Search found 102 matches
- Fri Mar 13, 2020 8:16 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Galvanic vs Electrolytic cells
- Replies: 6
- Views: 560
Re: Galvanic vs Electrolytic cells
A galvanic cell converts chemical energy into electrical energy, while an electrolytic cell converts electrical energy into chemical energy. In a galvanic cell, a spontaneous redox reaction (ΔG<0, energy is released) produces an electric current through an external circuit. However, in an electrolyt...
- Fri Mar 13, 2020 7:57 am
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: how to find pkb
- Replies: 2
- Views: 350
Re: how to find pkb
pKb is a measure of the strength of a base, while pOH is a direct measure of the hydroxide ion concentration of a substance. While they're related, you generally wouldn't be asked to calculate pKb from pOH. In the case of a basic buffer (ie a weak base and its salt), you could relate the two via the...
- Fri Mar 13, 2020 7:51 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: F in the equation for standard Gibbs energy
- Replies: 4
- Views: 390
Re: F in the equation for standard Gibbs energy
F is Faraday's constant (generally given as 96,485 C/mol). It is the magnitude of electric charge per mole of electrons.
- Thu Mar 12, 2020 12:14 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: determining k
- Replies: 13
- Views: 601
Re: determining k
Yeah, you just need to make sure that the concentration is the same for every other reactant than the one you're looking at. For example, in a reaction with reactants A, B, and C, if you're trying to figure out how [A] affects the rate of the reaction, you should compare two trials where [B] is the ...
- Tue Mar 10, 2020 2:38 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Intermediate
- Replies: 3
- Views: 504
Re: Intermediate
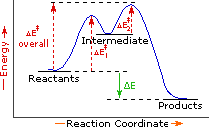
This image might help! Generally, the number of intermediates in a multi-step reaction can be determined by the number of troughs in the reaction profile.
- Tue Mar 10, 2020 2:34 pm
- Forum: Zero Order Reactions
- Topic: 0 order
- Replies: 14
- Views: 1544
Re: 0 order
An example of a zero order reaction is the decomposition of nitrous oxide in the presence of a hot platinum wire (a catalyst): 2N2O --> 2N2 + O2. Generally, a reaction involving a catalyst will be zero order. Using the stated reaction as an example, if we were to increase the concentration of the re...
- Wed Mar 04, 2020 11:08 am
- Forum: Van't Hoff Equation
- Topic: 5J.15
- Replies: 3
- Views: 433
Re: 5J.15
Data in appendix 2A is specific to the reaction occurring at 25C. Therefore, you can't use △G=-RTlnK for 150C, because the value of △G that you calculated using the appendix data will be different at 150C.
- Wed Mar 04, 2020 11:05 am
- Forum: Balancing Redox Reactions
- Topic: 6K.3 part D
- Replies: 3
- Views: 246
Re: 6K.3 part D
If you used Cl2 as written in the problem, no reduction would be occurring, since for Cl2 --> Cl2, the oxidation number of Chlorine is 0 on both the reactant and product side.
- Wed Mar 04, 2020 11:02 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 6N.9
- Replies: 1
- Views: 236
Re: 6N.9
(1) Consider what the two electrodes are. In this case it should be hydrogen (given in the problem), and tin (from Sn(NO3)2)) (2) Figure out which one is going to be at the cathode and which will be at the anode. The standard reduction potential of H+ (2H+ + 2e- --> H2) is 0 by definition. The oxida...
- Wed Mar 04, 2020 10:40 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: 5G.17
- Replies: 1
- Views: 118
Re: 5G.17
The lines plateau when the reaction has reached equilibrium. While there are some reactions that go to completion, most reactions will not and therefore the concentration of reactant won't fall to 0 exactly. [Reactants] continues to decrease and [Products] continues to increase as the reaction appro...
- Wed Mar 04, 2020 10:35 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6M.5
- Replies: 2
- Views: 265
Re: 6M.5
If you go to page A17, the standard potentials are listed in alphabetical order rather than numerical order, which should help you find the potential you're looking for much faster. The reason why we do not include H2O in the cell diagram is because the cell diagram already has species in aqueous so...
- Tue Feb 25, 2020 11:49 pm
- Forum: Van't Hoff Equation
- Topic: Van't Hoff (Thermo vs. Kinetic approach)
- Replies: 1
- Views: 177
Re: Van't Hoff (Thermo vs. Kinetic approach)
I believe we will probably have to know the Van't Hoff equation, considering the fact that Dr. Lavelle derived the equation during lecture.
- Tue Feb 25, 2020 11:46 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: 5G.17
- Replies: 2
- Views: 241
Re: 5G.17
From 5G.13, we get that the reaction Gibbs free energy for I2(g) --> 2I(g) at 1200 K when the partial pressures of I2 and I are 0.13 bar and 0.98 bar, respectively, is positive. This means that the forward reaction is not spontaneous, whereas the reverse is. This means that the production of I2 is f...
- Tue Feb 25, 2020 11:37 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: predict std. rxn for this galvanic cell
- Replies: 1
- Views: 127
Re: predict std. rxn for this galvanic cell
When calculating standard cell potential, the equation Ecathode - Eanode is used in reference to the convention of only including the standard reduction potentials of reactions (For example, in Appendix 2B in the textbook, all of the data is written as reduction half-reactions and never oxidation ha...
- Tue Feb 25, 2020 11:30 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: 5G.13 and 5G.19
- Replies: 1
- Views: 140
Re: 5G.13 and 5G.19
While 5G.19 is asking for the standard Gibbs free energy, 5G.13 is asking for the reaction Gibbs free energy. The difference is that if you consider the equation ΔG = ΔGnaught + RTlnQ, we know that depending on the concentration of the products and reactants, ΔG (the reaction Gibbs free energy) can ...
- Tue Feb 25, 2020 11:25 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Platinum
- Replies: 1
- Views: 122
Re: Platinum
Platinum is an inert electrode, meaning that it facilitates the conduction of electrons but does not actively participate in the redox reaction itself. In general, you need an inert electrode when the half-cell does not already have a solid metal that can act as the electrode itself (ex. Cu(s), Fe(s...
- Sun Feb 23, 2020 6:38 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6M.5a Cell diagram
- Replies: 1
- Views: 102
Re: 6M.5a Cell diagram
Usually the ions must be in the solid state in order to conduct electrons, but mercury is an important exception because it is the only liquid that can act as an electrode. Therefore, you don't need an inert electrode on the left side of the cell diagram.
- Sun Feb 23, 2020 6:29 pm
- Forum: Balancing Redox Reactions
- Topic: 6K.3 Part D
- Replies: 3
- Views: 232
Re: 6K.3 Part D
This one was very confusing for me as well, but I think since Cl2 has to serve as both the oxidizing and reducing agent, if Cl is getting oxidized in Cl2 --> HClO, then the Cl2 also needs to get reduced somehow. The easiest way for this to occur would be to make the other half reaction Cl2 --> 2 Cl-.
- Sun Feb 23, 2020 6:23 pm
- Forum: Balancing Redox Reactions
- Topic: 6K.3 b)
- Replies: 2
- Views: 189
Re: 6K.3 b)
The oxidation numbers for the hydrogen and oxygen present in each side of the reaction are the same, so hydrogen and oxygen specifically are not oxidized or reduced in this reaction. Therefore, you don't need a separate half reaction for them, since there is no oxidation or reduction of either the h...
- Sun Feb 23, 2020 6:17 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Platinum
- Replies: 4
- Views: 274
Re: Platinum
You only need an inert electrode when the half-cell does not have a solid substance to conduct electrons. In the case of Cu(s), it is in the solid state and therefore can serve as the electrode itself, which is why you only put Pt(s) on the right side of the cell diagram and not the left.
- Sun Feb 23, 2020 6:04 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: cell diagrams
- Replies: 1
- Views: 123
Re: cell diagrams
I believe the convention is different when there is a gas involved. For H2(g) --> 2H+ + 2e-, it is convention to put the H2(g) before the H+, since the anode is where oxidation occurs and in this case, H2(g) is the chemical species getting oxidized. You also generally put the electrodes on either th...
- Thu Feb 13, 2020 6:37 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: 4F.11
- Replies: 1
- Views: 150
Re: 4F.11
I think you have to calculate the ΔS due to change in temperature and change in volume separately for this problem
- Thu Feb 13, 2020 6:28 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Mean bond enthalpies
- Replies: 3
- Views: 286
Re: Mean bond enthalpies
When we are given mean bond enthalpy values, those values do not directly represent a change in energy coming about as a result of a specific reaction, unlike when we use the standard enthalpy of formation of a substance, which is by definition the change in energy resulting from the formation of a ...
- Thu Feb 13, 2020 6:20 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Delta U
- Replies: 5
- Views: 387
Re: Delta U
Technically, ΔU is only truly 0 in the case of an ideal gas. ΔU can be thought of as the gas's internal energy, comprising both potential and kinetic energy. By definition, an ideal gas does not have any interactions between particles, so potential energy is 0. That leaves kinetic energy. Temperatur...
- Thu Feb 13, 2020 6:15 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Delta S
- Replies: 4
- Views: 405
Re: Delta S
ΔS of the system is the same regardless of whether the process is reversible or irreversible. However, ΔS of the surroundings and ΔS total are different depending on the reversibility of the process. In a reversible process: ΔS of the surroundings = -ΔS of the system ΔS total = 0 In an irreversible ...
- Tue Feb 11, 2020 8:22 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: constant p & v
- Replies: 1
- Views: 116
Re: constant p & v
In an isothermal reversible compression, volume is decreased while pressure is increased. The opposite is true for an expansion, where volume is increased, and pressure is decreased. Whenever the process is reversible and isothermal, ΔH = 0. In contrast, for an isobaric process, ΔH = qp, and in an i...
- Tue Feb 04, 2020 11:34 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: enthalpy equations
- Replies: 1
- Views: 90
Re: enthalpy equations
ΔH is equal to q whenever the reaction is occurring at constant pressure, a condition which is specified in the problem. I think you may be referring to the fact that there has to be no expansion in order for ΔH to be equal to ΔU (internal energy). Hope this helps!
- Tue Feb 04, 2020 11:30 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: 4C.3
- Replies: 3
- Views: 139
Re: 4C.3
These values are all included on Dr. Lavelle's "Constants and Equations" sheet! The sheet can be found on the 14B website and is the same one that we will receive during any test or midterm.
- Tue Feb 04, 2020 11:27 pm
- Forum: Phase Changes & Related Calculations
- Topic: supercooling
- Replies: 3
- Views: 175
Re: supercooling
Supercooling is when a liquid is cooled below its standard freezing point without crystallizing. In order for crystallization (i.e. formation of a solid) to occur, generally a nucleus of some sort is needed around which a crystal will form. Thus, supercooling can occur when there are no nuclei of th...
- Tue Feb 04, 2020 11:17 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Negative Work Equation
- Replies: 1
- Views: 95
Re: Negative Work Equation
The equations for work all have a negative sign at the beginning in order to define the energy associated with doing work relative to the system rather than its surroundings. If the work equation did not have a negative sign (ie. w = PΔV), then when the volume of the gas increases (ie ΔV is positive...
- Mon Feb 03, 2020 6:15 pm
- Forum: Phase Changes & Related Calculations
- Topic: 4B. 3 part b
- Replies: 1
- Views: 163
Re: 4B. 3 part b
I'm pretty sure the answer key in the textbook for this problem is incorrect. If you copy and paste the problem into Google you will see many solutions to the problem that all say w = 490 J.
- Wed Jan 29, 2020 11:25 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 4D.3
- Replies: 2
- Views: 136
Re: 4D.3
The change in temperature should be proportional to the amount of carbon monoxide being reacted. First, convert 1.40 g of CO to moles, and you get roughly 0.0500 mol of CO. If you set up a proportion, then you get 0.0500 mol/1.00 mol = 0.636C/xC, with 0.636 being the change in temperature when 1.40 ...
- Wed Jan 29, 2020 12:46 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Cpm vs. Cvm
- Replies: 2
- Views: 386
Re: Cpm vs. Cvm
Cp is higher than Cv, because when pressure is constant, volume is not, so the gas is doing work via expansion. Therefore, you must account for the fact that for Cp, not all of the heat will be used toward increasing the temperature of the substance; some will be lost to work. To answer why pressure...
- Wed Jan 29, 2020 12:40 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: 4C.3
- Replies: 3
- Views: 139
Re: 4C.3
In the relevant section of the textbook, it is explained that for a monatomic ideal gas, Cp = 5/2R, while Cv = 3/2R, where R = 8.3145 J*K-1*mol-1.
- Mon Jan 27, 2020 10:13 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Acid and Bases
- Replies: 2
- Views: 261
Re: Acid and Bases
Sodium hydroxide
- Mon Jan 27, 2020 4:10 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Expansion work
- Replies: 2
- Views: 99
Re: Expansion work
When heat is added at constant volume, all of the heat is used to increase the temperature of the substance. However, when heat is added at constant pressure, the volume is changing and therefore more heat is needed to achieve the same temperature when volume is constant. This is because not only is...
- Sun Jan 26, 2020 1:28 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Kw Equations
- Replies: 10
- Views: 547
Re: Kw Equations
[H3O+][OH-] = Kw, and [Ka][Kb] = Kw. Keep in mind however that Kw will be different depending on temperature. We just tend to use 1*10^-14 b/c this is what Kw is equal to at the standard state temperature (25C)
- Sun Jan 26, 2020 1:25 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: 6C.13
- Replies: 3
- Views: 166
Re: 6C.13
The stronger the base, the weaker the conjugate acid. A weaker conjugate acid is denoted by a higher pKa, since pKa is the negative log of Ka (which is very small for a weak acid). Therefore, you would put them in order of increasing pKa. From weakest to strongest base, the answer should be aniline,...
- Sun Jan 26, 2020 1:20 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Bond Enthalpy vs. Standard Enthalpy of Formation
- Replies: 2
- Views: 170
Re: Bond Enthalpy vs. Standard Enthalpy of Formation
1. Bond enthalpy is the amount of energy required to break one mole of the given bond (ex. if the bond enthalpy of an O-H bond is 464 kJ/mol, then it would take 464 kJ to break 6.022*10^23 O-H bonds). 2. Standard enthalpy of formation of a substance is defined as the change in enthalpy when 1 mole o...
- Sun Jan 26, 2020 10:59 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: state property
- Replies: 1
- Views: 90
Re: state property
A state function can describe a particular state without knowing the path that was taken to reach this state. For example, it is possible to say that a system has a certain amount of internal energy, enthalpy, entropy, etc. given a set of specified conditions. However, for a path function (ex. work ...
- Wed Jan 22, 2020 4:57 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: 6D.5
- Replies: 2
- Views: 156
Re: 6D.5
I believe for (a)-(c), there is a table in the relevant section of the textbook that has the Kb values of common weak bases. If you look at the solutions manual, there are no calculations made for Kb, which seems to indicate that they assumed Kb was meant to be given for the problem.
- Thu Jan 16, 2020 6:13 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Autoprotolysis
- Replies: 1
- Views: 131
Re: Autoprotolysis
Autoprotolysis can occur with other molecules as well; not just water. For example, in some cases ammonia can undergo protolysis (2NH3 --> NH2- + NH4+) too. You are correct that water just happens to be the most common example.
- Thu Jan 16, 2020 5:49 pm
- Forum: Ideal Gases
- Topic: ph
- Replies: 10
- Views: 513
Re: ph
Yes! pH can definitely be above 14 or below 0. An example is 12 M HCl, which has a pH of about -1.08. If we consider mathematically that pH=-log[H+], then we know that if [H+] is greater than 1, pH has to be below 0.
- Wed Jan 15, 2020 5:37 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6A 21
- Replies: 2
- Views: 96
Re: 6A 21
Since the water is neutral (neither acidic nor basic), we know that [H3O+] must equal [OH-]. Normally, when we are assuming that temperature is 25C, Kw = 1*10^-14. This is why the pH of neutral water is said to be 7: at 25C, [H3O+]=[OH-]=1*10^-7; thus [H3O+][OH-]=1*10^-14. In the problem we are give...
- Mon Jan 13, 2020 8:48 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Homework 5l. 29
- Replies: 2
- Views: 110
Re: Homework 5l. 29
For this problem, you can set up an ICE table, but instead of using the concentrations of the compounds, you use the partial pressures instead. You get: 2HCl (g) --> H2(g) + Cl2(g) I 0.22 0 0 C -2x +x +x E 0.22-2x x x We know that Kp = PH2*PCl2/(PHCl)^2, which we can then plug our equilibrium expres...
- Mon Jan 13, 2020 8:41 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Homework 5.35
- Replies: 3
- Views: 175
Re: Homework 5.35
In the graph shown, the partial pressures of the gases gradually change until they eventually plateau. This plateau corresponds to the equilibrium concentrations of A, B, and C.
- Thu Jan 09, 2020 1:49 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Textbook Self-test 5G.3A
- Replies: 2
- Views: 129
Re: Textbook Self-test 5G.3A
The net ionic equation would be 2Ag+(aq) + 2OH-(aq) <-> Ag2O (s) + H2O (l). Na+ and NO3- are the spectator ions and do not directly participate in the reaction. We don't use solids or liquids in the expression for the equilibrium constant, so we end up with Kc = 1/[Ag+]^2[OH-]^2.
- Thu Jan 09, 2020 1:43 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5G. 3
- Replies: 5
- Views: 167
Re: 5G. 3
In addition, generally when you have a choice between Kp and Kc, Kp is assumed to be the default (ex. if you look at table 5G.2 which you had to use for many of the hw problems, they have a separate column for K and Kc. The K is assumed to be the same thing as Kp)
- Wed Jan 08, 2020 7:28 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Module 4 Q17
- Replies: 1
- Views: 135
Re: Module 4 Q17
Everything you've done so far should be correct; x should be equal to either 0.0828 or 0.126. It can't be 0.126 because then that would mean that the equilibrium concentration of CO and H2O are negative, so that means that x has to be equal to 0.0828. Therefore, [CO]=[H2O]=0.0172, and [CO2]=[H2]=0.0...
- Wed Jan 08, 2020 7:19 pm
- Forum: Ideal Gases
- Topic: Units for Pressure
- Replies: 6
- Views: 182
Re: Units for Pressure
They are both units of pressure, with 1 atm being approximately 1.01325 bar. As previously mentioned, you can use either unit as long as you ensure that the units cancel, but when dealing with the ideal gas law PV=nRT, we generally use atm instead of bar because we conventionally say that the gas co...
- Wed Jan 08, 2020 3:19 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: partial pressure
- Replies: 2
- Views: 138
Re: partial pressure
Whenever there is more than one gas involved, we use the term partial pressure to refer to the pressure that the gas would exert if it were by itself. The sum of the partial pressures of all of the gases involved is the total pressure. We can rearrange the ideal gas law, PV=nRT, to solve for the par...
- Sat Dec 07, 2019 4:58 pm
- Forum: Identifying Acidic & Basic Salts
- Topic: homework question 6D.11
- Replies: 3
- Views: 227
Re: homework question 6D.11
A salt with an acidic ion in it will produce a solution with pH < 7, and a salt with a basic ion in it will produce a solution with pH > 7. For each compound you would want to look at each ion in the compound and check whether it would affect pH. For example, with NH4Br, Br- would NOT have an effect...
- Mon Dec 02, 2019 7:23 pm
- Forum: Lewis Acids & Bases
- Topic: Salts containing conjugate acids/bases
- Replies: 2
- Views: 193
Re: Salts containing conjugate acids/bases
Additionally, if the conjugate acid is the conjugate acid of a strong base, then it will have no effect on pH. The same is true for the conjugate base of a strong acid.
- Mon Dec 02, 2019 7:20 pm
- Forum: Lewis Acids & Bases
- Topic: 6.13
- Replies: 2
- Views: 225
Re: 6.13
pKa is not the same as the pH scale. While pKa is also an indicator of the strength of the acid, it does not follow the 0-14 pH scale. pKa values can be as low as -5 and as high as 50, so knowing that the pKa value is greater than 7 has no significance (you can't know whether a compound is an acid o...
- Mon Dec 02, 2019 7:10 pm
- Forum: Amphoteric Compounds
- Topic: 6A.17
- Replies: 5
- Views: 460
Re: 6A.17
In general, metal oxides tend to act as bases while nonmetal oxides tend to act as acids. The oxides of elements on or near the diagonal metalloid line going down the periodic table have relatively equal acidic and basic character, so they are considered amphoteric.
- Mon Dec 02, 2019 7:07 pm
- Forum: Properties & Structures of Inorganic & Organic Bases
- Topic: 6C.17
- Replies: 2
- Views: 267
Re: 6C.17
Additionally, BrO- is the conjugate base of a weak acid (hypobromous acid HBrO), so it must be a pretty strong base. Another thing to consider is that the defining basic characteristic of morphine is the N atom with its lone pair, and compounds like these generally make for relatively weak bases.
- Mon Dec 02, 2019 7:01 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Anion stability
- Replies: 6
- Views: 496
Re: Anion stability
The Ka value is the equilibrium constant for the reaction involving the dissociation of an acid. It is equivalent to ([H+][A-]/[HA]). The higher the Ka, the higher the [H+] and therefore the stronger the acid. Normally you will only be given the Ka if the acid in question is weak, because a strong a...
- Sat Nov 30, 2019 10:18 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: 6b.9
- Replies: 3
- Views: 237
Re: 6b.9
If you have pH, [H3O+] is 10^-pH. For example, if pH is 3, [H3O+] is 10^-3. The same is true for pOH and [OH-]. It's also useful to know the relationship between pH and pOH and [H3O+] and [OH-]: pH + pOH = 14, and [H3O+][OH-] = 1*10^-14.
- Sat Nov 30, 2019 10:14 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Calculating pOH
- Replies: 2
- Views: 121
Re: Calculating pOH
Dr. Lavelle might go over it during lecture at some point; but either way it'll probably be useful information to know that pOH=-log[OH-], pOH = 14-pH, and [H3O+][OH-]=1*10^-14.
- Sat Nov 30, 2019 10:09 pm
- Forum: Conjugate Acids & Bases
- Topic: Product of Acid and Base
- Replies: 5
- Views: 360
Re: Product of Acid and Base
In a neutralization reaction, an H+ ion is given off by the acid while an OH- ion is given off by the base. These two ions will combine to produce water. The remaining ions while combine to form a salt, which is the general term for any ionic substance that doesn't have H+ as the cation or OH- as th...
- Fri Nov 29, 2019 4:54 pm
- Forum: Lewis Acids & Bases
- Topic: Homework 6A 17
- Replies: 2
- Views: 131
Re: Homework 6A 17
For this question, I believe the main concept they want you to understand is that nonmetal oxides tend to act as acids, whereas metal oxides tend to act as bases. The classification of oxides as acids or bases is more ambiguous when the other element is a metalloid, so As2O3 and Bi2O3 would be class...
- Tue Nov 26, 2019 12:22 am
- Forum: Bronsted Acids & Bases
- Topic: Equlibrium Constant Expression for Strong Acids/Bases
- Replies: 3
- Views: 152
Re: Equlibrium Constant Expression for Strong Acids/Bases
Ka and Kb are meant to be used to calculate equilibrium concentrations of weak acids and bases only, because generally only a very tiny fraction of the acid/base is dissociated in water if it is weak. Strong acids and bases will have very large Ka and Kb, because they are generally assumed to comple...
- Sun Nov 24, 2019 6:35 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Chelate Complex
- Replies: 1
- Views: 68
Re: Chelate Complex
A chelate is just a compound containing a ligand bonded to a central metal atom at multiple points. You can also think of it as any coordination compound that contains polydentate ligands. There is no such thing as a chelate shape, and since chelates are just a special type of coordination compound,...
- Sun Nov 24, 2019 6:29 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Coordination Sphere Clarification
- Replies: 1
- Views: 75
Re: Coordination Sphere Clarification
A coordination sphere is just the array of molecules and ions directly attached to the central metal atom. It's just a grouping of the central metal atom and all of the ligands attached to it; shape is separately determined. Coordination spheres aren't visual aids, but knowing which metal ion and li...
- Sun Nov 24, 2019 6:25 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Coordination compound vs covalent compound
- Replies: 1
- Views: 195
Re: Coordination compound vs covalent compound
The atoms in a coordination compound are held together by coordinate covalent bonds, while covalent compounds are not. Recall that a normal covalent bond is one in which 2 electrons are shared between 2 atoms. Usually each atom contributes one electron to the covalent bond, but in a coordinate coval...
- Sun Nov 24, 2019 6:18 pm
- Forum: Naming
- Topic: Order in Naming
- Replies: 12
- Views: 738
Re: Order in Naming
For coordinate compounds, the order of the atomic symbols does matter. The ligand names would be in alphabetical order (either using their prefixes or the name of the element), followed by the name of the transition metal cation name. I think the question was about going from the name of the coordi...
- Sun Nov 24, 2019 6:14 pm
- Forum: Naming
- Topic: HW problem 9C.3(d)
- Replies: 2
- Views: 97
Re: HW problem 9C.3(d)
When writing the formula of the coordination compound, there is no single correct way to order the ligands as long as they are all written after the central metal ion. However, there is only one right way to name a coordination compound. The central metal ion should come first, with the ligands list...
- Sun Nov 24, 2019 6:10 pm
- Forum: Naming
- Topic: Oxidation Number
- Replies: 3
- Views: 222
Re: Oxidation Number
If you were just given the ion, you would first find the total charge of the ligands and identify the overall charge of the ion. The charge of the metal + the total charge of the ligands should add up to the overall charge of the ion. For example, if you were given [Co(CN)6]-3, you would identify th...
- Sun Nov 24, 2019 6:05 pm
- Forum: Naming
- Topic: Trans and Cis
- Replies: 8
- Views: 506
Re: Trans and Cis
Yes, you wouldn't be able to tell if you were just given the molecular formula. Cis-trans isomerism is when there is a double bond and therefore restricted rotation, since pi bonds can't be rotated without being broken. Cis indicates that the functional groups appear on the same side of the double b...
- Sun Nov 24, 2019 5:54 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Definitions
- Replies: 1
- Views: 84
Re: Definitions
From the textbook: Complex: a species consisting of a central metal atom or ion to which a number of molecules or ions are attached by coordinate covalent bonds Coordination compound: electrically neutral compound in which at least one of the ions present is a complex Ligand: Lewis base attached to ...
- Wed Nov 20, 2019 8:05 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Problem 3F.3c
- Replies: 2
- Views: 109
Re: Problem 3F.3c
Generally when there is a carbon atom in the molecule it is in the center. You would arrange H and Cl around it tetrahedrally. It technically does not matter how you arrange the 2 H and 2 Cl atoms around the C atom in the Lewis structure, since in a tetrahedral molecular geometry it is impossible fo...
- Wed Nov 20, 2019 8:03 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Section 2E #27a
- Replies: 1
- Views: 129
Re: Section 2E #27a
If you're referring to the question that asks you to predict whether C5H5N would be polar, you technically don't need to draw the Lewis structure to figure it out. The C-H and C-N dipole moments are different, and there is a difference in electron pull between all of the atoms (N is more electronega...
- Sun Nov 17, 2019 1:39 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: vapor pressure
- Replies: 4
- Views: 336
Re: vapor pressure
I don't believe that we have to know what vapor pressure is for Test 2. But essentially, when a liquid is heated and the molecules begin to accumulate enough energy to escape into the gas phase, it results in a layer of gaseous molecules on top of the liquid that exerts a pressure (hence the term va...
- Sun Nov 17, 2019 1:28 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Strength of Repulsion
- Replies: 4
- Views: 324
Re: Strength of Repulsion
The electron cloud of a lone pair is dispersed over a larger area than that of a bonding pair, because while a bonding pair is held in place by two atoms, a lone pair is only held in place by one. This means that there is only one nucleus's positive charge drawing in and localizing the electron clou...
- Sun Nov 17, 2019 1:21 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Interactions
- Replies: 3
- Views: 126
Re: Interactions
Yes to both questions!
- Sun Nov 17, 2019 1:12 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Polarity 2E.25
- Replies: 2
- Views: 183
Re: Polarity 2E.25
For CH2Cl2, recall Dr. Lavelle's explanation of the molecular geometry of water during lecture. In lecture, he drew a Lewis structure of water with the lone pairs and hydrogen atoms on opposite sides of the oxygen atom, explaining why it was a misleading representation of the molecular geometry. In ...
- Sun Nov 17, 2019 12:39 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Dipoles
- Replies: 2
- Views: 135
Re: Dipoles
Additionally, dipoles can cancel each other out. For example, in a carbon dioxide molecule, even though there is a difference in electronegativity between the carbon atom and each of the two oxygen atoms, the dipoles are pointing in the opposite direction (each pointing toward an oxygen atom) and th...
- Sun Nov 03, 2019 7:57 pm
- Forum: Electronegativity
- Topic: memorizing tables
- Replies: 9
- Views: 342
Re: memorizing tables
I think it's dependent on the question. For example, if the question is asking about the numerical value of the electronegativity difference between two atoms of different elements, you probably wouldn't be expected to know this off the top of your head. However, it is pretty reasonable to be expect...
- Sun Nov 03, 2019 7:48 pm
- Forum: Lewis Structures
- Topic: 2.B.11
- Replies: 2
- Views: 246
Re: 2.B.11
Generally, when you see a molecular formula given like that (instead of say C2H5NO2), the formula is a structural formula and can give you a clue about how to arrange the atoms in the Lewis structure. H2C, NH2, and COOH are all segments of the actual Lewis structure, with H2C in the center and NH2 a...
- Sun Nov 03, 2019 7:41 pm
- Forum: Lewis Structures
- Topic: structure stability
- Replies: 2
- Views: 168
Re: structure stability
To add on, if you know that one of the atoms has to carry a nonzero formal charge (ie. the overall charge of the molecule is nonzero) but aren't sure which atom to put it on, put the more negative formal charge on the more electronegative atom
- Sun Nov 03, 2019 7:36 pm
- Forum: Lewis Structures
- Topic: expanding an octect
- Replies: 5
- Views: 190
Re: expanding an octect
Generally, you would expand an octet if it would make the molecule's formal charges more favorable. For example, the Xenon atom in XeF4 has an expanded octet because 1) F cannot accommodate an expanded octet as it is in the 2nd period and therefore does not have any d orbitals that could hold additi...
- Sun Nov 03, 2019 7:26 pm
- Forum: Sigma & Pi Bonds
- Topic: Versus
- Replies: 3
- Views: 146
Re: Versus
Every molecule has sigma bonds, but not every molecule has pi bonds. Molecules with double (1 sigma and 1 pi bond) or triple (1 sigma and 2 pi bonds) bonds will have pi bonds, which are the result of the overlapping of p orbitals.
- Sat Oct 26, 2019 9:24 pm
- Forum: Trends in The Periodic Table
- Topic: Electron affinity of Chlorine vs Fluorine
- Replies: 1
- Views: 260
Electron affinity of Chlorine vs Fluorine
Why is the electron affinity of chlorine greater than that of fluorine? I understand that electronegativity and electron affinity are different, with electronegativity being the tendency to attract electrons and electron affinity being the amount of energy released when an electron is gained, but th...
- Sat Oct 26, 2019 9:19 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Stability
- Replies: 9
- Views: 562
Re: Stability
It's all about charge distribution. The closer to 0 the formal charge is, the more evenly distributed the electrons are about the molecule. If the absolute values of the formal charges of the atoms in a molecule are high, this will lead to the molecule having regions that are more negatively or posi...
- Sat Oct 26, 2019 9:09 pm
- Forum: Lewis Structures
- Topic: Resonance and lower energy levels
- Replies: 2
- Views: 92
Re: Resonance and lower energy levels
In resonance, the electrons are delocalized and therefore aren't fixed to a particular atom or bond. Because delocalization allows for the charge of the electrons to be distributed over a larger amount of space, the overall energy of the molecule is lowered and thus more stable.
- Sat Oct 26, 2019 9:04 pm
- Forum: Sigma & Pi Bonds
- Topic: orbitals
- Replies: 3
- Views: 675
Re: orbitals
A simple trick to solve for the number of orbitals in the nth shell of an atom is to know that it is equal to n^2. So the 1st shell has 1 orbital, 2nd shell has 4, 3rd shell has 9, etc. You can also adjust this equation to 2n^2 to solve for the number of electrons that a given shell of an atom can h...
- Sat Oct 26, 2019 9:00 pm
- Forum: Sigma & Pi Bonds
- Topic: Pi bonds
- Replies: 3
- Views: 184
Re: Pi bonds
The number of pi bonds in a molecule can be easily determined by looking at its Lewis structure. Every single covalent bond has 1 sigma bond, every double bond has 1 sigma and 1 pi bond, every triple bond has 1 sigma and 2 pi bonds, etc. For example, ethane (C2H6) has 7 single bonds, so it would hav...
- Sat Oct 19, 2019 10:20 pm
- Forum: DeBroglie Equation
- Topic: Deriving the DeBrogile Equation
- Replies: 8
- Views: 394
Re: Deriving the DeBrogile Equation
It is probably fine that you have a basic understanding of what it does, but I guess the main takeaway in deriving the DeBroglie Equation is that you can combine E=pc and E=hv to get hc/λ = pc --> h/λ = p --> h/λ = mv --> λ = h/mv
- Sat Oct 19, 2019 10:15 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Frequencies
- Replies: 7
- Views: 484
Re: Frequencies
You should definitely memorize that UV is from 10-400 nm and that visible is from 400-700 nm in case you get a question about the Lyman or Balmer series, respectively.
- Sat Oct 19, 2019 9:57 pm
- Forum: *Black Body Radiation
- Topic: Applying Wein's Law
- Replies: 3
- Views: 292
Re: Applying Wein's Law
The most basic concept of Wien's law is that the wavelength of peak emission is inversely proportional to temperature on an object's blackbody radiation curve. Therefore, the higher the temperature, the shorter the wavelength (aka the hotter it is, the bluer it appears), and vice versa (aka the cold...
- Sat Oct 19, 2019 9:44 pm
- Forum: Properties of Electrons
- Topic: 1B. 7 Homework help
- Replies: 3
- Views: 184
Re: 1B. 7 Homework help
For a) You can combine E=hv and c=λv to get E=hc/λ to find the amount of energy emitted. You know planck's constant (h), the speed of light (c), and wavelength (λ), so you just have to plug the numbers in to get the amount of energy emitted. For b) You have to first find how many moles of Na are in ...
- Sat Oct 19, 2019 9:34 pm
- Forum: Properties of Light
- Topic: 1A. 15
- Replies: 3
- Views: 185
Re: 1A. 15
1), First, you need to know that the ultraviolet spectrum of atomic hydrogen corresponds to the Lyman series (defined as a transition between n=1 and n>=2). For this class, I would remember this in addition to the fact that the visible spectrum corresponds to the Balmer series (transition between n=...
- Sat Oct 12, 2019 11:14 pm
- Forum: Properties of Light
- Topic: Rydberg constant
- Replies: 7
- Views: 286
Re: Rydberg constant
The Rydberg equation is used in instances in which we want to calculate the energy of a given energy level (n) in a Hydrogen atom. We use the second equation you listed, ΔE=Ef-Ei, when we want to find the energy difference when an electron transitions from one energy level to another. However, the v...
- Sat Oct 12, 2019 11:06 pm
- Forum: Photoelectric Effect
- Topic: Photoelectric Effect and Photons
- Replies: 6
- Views: 272
Re: Photoelectric Effect and Photons
A photon is an elementary particle representing a unit or quanta of light. The photoelectric effect suggested that light exhibited the behavior of a particle (ie photon), because a change in the intensity of light did not result in a change in the kinetic energy of the electrons emitted from the met...
- Sat Oct 12, 2019 10:58 pm
- Forum: Properties of Light
- Topic: Equations We’ve Learned So Far
- Replies: 11
- Views: 687
Re: Equations We’ve Learned So Far
A good rule of thumb is that any equation with mass (m) as one of the variables CANNOT be used for light, as light particles (aka photons) have no mass!
- Sat Oct 12, 2019 10:28 pm
- Forum: Properties of Light
- Topic: 1A 15
- Replies: 2
- Views: 159
Re: 1A 15
The Lyman Series is defined as an electron transition from any n greater than or equal to 2 to n = 1 (or vice versa). You would want to use the Rydberg equation in this case: E = -(hR)/n^2. You can substitute E for hv, and then the h's on both sides of the equation cancel out and you're left with v=...
- Sat Oct 12, 2019 10:04 pm
- Forum: *Black Body Radiation
- Topic: Black Body Radiation
- Replies: 12
- Views: 753
Re: Black Body Radiation
Blackbody radiation is essentially thermal EM radiation. The reason why it is called "blackbody" is because an ideal blackbody (which does NOT exist in nature -- the closest thing would probably be a black hole) absorbs all radiation (and thus reflects none) and appears to be black because...
- Sat Sep 28, 2019 3:07 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: HW Question: G5 (Part A)
- Replies: 3
- Views: 134
Re: HW Question: G5 (Part A)
Once you find the molarity of sodium carbonate solution (should be 0.07967 M), you can write out the following equation to find the amount of solution you need to get 2.15 mmol (or 0.00215 mol) of Na2CO3: 0.07967 mol/L * x L = 0.00215 mol. Once we solve for x, we get 0.02699 L. This is the amount of...
- Sat Sep 28, 2019 2:50 pm
- Forum: SI Units, Unit Conversions
- Topic: Formula units vs molecules vs atoms? [ENDORSED]
- Replies: 7
- Views: 327
Re: Formula units vs molecules vs atoms? [ENDORSED]
46 g of NaCl * (1 mol of NaCl/58.44 g of NaCl) = 0.787 mol of NaCl (6.022 * 10^23 formula units/1 mol of NaCl) = 4.7 * 10^23 formula units of NaCl. You would essentially solve it the same way as you would for any molecular compound; you are just substituting the word "molecule" for "f...
- Fri Sep 27, 2019 11:34 pm
- Forum: SI Units, Unit Conversions
- Topic: Formula units vs molecules vs atoms? [ENDORSED]
- Replies: 7
- Views: 327
Re: Formula units vs molecules vs atoms? [ENDORSED]
A formula unit is the empirical formula of any ionic solid (or covalent network solid). Since these solids (ex. NaCl or SiO2) don't exist as individual molecules, we use the term "formula unit" instead. In the example you stated, alumina is an ionic compound so the technically correct unit...

