https://www.toppr.com/content/concept/z ... ns-203348/
here is a link with an example
Search found 103 matches
- Tue Mar 10, 2020 6:25 pm
- Forum: Zero Order Reactions
- Topic: 0 order
- Replies: 14
- Views: 1533
- Tue Mar 10, 2020 6:18 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: catalysts in balanced equations
- Replies: 6
- Views: 493
Re: catalysts in balanced equations
Tai Metzger 3K wrote:Yes, in other words, catalysts are present before and after a reaction whereas intermediates are only present during the reaction.
this is helped thank you!
- Tue Mar 10, 2020 6:17 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Intermediate
- Replies: 3
- Views: 503
Re: Intermediate
Qiu Ya Wu 4I wrote:For the image above, it's good to know that only one intermediate will be formed since the reaction profile indicates a 2 step process and the intermediate will be created in the first step and used as a reactant for the second step.
Thank you for clarifying on this
- Tue Mar 10, 2020 6:12 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Saying Thank You to Dr. Lavelle
- Replies: 490
- Views: 547163
Re: Saying Thank You to Dr. Lavelle
Thank you Dr.Lavelle for yet another amazing quarter!!
- Tue Mar 10, 2020 6:10 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Review Sessions
- Replies: 2
- Views: 260
Re: Review Sessions
Dr.Lavelle should send out an email soon but I feel like they might be
- Wed Mar 04, 2020 10:40 pm
- Forum: Second Order Reactions
- Topic: [A] v. Time
- Replies: 27
- Views: 1215
Re: [A] v. Time
Angela Patel 2J wrote:Would the slope of the graph be k?
yes the slope would be k
- Wed Mar 04, 2020 10:37 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3589504
Re: Post All Chemistry Jokes Here
When the chemical kinetics specialist is asked why he ran slowly, his reply was “I always wanted to be the significant rate-determining step”.
- Wed Mar 04, 2020 10:36 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Rate-Determining Step
- Replies: 4
- Views: 410
Re: Rate-Determining Step
The rate-determining step is equivalent to the slow step. Therefore, by comparing each of the elementary steps' rate laws with the overall reaction rate law, you can determine which step is the rate-determining step: it's whatever step gives you a rate law identical to the overall reaction rate law...
- Wed Mar 04, 2020 10:34 pm
- Forum: First Order Reactions
- Topic: Graph
- Replies: 9
- Views: 751
Re: Graph
should look linear and have a negative slope which is -k
- Wed Mar 04, 2020 10:28 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Excellence in Chemistry Award!
- Replies: 27
- Views: 10006
Re: Excellence in Chemistry Award!
Congrats for doing so great in these classes!!
- Tue Feb 25, 2020 8:43 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3589504
Re: Post All Chemistry Jokes Here
JosephineF wrote:chem final outfit ideas
I love this so much !!
- Tue Feb 25, 2020 8:42 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Pressure
- Replies: 2
- Views: 257
Re: Pressure
For cells that involve gases, such as hydrogen gas or chlorine gas, the pressure of these species matters. In these cases, the partial pressures of gases would probably be used over concentrations. Depending on the partial pressures, E would differ because of the nernst equation, E = Enot - (RT/NF)...
- Tue Feb 25, 2020 8:39 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Today in lecture, Dr. Lavelle
- Replies: 4
- Views: 336
Re: Today in lecture, Dr. Lavelle
Dr.Lavelle always picks good songs to end the lecture with
- Tue Feb 25, 2020 8:33 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: homework question 6M.3
- Replies: 2
- Views: 342
Re: homework question 6M.3
galvanic cells have a positive potential difference so find the cathode and anode that makes E (naught) cell positive using the equation E(naught) cell= E (naught) cathode- E(naught) anode
- Tue Feb 25, 2020 8:29 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: homework question 6L.9
- Replies: 2
- Views: 345
Re: homework question 6L.9
a) MnO4-(aq) +8H+(aq)+5e- yields Mn2+(aq) +4H2O (cathode half reaction)
5[Fe2+ (aq) yields Fe3+(aq)+e-] anode half reaction
Two equations together:
MnO4-(aq)+fFe2+(aq)+8H+(aq) yields Mn2+(aq) + Fe3+(aq) +4H20(l)
5[Fe2+ (aq) yields Fe3+(aq)+e-] anode half reaction
Two equations together:
MnO4-(aq)+fFe2+(aq)+8H+(aq) yields Mn2+(aq) + Fe3+(aq) +4H20(l)
- Wed Feb 19, 2020 11:32 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Anode and Cathode
- Replies: 14
- Views: 1034
Re: Anode and Cathode
Lydia Luong 4L wrote:Cathode is always on the right side of a cell diagram and anode is always on the left side.
Thank you!!
- Wed Feb 19, 2020 11:20 am
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3589504
Re: Post All Chemistry Jokes Here
Why was the electrochemical cell arrested?
Because it was charged with battery.
Because it was charged with battery.
- Wed Feb 19, 2020 11:15 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Platinum Electrode
- Replies: 3
- Views: 521
Re: Platinum Electrode
Matthew Tran 1H wrote:You need a solid platinum electrode when there is no conducting solid (except liquid mercury) in the cathode or anode reaction. That's all you need to know.
THANKS!!
- Wed Feb 19, 2020 11:05 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: n in -nFE
- Replies: 14
- Views: 887
Re: n in -nFE
Thanks for clarifying what n was. I was lost when doing the homework problems
- Wed Feb 19, 2020 11:04 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Relationship between free energy and cell potential?
- Replies: 2
- Views: 261
Re: Relationship between free energy and cell potential?
Gibbs free energy is related to cell potential by the equation: ΔG°cell = −nFE°cell. If E°cell > 0, then the process is spontaneous. If E°cell < 0, then the process is nonspontaneous
- Tue Feb 11, 2020 3:53 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3589504
Re: Post All Chemistry Jokes Here
Did you hear that Oxygen and Magnesium hooked up last night?
OMg
OMg
- Tue Feb 11, 2020 3:51 pm
- Forum: Phase Changes & Related Calculations
- Topic: Negative Work
- Replies: 18
- Views: 1473
Re: Negative Work
CynthiaLy4F wrote:Work will be negative when the system is doing the work, such as through expansion. Work is positive when is being done on the system, such as through compression.
this helps a lot thank you! I have been confused about this
- Tue Feb 11, 2020 3:38 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Advice from a Medical Student - Part II [ENDORSED]
- Replies: 298
- Views: 260160
Re: Advice from a Medical Student - Part II [ENDORSED]
Thanks for sharing your experience !!
- Tue Feb 11, 2020 3:34 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: C in nCv ln (T2/T1)
- Replies: 8
- Views: 1226
Re: C in nCv ln (T2/T1)
If it is under constant pressure C equals Cp=5/2*R for a monatomic gas
- Tue Feb 11, 2020 3:32 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: qp=deltaH
- Replies: 6
- Views: 374
Re: qp=deltaH
TarynD_1I wrote:qp is equal to delta h when a system is at constant pressure, so no nonexpansion work is being done.
Thank you for clarifying!!
- Tue Feb 04, 2020 6:19 pm
- Forum: Phase Changes & Related Calculations
- Topic: -w vs w
- Replies: 15
- Views: 664
Re: -w vs w
Brianna Becerra 1B wrote:When w is negative, it means that work is being done (expansion), while when it is positive, it is compression.
thanks for clarifying I have been so confused!!
- Tue Feb 04, 2020 6:17 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: FORMULAS
- Replies: 3
- Views: 529
Re: FORMULAS
The equation sheet on Lavelle's website should have all the equations you need to use on an exam.
- Tue Feb 04, 2020 6:12 pm
- Forum: Calculating Work of Expansion
- Topic: second equation
- Replies: 8
- Views: 255
Re: second equation
and this equation is used for reversible reactions only?
- Tue Feb 04, 2020 6:11 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: isolated system
- Replies: 8
- Views: 500
Re: isolated system
Brooke Yasuda 2J wrote:a bomb calorimeter is tightly sealed and insulated which means that heat cannot be transferred with the surroundings. Also, the volume of the system cannot change so there can be no work done. This means that it is an isolated system.
thanks for explaining so well!!
- Tue Feb 04, 2020 6:09 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Useful Summary of Thermodynamic Definitions
- Replies: 55
- Views: 18556
Re: Useful Summary of Thermodynamic Definitions
Nathan Rothschild_2D wrote:Will we have to know these terms for the test?
I believe so because they can be used to describe something. For example yesterday during Lyndon workshop one of these words was used to describe the system and it was needed to solve the problem.
- Mon Jan 27, 2020 9:28 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3589504
Re: Post All Chemistry Jokes Here
why do white bears dissolve in water?
because they are polar
because they are polar
- Mon Jan 27, 2020 9:26 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Test 2
- Replies: 7
- Views: 252
Re: Test 2
everything after the midterm until test 2 begins
- Mon Jan 27, 2020 9:22 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Units for enthalpy
- Replies: 3
- Views: 140
Re: Units for enthalpy
thanks for clarifying I was also confused about this
- Mon Jan 27, 2020 9:19 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: bomb calorimeter
- Replies: 8
- Views: 1220
Re: bomb calorimeter
Luis_Yepez_1F wrote:Work would be equal to zero, since volume is constant.
so when volume is constant work is equal to 0 in any case?
- Mon Jan 27, 2020 9:17 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Week 4 Homework
- Replies: 11
- Views: 350
Re: Week 4 Homework
I would follow outline three from Lavelle's website
- Tue Jan 21, 2020 9:00 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: van't hoff's equation
- Replies: 3
- Views: 156
Re: van't hoff's equation
we do not have to know this for the test since we did not learn this in class
- Tue Jan 21, 2020 8:59 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: X was ignored
- Replies: 27
- Views: 1126
Re: X was ignored
you usually ignore the -x when the Ka or Kb is less than 10^-3 because it has little effect to the concentrations
- Tue Jan 21, 2020 8:56 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Sig Figs for pH/pOH
- Replies: 7
- Views: 371
Re: Sig Figs for pH/pOH
significant figures for pH/pOH only counts after the decimal.
- Tue Jan 21, 2020 8:55 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: H20 in the ICE table
- Replies: 26
- Views: 1499
Re: H20 in the ICE table
when performing a ice table you should only worry about the gasses and aqueous molecules
- Tue Jan 21, 2020 8:50 pm
- Forum: Ideal Gases
- Topic: homework #3
- Replies: 16
- Views: 905
Re: homework #3
homework three can probably be on chemical equilibria as well since it will be on test one so it can be helpful to prepare with the hw questions
- Wed Jan 15, 2020 10:59 am
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3589504
Re: Post All Chemistry Jokes Here
Why does hamburger yield lower energy than steak?
A: Because it's in the ground state.
A: Because it's in the ground state.
- Wed Jan 15, 2020 9:48 am
- Forum: Ideal Gases
- Topic: Partial Pressure
- Replies: 8
- Views: 322
Re: Partial Pressure
It is called partial pressure instead of just pressure because there are several gases in a given volume at once. Each gas has a certain amount of moles that occupies part of the total pressure. The sum of all the partial pressures of the gases present equals the total pressure. If only one gas was...
- Wed Jan 15, 2020 9:45 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: ICE charts on tests/exams
- Replies: 8
- Views: 273
Re: ICE charts on tests/exams
You should use ICE charts when needed! In my discussion, we mostly did ICE charts for weak acids and bases.
- Wed Jan 15, 2020 9:44 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Which liquids to use
- Replies: 7
- Views: 201
Re: Which liquids to use
To add on, we don't include a pure liquid in an equilibrium calculation because it acts as the solvent in a chemical reaction and its change in concentration is relatively insignificant so in the K expression, the product concentration and reactant concentration would just cancel out. Thanks for ex...
- Wed Jan 15, 2020 9:41 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Real reason explaining Le Chatelier's principle
- Replies: 4
- Views: 152
Re: Real reason explaining Le Chatelier's principle
When using PV=nRT you see there is an inverse relationship between P and V so when P increases V decreases and the reverse is also true.
- Thu Jan 09, 2020 3:34 pm
- Forum: Ideal Gases
- Topic: Understanding Q
- Replies: 19
- Views: 743
Re: Understanding Q
You solve for Q the same way you solve for K.
- Thu Jan 09, 2020 3:27 pm
- Forum: Ideal Gases
- Topic: R constant in PV=nRT
- Replies: 9
- Views: 298
Re: R constant in PV=nRT
Gas constant, R = 8.314 J·K-1·mol-1 = 8.206 x 10-2 L·atm·K-1·mol-1 = 8.314 x 10-2 L·bar·K-1·mol-1= 62.364 L·Torr·K-1·mol-1
This is what was on the constants and formualas sheet on Dr. Lavelle's website.
This is what was on the constants and formualas sheet on Dr. Lavelle's website.
- Thu Jan 09, 2020 3:24 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Direction
- Replies: 4
- Views: 148
Re: Direction
If Q<K, then we know that the reaction will favor the forward reaction because there is not enough product for the reaction to be at equilibrium. If Q>K, then we know the reaction will favor the reverse reaction because there is not enough reactant for the reaction to be at equilibrium. Thanks for ...
- Thu Jan 09, 2020 3:20 pm
- Forum: Ideal Gases
- Topic: K and Q
- Replies: 8
- Views: 1130
Re: K and Q
NatBrown1I wrote:Because the concentration of solids and liquids are constant throughout the reaction.
Thank you!
- Thu Jan 09, 2020 3:17 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3589504
Re: Post All Chemistry Jokes Here
What animal is made up of calcium, nickel and neon?
A: A CaNiNe
A: A CaNiNe
- Thu Dec 05, 2019 1:00 pm
- Forum: Air Pollution & Acid Rain
- Topic: final?
- Replies: 5
- Views: 539
Re: final?
I think it would be good to know it because in lecture Dr.Lavelle notes state to look at the acid rain topics in the textbooks
- Thu Dec 05, 2019 12:57 pm
- Forum: Hybridization
- Topic: writing hybridization
- Replies: 3
- Views: 302
Re: writing hybridization
the hybridization for the C atom is sp
- Thu Dec 05, 2019 12:55 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3589504
Re: Post All Chemistry Jokes Here
What do you call an acid with an attitude?
a-mean-oh-acid
a-mean-oh-acid
naming
Can someone explain when do you use the prefixes bis,-tris-,tetrakis-, pentakis-?
- Thu Dec 05, 2019 12:50 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Saying Thank You to Dr. Lavelle
- Replies: 490
- Views: 547163
Re: Saying Thank You to Dr. Lavelle
Thank you Dr. Lavelle for being such an amazing professor and can not wait to be in chem 14B with you next quarter!
- Mon Nov 25, 2019 5:45 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Why HF is a weaker acid than HCl
- Replies: 17
- Views: 16268
Re: Why HF is a weaker acid than HCl
In this case, you are looking at the HF bond length vs the HCL bong length. Since Florine is more elctronegative than Cl, it will have a stronger pull on the H make it harder to donate the H. The longer the bond, the easier it is to remove the H. It follows one of three rules in the book for strong...
- Mon Nov 25, 2019 5:44 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3589504
Re: Post All Chemistry Jokes Here
Why did the military use acid?
To neutralize the enemy base.
To neutralize the enemy base.
- Mon Nov 25, 2019 5:43 pm
- Forum: Bronsted Acids & Bases
- Topic: List of strong/weak acids/bases
- Replies: 3
- Views: 483
Re: List of strong/weak acids/bases
Here is a list of the strong bases:
LiOH = lithium hydroxide, NaOH = sodium hydroxide, KOH = potassium hydroxide, RbOH = rubidium hydroxide, CsOH = cesium hydroxide, Ca(OH)2 = calcium hydroxide , Sr(OH)2 = strontium hydroxide, Ba(OH)2 = barium hydroxide
LiOH = lithium hydroxide, NaOH = sodium hydroxide, KOH = potassium hydroxide, RbOH = rubidium hydroxide, CsOH = cesium hydroxide, Ca(OH)2 = calcium hydroxide , Sr(OH)2 = strontium hydroxide, Ba(OH)2 = barium hydroxide
- Mon Nov 25, 2019 5:38 pm
- Forum: Bronsted Acids & Bases
- Topic: Sig Figs
- Replies: 4
- Views: 505
Re: Sig Figs
Every time my TA does a problem he always tells us to follow sig fig rules so I do not think you would get a lot of points taken off but it is important to know
- Mon Nov 25, 2019 5:33 pm
- Forum: Hybridization
- Topic: Sigma and Pi Bonds
- Replies: 21
- Views: 1067
Re: Sigma and Pi Bonds
a single bond has one sigma bond, a double bond has one sigma and one pi bond, and a triple bond has one sigma and two pi bonds
- Mon Nov 25, 2019 5:26 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Ground state? [ENDORSED]
- Replies: 8
- Views: 1939
Re: Ground state? [ENDORSED]
it is the lowest energy state of an atom
- Fri Nov 22, 2019 11:59 am
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3589504
Re: Post All Chemistry Jokes Here
Why can you never trust atoms?
A: They make up everything!
A: They make up everything!
- Fri Nov 22, 2019 11:57 am
- Forum: Hybridization
- Topic: hybridizing oxygen
- Replies: 5
- Views: 414
Re: hybridizing oxygen
To find the hybridization of an atom, you need to find its steric number. The steric number is calculated by adding the number of atoms bonded to the central atom plus the number of lone pairs on the central atom. In this case, because oxygen has 1 carbon bonded to it + 2 lone pairs, its steric num...
- Fri Nov 22, 2019 11:53 am
- Forum: Administrative Questions and Class Announcements
- Topic: Final Studying
- Replies: 14
- Views: 904
Re: Final Studying
I plan on doing the homework questions, reviewing the midterm, and looking at past final tests if they are available.
- Fri Nov 22, 2019 11:46 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSEPR Bond Angles for Shapes with Lone Pairs
- Replies: 5
- Views: 383
Re: VSEPR Bond Angles for Shapes with Lone Pairs
looks to be all correct
- Fri Nov 22, 2019 11:44 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Tetrahedral and its polarity
- Replies: 6
- Views: 412
Re: Tetrahedral and its polarity
Kassidy Ford 1J wrote:If you have a molecule like CHF3, it will be polar because not all of the atoms surrounding the C are the same, but will that affect the bond angles or do you still assume that it is 109.5
I believe it will have bond angles of 109.5 since the shape is still tetrahedral.
- Fri Nov 15, 2019 1:20 pm
- Forum: Dipole Moments
- Topic: London forces
- Replies: 9
- Views: 549
Re: London forces
London forces are always present in each molecule.
- Fri Nov 15, 2019 1:14 pm
- Forum: Dipole Moments
- Topic: Hydrogen Bond
- Replies: 7
- Views: 412
Re: Hydrogen Bond
Hydrogen bonds are formed when H is bonded to F,O,N. Easy way I was taught to remember if it is a hydrogen bond was if Hydrogen was bonded to Freak Of Nature.
- Fri Nov 15, 2019 1:10 pm
- Forum: Dipole Moments
- Topic: Test 2
- Replies: 3
- Views: 340
Re: Test 2
TA's have been saying to study everything we have learned after the midterm until lecture this Friday.
- Fri Nov 15, 2019 1:08 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3589504
Re: Post All Chemistry Jokes Here
What do you call a tooth in a glass of water?
A one molar solution.
A one molar solution.
- Fri Nov 15, 2019 1:06 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Lewis Structures & VSEPR
- Replies: 9
- Views: 482
Re: Lewis Structures & VSEPR
It is not totally necessary but can be helpful to determine the shape of the molecule.
- Fri Nov 08, 2019 2:45 pm
- Forum: Coordinate Covalent Bonds
- Topic: Elements that can form coordinate covalent bonds
- Replies: 2
- Views: 263
Re: Elements that can form coordinate covalent bonds
I think two factors that help determine whether or not an element forms coordinate covalent bonds is electronegativity and the octet rule. Atoms that accept the two electrons should be "hungry" enough to take them, but this can also mean that they don't have a complete electron shell. Tha...
- Fri Nov 08, 2019 2:44 pm
- Forum: Sigma & Pi Bonds
- Topic: Single vs. Double bonds
- Replies: 15
- Views: 1952
Re: Single vs. Double bonds
There are more electrons being shared so there is a greater attraction causing for shorter bonds.
- Fri Nov 08, 2019 2:36 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3589504
Re: Post All Chemistry Jokes Here
kevinolvera1j wrote:nice joke,
Helium Helium Helium
This made me hehehe!! haha
- Fri Nov 08, 2019 2:34 pm
- Forum: Resonance Structures
- Topic: Resonance Structures
- Replies: 18
- Views: 1136
Re: Resonance Structures
There is more than one way to draw a lewis structure. For example the double bonds can move to another same element in the drawing.
- Fri Nov 08, 2019 2:29 pm
- Forum: Dipole Moments
- Topic: Dipole moments on Lewis structures
- Replies: 3
- Views: 267
Re: Dipole moments on Lewis structures
I feel like the question would ask for you to include the dipole moment but maybe include it just in case.
- Thu Oct 31, 2019 7:42 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3589504
Re: Post All Chemistry Jokes Here
A neutron walks into a bar. He asks the bartender, "How much for a beer?"
The bartender gives him a smile and says, "For you, no charge"
The bartender gives him a smile and says, "For you, no charge"
- Thu Oct 31, 2019 7:41 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Why does formal charge indicate stability?
- Replies: 3
- Views: 276
Re: Why does formal charge indicate stability?
Zoya Mulji 1F wrote:since formal charge indicates the gain/loss of electrons while forming a covalent bond, it serves as a predictor for stability in a lewis structure. The structure with the least formal charge should be lower in energy and thereby be the better Lewis structure.
Thank you for explaining very well.
- Thu Oct 31, 2019 6:47 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3589504
Re: Post All Chemistry Jokes Here
Why did the attacking army use acid?
To neutralize the enemy's base!
To neutralize the enemy's base!
- Thu Oct 31, 2019 5:05 pm
- Forum: Lewis Structures
- Topic: Drawing lewis structures
- Replies: 8
- Views: 267
Re: Drawing lewis structures
since he has not gone over it I would not worry to much about it
- Thu Oct 31, 2019 5:04 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3589504
Re: Post All Chemistry Jokes Here
Did you hear about oxygen’s date with potassium?
It went OK.
It went OK.
- Thu Oct 31, 2019 5:02 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3589504
Re: Post All Chemistry Jokes Here
Did you hear about oxygen’s date with potassium?
It went OK.
It went OK.
- Thu Oct 24, 2019 8:40 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: order of electron configuration
- Replies: 4
- Views: 283
Re: order of electron configuration
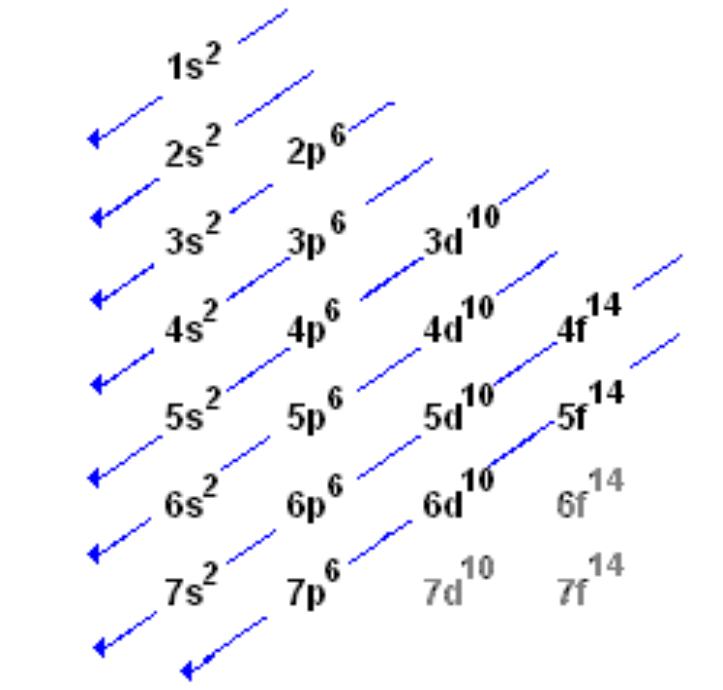
This should help in writing electron configurations
- Thu Oct 24, 2019 8:35 pm
- Forum: Trends in The Periodic Table
- Topic: Homework Question 1F.19
- Replies: 5
- Views: 287
Re: Homework Question 1F.19
Since s-block has a much lower ionization energy than the p-block so they lose electrons with less energy making them highly reactive
- Thu Oct 24, 2019 8:29 pm
- Forum: Lewis Structures
- Topic: Where to start putting dots for electrons
- Replies: 10
- Views: 590
Re: Where to start putting dots for electrons
There are no rules but I was taught that you should put a pair of valence electrons on each element before putting another pair on the first element you did
- Thu Oct 24, 2019 8:23 pm
- Forum: Student Social/Study Group
- Topic: Midterm
- Replies: 17
- Views: 897
Re: Midterm
I feel like everything we have learned so far will be on the midterm, but hopefully they mention what the midterm will consist of soon.
- Thu Oct 24, 2019 8:22 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3589504
Re: Post All Chemistry Jokes Here
Why do chemists like nitrates so much?
They're cheaper than day rates.
They're cheaper than day rates.
- Fri Oct 18, 2019 3:25 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Exceptions
- Replies: 4
- Views: 239
Re: Exceptions
There are other exceptions but Dr.Lavelle mentioned that we will only be looking at the first row of the d-block so Cr and and Cu are the only ones we need to know.
- Fri Oct 18, 2019 3:16 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3589504
Re: Post All Chemistry Jokes Here
Adelpha Chan 1B wrote:Q: Wanna hear a joke about sodium hypobromite?
A: NaBrO.
This was such a good joke. Thanks for the laugh.
- Fri Oct 18, 2019 3:15 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3589504
Re: Chemistry Jokes
I heard Potassium and Oxygen went on a date....It went OK Q: What do you do with a sick chemist? A: If you can't helium, and you can't curium, then you might as well barium Q: What is the name of 007's Eskimo cousin? A: Polar Bond My high school chemistry had the first joke up all year and it alway...
- Fri Oct 18, 2019 3:14 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: G orbital
- Replies: 6
- Views: 438
Re: G orbital
Chantel_4B wrote:The G orbital does exist, but in their ground state, electrons are not in the G orbital, so it is therefore not represented on the periodic table.
Thank you for saying this. I had never heard of the G orbital before
- Fri Oct 18, 2019 3:12 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: electron configuration
- Replies: 4
- Views: 216
electron configuration
Why does 3d go before 4s in the electron configuration? I learned before it was 4s then 3d.
- Fri Oct 11, 2019 11:14 am
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Uncertainty in Speed [ENDORSED]
- Replies: 31
- Views: 17232
Re: Uncertainty in Speed [ENDORSED]
andrewcj 4I wrote:One additional thing for my reply, make sure you convert nm to m.
thanks for clarifying this.
- Fri Oct 11, 2019 11:03 am
- Forum: Photoelectric Effect
- Topic: Photoelectric Effect vs Atomic Spectrum
- Replies: 2
- Views: 178
Re: Photoelectric Effect vs Atomic Spectrum
The photoelectric effect is an experiment that discovered light has the properties of particles. The equation of calculating the energy of a photon E=hv comes from this experiment. The electrons will only be ejected when the energy is greater than or equal to the threshold energy (work function), a...
- Fri Oct 11, 2019 11:02 am
- Forum: Einstein Equation
- Topic: Planck's constant
- Replies: 9
- Views: 670
Re: Planck's constant
Planck's constant is 6.626 x10^-34 J s. It is used to relate the energy of a particle to its frequency. The equation E=hv, we can be manipulated to h=E/v. Therefore, h or Planck's constant gives the ratio of energy to frequency and its relationship between these two variables. Thank you for your ex...
- Fri Oct 11, 2019 10:59 am
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Wavelength Plausibility
- Replies: 31
- Views: 2682
Re: Wavelength Plausibility
APatel_4A wrote:He said that it is 720 to 400 but that we can just remember 700 to 400 since it's easier!
Thank you for clarifying .
- Fri Oct 11, 2019 10:57 am
- Forum: Administrative Questions and Class Announcements
- Topic: Week 2 Homework Problems [ENDORSED]
- Replies: 67
- Views: 7616
Re: Week 2 Homework Problems [ENDORSED]
For week 3 we should only be submitting problems from the quantum section. We were allowed to submit fundamentals problems for week 2 because so many students were still preparing for the test, and he didn't want us to have to confuse ourselves with new material before it. Yes you should be doing t...
- Fri Oct 11, 2019 10:54 am
- Forum: Administrative Questions and Class Announcements
- Topic: KAREN SUN 5-7PM WORKSHOP - DOWNLAOD WORKSHEETS HERE
- Replies: 53
- Views: 5922
Re: KAREN SUN 5-7PM WORKSHOP - DOWNLAOD WORKSHEETS HERE
What was the scam for workshops that Dr. Lavelle mentioned in lecture? Something to do with asking for our phone numbers? Yeah he mentioned it in my lecture which was at 2. He said in one of his earlier lectures there was a piece of paper going around to sign up for workshops. It asked for your nam...
- Thu Oct 03, 2019 5:27 pm
- Forum: Properties of Electrons
- Topic: Wave Properties of Electrons- Module Question
- Replies: 3
- Views: 622
Re: Wave Properties of Electrons- Module Question
The electrons have both wave-like and particle-like properties, and the photoelectric effect is a key example showing the particle-like property. The diffraction pattern observed during the experiment we went over in class is evidence that electrons have wave-like properties. As for the particle na...
- Thu Oct 03, 2019 5:26 pm
- Forum: Properties of Light
- Topic: Relationship Between Velocity and Wavelength
- Replies: 6
- Views: 464
Re: Relationship Between Velocity and Wavelength
In terms of the original question you asked - the relationship between velocity and wavelength for light is given by this equation: V = f\lambda Where V is the velocity of the wave in meters per second, \lambda (lambda) is the wavelength in meters, and f is the frequency, or cycles per second. I be...

