen, but it has a smaller radius than oxygen. That is why the size becomes smaller if you go down a group. However, just adding electrons to an atom will make it bigger. A fluorine i...
- Sun Oct 27, 2019 11:39 am
- Forum: Trends in The Periodic Table
- Topic: Question on 1F.5 b
- Replies: 3
- Views: 169
Re: Question on 1F.5 b
A good way to remember is that the farther away an electron is, the easier it is to remove. Since Na has a greater size than Mg, it is easier to remove an electron; therefore, Na has a lower ionization energy.
- Sat Oct 19, 2019 2:16 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Question about last lecture
- Replies: 4
- Views: 145
Re: Question about last lecture
Both are correct, but the other one is more specific since it showed each electron in each subshell. The problem in the first one is that one could misunderstand and put 2 electrons in one subshell before filling all of the other ones first.
- Sat Oct 19, 2019 2:14 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: spin state (ms) quantum number
- Replies: 2
- Views: 166
Re: spin state (ms) quantum number
There is a way to prove that an atom's electron can spin up or spin down through the beaming of silver atoms shown in one of Lavelle's lectures. The electrons spin dictated where the atom would go. However,I think you just need to know that paired electrons have the opposite spin.
- Sat Oct 19, 2019 2:11 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Hund's rule
- Replies: 5
- Views: 161
Re: Hund's rule
It is energetically favorable for electrons to have that configuration
- Sat Oct 19, 2019 2:09 pm
- Forum: Photoelectric Effect
- Topic: One photon one atom interaction
- Replies: 14
- Views: 595
One photon one atom interaction
So after the energy of light surpasses the minimum energy needed to eject an electron, would increasing the energy of light (the frequency) increase the amount of electrons ejected at that point or would the amount of electrons ejected stay the same?
- Sat Oct 19, 2019 2:07 pm
- Forum: Photoelectric Effect
- Topic: Problem 1B.15
- Replies: 1
- Views: 103
Re: Problem 1B.15
a) Use the DeBroglie equation for this problem since it both relates wavelength and the velocity of an electron. b)Use the photoelectric equation that relates to the frequency and energy in this case it would be E=h\nu + Kinetic energy but since there is no kinetic energy of an electron the kinetic ...
- Sun Oct 06, 2019 11:22 am
- Forum: Empirical & Molecular Formulas
- Topic: What decimal place to round to when taking masses from the Periodic Table?
- Replies: 19
- Views: 2983
Re: What decimal place to round to when taking masses from the Periodic Table?
Atomic numbers are usually carried out to four significant figures such as with hydrogen being 1.008 and carbon being 12.01. So I would just use four significant figures, and it should be fine.
- Sun Oct 06, 2019 11:19 am
- Forum: Empirical & Molecular Formulas
- Topic: Empirical to Molecular Formulas [ENDORSED]
- Replies: 6
- Views: 455
Re: Empirical to Molecular Formulas [ENDORSED]
You would need the molar mass of the molecule you are trying to find. Then you would divide that molar mass by the molar mass of the empirical formula and get a number. If the number is like 2.9 or 5.1, you can round it to a whole number. If it is not a whole number, I suggest checking your work bec...
- Sun Oct 06, 2019 11:08 am
- Forum: Empirical & Molecular Formulas
- Topic: Polyatomic Ions/Naming Compounds
- Replies: 6
- Views: 650
Re: Polyatomic Ions/Naming Compounds
I recommend putting them into groups and recognizing the patterns in the prefixes and the charges. Hypochlorite, Chlorite, Chlorate, and Perchlorate all have charges of -1. Polyatomic ions that start with hypo- and end with -ite have one oxygen atom. Polyatomic ions that just end in -ite have 2 oxyg...
- Sun Oct 06, 2019 11:02 am
- Forum: Balancing Chemical Reactions
- Topic: Advice for Test 1
- Replies: 7
- Views: 435
Re: Advice for Test 1
In the syllabus, it says that he uses problems from the homework. I suggest if you want to practice more for the test is to do those problems listed in the syllabus.
- Mon Sep 30, 2019 10:04 pm
- Forum: Limiting Reactant Calculations
- Topic: 2 Limiting Reactants
- Replies: 9
- Views: 379
Re: 2 Limiting Reactants
A little unrelated, but if there is a reaction that requires a catalyst, and the catalyst is present in very small quantities relative to the reactants, is it called a limiting reactant because it severely slows the reaction, or do we classify it as something else? The catalyst is not a limiting re...

