Search found 101 matches
- Thu Mar 12, 2020 4:56 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Just a reminder about pH and pKa!
- Replies: 2
- Views: 349
Re: Just a reminder about pH and pKa!
Comparing pKa/pKb with pH/pOH will be important to know how to do on the final - I think! See...pKa = -log(Ka), so the higher the pKa, the lower the Ka. Ka = \frac{[A-][H+]}{[HA]} Lower Ka means higher products - and lower H+ pH = -log(H+), which means the higher the pH, the lower the H+ Therefore,...
- Thu Mar 12, 2020 4:53 pm
- Forum: Balancing Redox Reactions
- Topic: Oxidizing and Reducing
- Replies: 5
- Views: 372
Re: Oxidizing and Reducing
It depends on the situation that is being set up. If the cell is a galvanic cell then the half-reaction with the more negative potential will be oxidized and the half-reaction with the more positive potential will be reduced. If the set-up is for electrolysis, then the more negative potential will b...
- Thu Mar 12, 2020 4:48 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Pre-Equilibrium Method
- Replies: 4
- Views: 342
Re: Pre-Equilibrium Method
The pre-equilibrium method is used to eliminate an intermediate in the rate law. In the pre-equilibrium method, the step before the slow step can be assumed to bottle-neck, creating a buildup of products. This allows products to go back to reactants and the reaction to be at equilibrium. From then y...
- Thu Mar 12, 2020 4:43 pm
- Forum: General Rate Laws
- Topic: Fast and Slow Step Reactions
- Replies: 5
- Views: 449
Re: Fast and Slow Step Reactions
The slow step is the step with the highest activation energy. With regard to solving problems we use the slow step to determine if a given mechanism is correct for a reaction and to determine the order and rate law of a reaction.
- Thu Mar 12, 2020 4:40 pm
- Forum: General Rate Laws
- Topic: 7A.9
- Replies: 4
- Views: 418
Re: 7A.9
You can tell because of the units of k, but you can also know that it is a first-order reaction because it tells you in the problem.
- Thu Mar 12, 2020 4:38 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Textbook question 7.17
- Replies: 4
- Views: 362
Re: Textbook question 7.17
Since the slow step is the rate-determining step, if the catalyst doesn't accelerate the slow step, then the rate of the reaction won't change.
- Wed Mar 11, 2020 12:16 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: residual entropy interpretation
- Replies: 2
- Views: 275
Re: residual entropy interpretation
At T=0 K, the entropy that a molecule has is just due to the different microstates that the molecule could be in. At t=0 there are no other forms of entropy so you just have to calculate the entropy that arises from the molecule occupying different positions.
- Wed Mar 11, 2020 12:10 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Activation energies for multi-step reactions
- Replies: 2
- Views: 244
Re: Activation energies for multi-step reactions
If there is a graph, you can look at the difference between the top of the bump to the starting energy level of the different fast steps to determine which fast step has a higher activation energy. In other words, the fast step with the largest "uphill section" will have the higher activat...
- Wed Mar 11, 2020 12:05 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Arrhenius Equation A variable
- Replies: 2
- Views: 192
Re: Arrhenius Equation A variable
You can solve for k using this version of the Arrhenius equation: 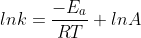 . To calculate k you need another k value for a different temperature and then you can subtract
. To calculate k you need another k value for a different temperature and then you can subtract 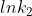 from
from  and the lnA term will cancel.
and the lnA term will cancel.
- Wed Mar 11, 2020 12:01 pm
- Forum: General Rate Laws
- Topic: intermediates
- Replies: 12
- Views: 744
Re: intermediates
Correct, they should be replaced using the equilibrium equation solved for the intermediate.
- Mon Mar 09, 2020 11:58 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Rate determining step and Ea
- Replies: 2
- Views: 218
Re: Rate determining step and Ea
Yes, the rate-determining step will always have the highest activation energy because the step with the largest activation energy is the slowest step so it is the rate-determining step. To find the rate determining step when looking at the reaction profile, find the step with the largest difference ...
- Mon Mar 09, 2020 11:06 pm
- Forum: Second Order Reactions
- Topic: 7.21 part H
- Replies: 2
- Views: 245
Re: 7.21 part H
Yes, it is because you have to account for the fact that the rate = A^{2} . From this equation, you can get the half-life equation which is t_{1/2}= \frac{1}{k[A]_{0}} . From this equation you can clearly see that with respect to [A], the half-life has an inverse relationship, so the plot won't be l...
- Mon Mar 09, 2020 6:44 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: reaction profile
- Replies: 2
- Views: 216
reaction profile
How do you know if in a multistep reaction profile each starting energy for each step increases or decreases? For example in question 7.11, why doesn't the energy decrease each step instead of increasing?
- Mon Mar 09, 2020 6:39 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Pre-Equilibrium Approach Constants
- Replies: 1
- Views: 147
Re: Pre-Equilibrium Approach Constants
the constant in the pre equilibrium approach is dependent on the coefficients of the species that you are using to determine the rate of. In class, this was the 2 NO. Also the constant depends on the rate constant of the slow step as well as the equilibrium expression that you solve for to replace t...
- Sun Mar 08, 2020 9:00 pm
- Forum: General Rate Laws
- Topic: Arrhenius Equation
- Replies: 3
- Views: 337
Re: Arrhenius Equation
You can use the Arrhenius equation to find the activation energy as well as the rate constant at different temperatures.
- Sun Mar 08, 2020 3:15 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: 7D.7
- Replies: 1
- Views: 110
Re: 7D.7
The equilibrium constant is the forward reaction constant over the reverse rate constant. For part b) you can think of it in terms of le chatlier's principle. Since the equilibrium constant is less than 1 you know that the reactant are favored and the reaction is endothermic. When you raise the temp...
- Mon Mar 02, 2020 7:26 pm
- Forum: Balancing Redox Reactions
- Topic: Balancing half reactions in acidic conditions
- Replies: 8
- Views: 576
Balancing half reactions in acidic conditions
For a redox reaction in an acidic half-reaction, do we use H3O+ or just H+ to balance the hydrogens?
- Sun Mar 01, 2020 9:48 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Galvanic and Voltaic Cells
- Replies: 7
- Views: 521
Re: Galvanic and Voltaic Cells
Galvanic and Voltaic cells are the same. They both get their energy from spontaneous reactions. The E of the cell will be positive and the  is negative.
is negative.
- Sun Mar 01, 2020 9:44 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Reducing/oxidizing agent
- Replies: 8
- Views: 577
Re: Reducing/oxidizing agent
The reducing agent ends up becoming oxidized because it donates its electron to reduce another species. The oxidizing agent becomes reduced because it gains an electron from another species that becomes oxidized.
- Sun Feb 23, 2020 6:24 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6L. 7 c
- Replies: 2
- Views: 237
6L. 7 c
Cd + 2Ni(OH)3  Cd(OH), why is there KOH on the anode side and Ni on the cathode side?
Cd(OH), why is there KOH on the anode side and Ni on the cathode side?
- Sun Feb 23, 2020 5:47 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: cell diagrams
- Replies: 1
- Views: 123
cell diagrams
Is there a convention for which order you write the states in when there is an inert electrode. For example, for CL2 + H2  HCl, is there any order in which you have to write the H2 and H+ on the anode side?
HCl, is there any order in which you have to write the H2 and H+ on the anode side?
- Sun Feb 23, 2020 5:39 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6L.5 part b)
- Replies: 2
- Views: 190
6L.5 part b)
For  , how can you tell that the are in the same solution and therefore need a inert electrode?
, how can you tell that the are in the same solution and therefore need a inert electrode?
- Sun Feb 23, 2020 2:41 pm
- Forum: Balancing Redox Reactions
- Topic: 6k. d
- Replies: 1
- Views: 130
6k. d
Will someone explain how to find the half-reactions for 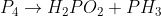 ?
?
- Sun Feb 23, 2020 1:59 pm
- Forum: Balancing Redox Reactions
- Topic: 6K.5 a
- Replies: 1
- Views: 111
6K.5 a
How do you know that O3 is oxidized? 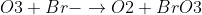
- Sun Feb 23, 2020 1:41 pm
- Forum: Balancing Redox Reactions
- Topic: 6K.3
- Replies: 1
- Views: 100
6K.3
Will someone explain how to do part d? Cl2 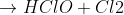
- Tue Feb 11, 2020 2:18 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: 4I.5
- Replies: 1
- Views: 81
Re: 4I.5
You can just keep the moles as grams in the calculations. However, you will need to convert to moles to find the heat to melt the ice as the  is given in KJ/mol.
is given in KJ/mol.
- Tue Feb 11, 2020 2:14 pm
- Forum: Phase Changes & Related Calculations
- Topic: 4.C.13
- Replies: 1
- Views: 126
Re: 4.C.13
Because energy isn't lost you know that q1+q2=0 which means that q1=-q2. q1 is the heat required to first melt the ice and then increase its temperature to the final temp. So q_{1}=n\Delta H_{fus} +mC\Delta T . Also, q2 is the heat lost by the water when it is cooled down. So, q_{2}=mC\Delta T . Rem...
- Tue Feb 11, 2020 2:10 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Constant R
- Replies: 15
- Views: 1082
Re: Constant R
When do you use the constant R=8.314 vs 8.206x10^-2 and what are the units for each
The units are R = 8.314 J/(K mol) and 8.206x10^-2 L atm/(K mol). You use 8.314 whenever you are working with J and you use 8.206x10^-2 when using atm and L.
The units are R = 8.314 J/(K mol) and 8.206x10^-2 L atm/(K mol). You use 8.314 whenever you are working with J and you use 8.206x10^-2 when using atm and L.
- Tue Feb 11, 2020 2:07 pm
- Forum: Phase Changes & Related Calculations
- Topic: 4C.7
- Replies: 3
- Views: 327
Re: 4C.7
You should be able to use q=n . For part a)
. For part a)  = 4.76 KJ/ .579 mol = 8.22 KJ/mol. Part b is the same except you need to convert the mass given into moles using the molar mass.
= 4.76 KJ/ .579 mol = 8.22 KJ/mol. Part b is the same except you need to convert the mass given into moles using the molar mass.
- Tue Feb 11, 2020 2:04 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Test#1 Problem#6
- Replies: 1
- Views: 152
Re: Test#1 Problem#6
The correct kA value was 1.9 x10^-5.
- Tue Feb 11, 2020 2:02 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Test#1 Problem#5
- Replies: 1
- Views: 135
Re: Test#1 Problem#5
After finding the Ka set up your ice table. You should get that the equilibrium conc. are [HClO2]= .15-x, [ClO2-]=x and [H3O+]=x. Then set up your equilibrium equation with the appropriate values. The Kc should = x^2/(.15-x) which also equals the Ka that is .012. Then solve the quadratic and you get...
- Tue Feb 11, 2020 1:55 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: 4C.3
- Replies: 1
- Views: 97
Re: 4C.3
The correct answer for part a is 343 K and the correct answer for part b is 373K
- Sun Feb 02, 2020 10:24 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: 4B.5
- Replies: 3
- Views: 134
Re: 4B.5
First, U=q+w. So you know that q= 5.50KJ. Then you know that there is a constant pressure so 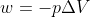 .
.
- Sun Feb 02, 2020 10:21 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Constant volume vs constant pressure
- Replies: 1
- Views: 76
Re: Constant volume vs constant pressure
By definition, the enthalpy is the same as the heat transfer under constant pressure. Under conditions of constant volume, there is no work done, so the change in internal energy equals the enthalpy.
- Sun Feb 02, 2020 10:18 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Adiabatic Wall
- Replies: 3
- Views: 199
Re: Adiabatic Wall
Adiabatic mean that 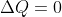 , so a reaction can still absorb or release energy if work is done on the system or the system does work.
, so a reaction can still absorb or release energy if work is done on the system or the system does work.
- Sun Feb 02, 2020 10:16 pm
- Forum: Calculating Work of Expansion
- Topic: -PdeltaV
- Replies: 2
- Views: 90
Re: -PdeltaV
Because you are calculating the work done by the system, which is equal to the negative work done on the system. If a gas does work on its surrounding then the final volume is greater than the initial volume so in order to convey that the system lost energy the work must equal 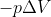
- Sun Feb 02, 2020 10:09 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Expansion Work
- Replies: 2
- Views: 111
Re: Expansion Work
Non-expansion work is work where the volume doesn't change. This could be muscle contractions or transmission of nerve impulses. expansion work is work that occurs when there is a change in volume. Work is for the most part independent of enthalpy except for when heat is added or taken away from a s...
- Sun Feb 02, 2020 9:57 pm
- Forum: Calculating Work of Expansion
- Topic: Work vs. Enthalpy
- Replies: 1
- Views: 74
Re: Work vs. Enthalpy
Yes, there are scenarios where a closed reaction would do work on the system but still have positive enthalpy because the sign of the enthalpy doesn't indicate anything about the work. It is possible for a system to lose heat, having a negative q but do work on the surroundings by expanding. Exother...
- Sun Jan 26, 2020 10:36 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Standard Reaction Enthalpies
- Replies: 2
- Views: 54
Re: Standard Reaction Enthalpies
It does not matter if the products and reactants are in different states. However, you do have to differentiate between the states because there can be different enthalpies of formation for different phases of the same molecule.
- Sun Jan 26, 2020 3:15 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: bond v. standard
- Replies: 5
- Views: 319
Re: bond v. standard
Bond enthalpies are the energy required to break a bond, while standard enthalpies of formation are the reaction enthalpies for the formation of 1 mol of a substance from its elements in their most table form.
- Sun Jan 26, 2020 1:07 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Method 3 - Enthalpy
- Replies: 2
- Views: 127
Re: Method 3 - Enthalpy
Basically you take the sum of the enthalpies of formation of the products and subtract the sum of enthalpies of formation of the reactants.
- Mon Jan 20, 2020 2:27 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6E.1
- Replies: 2
- Views: 99
Re: 6E.1
The concentration of H30+ is initially .15 M for the second protonation because .15 M H2SO4 produces .15 M H3O+ so when the second protonation starts there is already .15M H3O+ in solution from the first protonation.
- Mon Jan 20, 2020 2:22 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Percent ionization
- Replies: 2
- Views: 76
Re: Percent ionization
Partially. The percent ionization is the amount of ionized product divided by the amount of reactant. So it tells us the ratio of ionized product to unionized reactant.
- Sun Jan 19, 2020 1:44 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Homework 6E.3
- Replies: 3
- Views: 179
Homework 6E.3
The question is: Calculate the pH of each of the following solutions of diprotic acids at 25 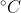 ignoring second deprotonations only when the approximation is justified. How do you tell if ignoring the second deprotonation is justified?
ignoring second deprotonations only when the approximation is justified. How do you tell if ignoring the second deprotonation is justified?
- Mon Jan 13, 2020 7:38 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Homework 5.39
- Replies: 1
- Views: 85
Homework 5.39
What information were we supposed to find in table 5E. 2? My book didn't have a table 5E. 2.
- Mon Jan 13, 2020 7:36 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Homework 5.35
- Replies: 3
- Views: 175
Homework 5.35
The following plot shows how the partial pressures of reactant and products vary with time for the decomposition of compound A into compounds B and C. All three compounds are gases. Use this plot to do the following: write the balanced chemical equation for the reaction. b) calculate the equilibrium...
- Mon Jan 13, 2020 7:33 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Homework 5l. 29
- Replies: 2
- Views: 112
Homework 5l. 29
At 25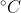 K = 3.2 x
K = 3.2 x 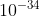 for the reaction 2HCl
for the reaction 2HCl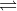 H2 + Cl2. If a reaction vessel of volumme 1.0L is filled with HCl at .22 bar, what are the equilibrium partial pressures of HCl, H2, and Cl2.
H2 + Cl2. If a reaction vessel of volumme 1.0L is filled with HCl at .22 bar, what are the equilibrium partial pressures of HCl, H2, and Cl2.
- Sun Jan 12, 2020 11:39 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: pressure change
- Replies: 4
- Views: 154
Re: pressure change
The general rule for change in pressure is that if the pressure has increased the side of the reaction with fewer moles is favored. This, however, only applies if the pressure is changed by changing the volume because then the concentrations change.
- Sun Jan 12, 2020 11:37 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: appliction of principle
- Replies: 5
- Views: 256
Re: appliction of principle
It applies to changes in concentration, pressure, or temperature.
- Sun Jan 12, 2020 11:36 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: ICE
- Replies: 5
- Views: 154
Re: ICE
The ice tables are mainly used when we only know the initial concentration(s) and the equilibrium constant. Basically, we use ice tables when we are unable to just use the equilibrium concentrations and equilibrium constant to find the other equilibrium concentrations.
- Fri Dec 06, 2019 10:55 am
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: pKa and Ka
- Replies: 10
- Views: 636
Re: pKa and Ka
Ka is the acid dissociation constant and is equal to [H+][A-]/[HA]. The pKa is the -log of the Ka. The Ka and pKa tell you the strength of a weak acid. The larger the Ka and the smaller the pKa the stronger the acid.
- Fri Dec 06, 2019 10:52 am
- Forum: Bronsted Acids & Bases
- Topic: Strong Acids and Bases
- Replies: 2
- Views: 224
Re: Strong Acids and Bases
I'm not sure if these are all the ones we need to know but here are some: strong acids: HCl, HI, HBr, H2SO4, HNO3, HClO4. Strong bases: LiOH, NaOH, KOH, RbOH, CsOH, Ca(OH)2, Sr(OH)2, and Ba(OH)2.
- Fri Dec 06, 2019 10:48 am
- Forum: Dipole Moments
- Topic: Dipole Moments Cancel in Tetrahedral?
- Replies: 5
- Views: 1053
Re: Dipole Moments Cancel in Tetrahedral?
No, they would not cancel. The only way to have a nonpolar tetrahedral molecule is if all the atoms attached to the central atom are the same.
- Fri Dec 06, 2019 10:43 am
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: How to tell the strength of a base
- Replies: 2
- Views: 126
How to tell the strength of a base
Are the guidelines for determining how strong a base is the same as the guidelines for relative acidity?
- Fri Dec 06, 2019 10:41 am
- Forum: Hybridization
- Topic: pi bonds
- Replies: 1
- Views: 97
Re: pi bonds
Pi bonds are formed using unhybridized orbitals. If a bond is using sp2 orbitals then the pi bonds are formed using p orbitals.
- Sun Dec 01, 2019 6:55 pm
- Forum: Conjugate Acids & Bases
- Topic: Conjugate acids/bases
- Replies: 1
- Views: 105
Re: Conjugate acids/bases
'm still not exactly sure what a conjugate acid is. If a molecule gives off a proton in a reaction, in the reverse reaction is it then the conjugate base since it receives a proton? A conjugate acid is a compound that starts out as a base and then gains an H+. If a molecule gives off an H+ in a reac...
- Sun Dec 01, 2019 6:48 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Calculating pH
- Replies: 4
- Views: 298
Re: Calculating pH
To calculate the pH of a solution take the -log of the concentration of H3O+ ions.
- Sun Dec 01, 2019 6:46 pm
- Forum: Amphoteric Compounds
- Topic: 6A.11
- Replies: 1
- Views: 98
Re: 6A.11
Amphiprotic means that the compound can act as both an acid and a base by either accepting or donating an H+. The easiest way to answer this question is to write the chemical reaction between the given compound and H2O. Then show the products when the compound is acting as an acid and as a base. For...
- Sat Nov 30, 2019 5:12 pm
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: Homework 6C.21
- Replies: 1
- Views: 162
Homework 6C.21
Why is HCOOH a stronger acid than CH3COOH? I would assume that CH3COOH would be stronger because of the larger delocalization of charge caused by the CH3 group?
- Sat Nov 30, 2019 5:02 pm
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: Homework 6c.19
- Replies: 1
- Views: 103
Homework 6c.19
part c asks whether HBrO2 or HClO2 is a stronger acid. the H-Br bond is longer and therefore weaker than the H-Cl bond, so why is HClO2 the stronger acid?
- Sun Nov 24, 2019 7:02 pm
- Forum: Lewis Acids & Bases
- Topic: HCl vs HF
- Replies: 19
- Views: 1402
Re: HCl vs HF
F is more electronegative than Cl so it is less likely to give off the H+ ion. This means that HCl can disassociate easier, making it the stronger acid.
- Sun Nov 24, 2019 6:58 pm
- Forum: Hybridization
- Topic: Hybridization
- Replies: 1
- Views: 95
Re: Hybridization
You can count the number of regions of high electron density (a bond or lone pair) and then from there assign it a hybridization with the sample number of orbitals as electron densities. For example if the molecule had 3 areas of high electron density then you would choose a hybridization that has 3...
- Sun Nov 24, 2019 6:54 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: 9C.9
- Replies: 1
- Views: 121
Re: 9C.9
To find the coordination number count the number of bonds that the transition metal forms. For a) the Ni is bonded to 4 Cl so the coordination number is 4. Remember that en is bidentate and edta is hexadentate.
- Sun Nov 24, 2019 6:52 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: [PtCl2(en)2] 2+
- Replies: 3
- Views: 344
Re: [PtCl2(en)2] 2+
It has a coordination number of 6 because it forms a bond with each of the Cl's and then each of the en is bidentate so each en bonds twice to the Pt and since there's 2 of them that's 4 bonds. Then the total number of bonds the Pt has is 6.
- Sun Nov 24, 2019 6:49 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Chelating Ligand
- Replies: 1
- Views: 132
Re: Chelating Ligand
Cheating ligand form a ring of atoms that include the central metal atom. Chelating ligands have to be multidentate the chelate.
- Sun Nov 17, 2019 8:46 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Drawing the Lewis Structure of N20 (2E.13d)
- Replies: 5
- Views: 901
Re: Drawing the Lewis Structure of N20 (2E.13d)
First, you count up all the valence electrons, 2(5) + 6= 16. Then you put the less electronegative atom in the middle, which is N. Then you arrange the O and other N symmetrically around the central O. Then you put the 16 electrons around the atoms. You find out that with just single bonds, not all ...
- Sun Nov 17, 2019 8:31 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: ion-ion intermolecular forces and ionic bonds
- Replies: 2
- Views: 188
ion-ion intermolecular forces and ionic bonds
What is the difference between an ion-ion molecular force and an ionic bond or are they the same thing?
- Sun Nov 17, 2019 8:27 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Terminal Atom
- Replies: 2
- Views: 222
Re: Terminal Atom
Terminal atoms are just the atoms surrounding the central atom in a Lewis Structure. For example, Cl is a terminal atom in CCl4.
- Sun Nov 17, 2019 8:26 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSEPR
- Replies: 7
- Views: 367
Re: VSEPR
Correct. We will not be asked to draw them but we will need to name the shapes.
- Sun Nov 17, 2019 8:25 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Molecular shape
- Replies: 3
- Views: 244
Re: Molecular shape
Yes, seesaw and sawhorse both have the VSEPR model AX4E
- Thu Nov 14, 2019 1:28 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Homework 2E.11
- Replies: 3
- Views: 124
Homework 2E.11
Use Lewis Structures and the VSEPR model to give the VSEPR formula for each of the following species and predict its shape:
a)sulfur tetrachloride
b)iodine trichloride
c) IF4-
d)xenon trioxide
What is the VSEPR formula for each part and how do you find it?
a)sulfur tetrachloride
b)iodine trichloride
c) IF4-
d)xenon trioxide
What is the VSEPR formula for each part and how do you find it?
- Sun Nov 10, 2019 8:48 pm
- Forum: Dipole Moments
- Topic: 3F.3 c
- Replies: 1
- Views: 118
Re: 3F.3 c
It experiences a dipole moment because the Cl's and H's are next to each other and so there is a dipole moment pointing from the H to the C and from the C to the Cl. So, therefore, the dipole moments add in both directions so the dipole moment points from the H to the Cl.
- Sun Nov 10, 2019 8:33 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Polarizability
- Replies: 3
- Views: 131
Re: Polarizability
The bigger the atom, the more polarizable it will be because the outer electrons feel the pull of the nucleus less so they can be more distorted. The number of electrons is indicative of the size.
- Sun Nov 10, 2019 8:04 pm
- Forum: Lewis Structures
- Topic: CH2Cl2 structure
- Replies: 1
- Views: 229
CH2Cl2 structure
How do you determine that in the lewis structure of CH2Cl2 the Cl's are next to each other and the H's are next to each other instead of the Cl's and H's being across from each other?
- Sun Nov 10, 2019 7:56 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: dipole dipole of H2SeO4
- Replies: 1
- Views: 189
dipole dipole of H2SeO4
Why does H2SeO4 have a dipole moment?
- Sun Nov 03, 2019 12:59 pm
- Forum: Electronegativity
- Topic: Electron Affinity
- Replies: 3
- Views: 126
Re: Electron Affinity
Electron affinity is the energy released when an electron is added to a gas-phase atom. Electronegativity is the electron-pulling power of an atom when it is part of a molecule. Electronegativity is the average of the ionization energy and electron affinity of an element.
- Sun Nov 03, 2019 12:53 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Degenerate orbitals?
- Replies: 1
- Views: 152
Re: Degenerate orbitals?
Yes, all 3 of the 2p orbitals and all 5 of the 3d orbitals are degenerate because they are at the same energy level.
- Sun Nov 03, 2019 12:50 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: general question
- Replies: 2
- Views: 191
Re: general question
Polarizability is how much an electron cloud of an atom or ion can undergo a large distortion. Atoms or ions with a large distortion are highly polarizable. The larger the atom the more polarizable it is.
- Sun Nov 03, 2019 12:46 pm
- Forum: Bond Lengths & Energies
- Topic: 2D.19
- Replies: 1
- Views: 144
Re: 2D.19
"Use the covalent radii in Fig. 2D.11 to calculate the bond lengths in the following molecules. Account for the trends in your calculated values: (a) CF 4; (b) SiF 4; (c) SnF 4" In order to find the bond length of covalent bonds you need to add together the 2 covalent radii for the element...
- Mon Oct 28, 2019 2:34 pm
- Forum: Ionic & Covalent Bonds
- Topic: Homework 2B.11
- Replies: 1
- Views: 81
Homework 2B.11
Draw the complete Lewis structure for each of the following compounds:
c) H2C(NH2)COOH
How do you determine the arrangement of the atoms and which atoms are bonded to another?
c) H2C(NH2)COOH
How do you determine the arrangement of the atoms and which atoms are bonded to another?
- Sun Oct 27, 2019 10:36 pm
- Forum: Ionic & Covalent Bonds
- Topic: 2A. 9
- Replies: 2
- Views: 147
Re: 2A. 9
This is the question: Which M2+ ions (where M is a metal) are predicted to have the following ground-state electron configurations: (a) [Ar]3d7; (b) [Ar]3d6; (c) [Kr]4d4; (d) [Kr]4d3? a) Because there is a 2+ charge we need to add 2 electrons to the given number of valence electrons, which in this c...
- Sun Oct 27, 2019 10:25 pm
- Forum: Ionic & Covalent Bonds
- Topic: Valence Electrons
- Replies: 3
- Views: 127
Re: Valence Electrons
You start from the left side of the periodic table and count across a period until you reach the element you're trying to find. The count is the number of valence electrons. If you're trying to find the number of valence electrons for a cation then you find the number of valence electrons and subtra...
- Sun Oct 27, 2019 10:19 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Elements having octets
- Replies: 4
- Views: 267
Re: Elements having octets
C,N,O,F always form an octect and anything with an atomic number above F has a d-shell, so they can form expanded octects.
- Sun Oct 27, 2019 10:16 pm
- Forum: Trends in The Periodic Table
- Topic: Cations vs Parent atoms
- Replies: 4
- Views: 219
Re: Cations vs Parent atoms
Cations are smaller than their parent atoms because there is less electron-electron repulsion but there is still the same number of protons so the electrons are closer to the nucleus.
- Sun Oct 27, 2019 10:13 pm
- Forum: Ionic & Covalent Bonds
- Topic: 2A. 1 Question
- Replies: 2
- Views: 116
Re: 2A. 1 Question
Sb and Mn have electrons in the d-shell so those electrons are counted as valence electrons because they are in the outermost shell.
- Sun Oct 20, 2019 7:46 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: 1E.17 Ion formation
- Replies: 1
- Views: 66
Re: 1E.17 Ion formation
Electrons are removed from the highest energy orbital and the energies of orbitals go s<p<d<f.
- Sun Oct 20, 2019 7:42 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Problem 1E.25
- Replies: 2
- Views: 81
Re: Problem 1E.25
1E.25 Give the notation for the valence-shell configuration
(including the outermost d-electrons) of (a) the alkali metals;
(b) Group 15 elements; (c) Group 5 transition metals; (d) the
“coinage” metals (Cu, Ag, Au).
a) ns^1
b) ns^2 np^3
c) omit
d) (n-1)d^10 ns^1
(including the outermost d-electrons) of (a) the alkali metals;
(b) Group 15 elements; (c) Group 5 transition metals; (d) the
“coinage” metals (Cu, Ag, Au).
a) ns^1
b) ns^2 np^3
c) omit
d) (n-1)d^10 ns^1
- Sun Oct 20, 2019 7:34 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Problem 1E.5
- Replies: 2
- Views: 181
Re: Problem 1E.5
Electrons in an s-orbital are more effective at shielding other electrons because the penetration levels go ns > np > nd > nf. The higher the penetration the more effective an electron is at shielding others. The s-orbital is closest to the nucleus so it is the most effective at shielding other elec...
- Sun Oct 20, 2019 7:28 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: electron configuration exceptions
- Replies: 3
- Views: 113
Re: electron configuration exceptions
what were the electron configuration exceptions, and does anyone know why they are exceptions? additionally, other than asking what the exceptions are on an exam, what kind of questions would test this knowledge of exceptions? The exceptions that we need to know are Cr whose electron configuration i...
- Sun Oct 20, 2019 7:21 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Orbital orders
- Replies: 2
- Views: 186
Re: Orbital orders
The s < p < d < f is referring to the energy levels of different subshells. They can all exist at the same time for example boron has electrons in the 2s and 2p orbitals. The only limitation is if the atom doesn't have enough electrons to need to fill the p, d, or f levels. For example, magnesium do...
- Sun Oct 20, 2019 7:16 pm
- Forum: Photoelectric Effect
- Topic: 1B.15
- Replies: 2
- Views: 162
Re: 1B.15
keV is kilo-electron volts, which is a unit for energy. The conversion from kilo- electron volts to electron volts is (1000 eV/keV) and then the conversion from eV to J is ) J/ 1eV.
J/ 1eV.
- Sun Oct 13, 2019 1:20 pm
- Forum: Properties of Light
- Topic: 1A3
- Replies: 5
- Views: 221
Re: 1A3
The speed in the question is referring to the speed of light which is always 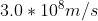
- Sat Oct 12, 2019 6:09 pm
- Forum: Properties of Light
- Topic: Homework 1A 15
- Replies: 2
- Views: 126
Homework 1A 15
Will someone explain how to find the final and initial energy levels of an electron during the emission of energy that leads to a spectral line observed at 102.6 nm in the ultraviolet spectrum of atomic hydrogen using the equation from class?
- Sat Oct 12, 2019 5:54 pm
- Forum: Properties of Light
- Topic: ∝ notation
- Replies: 2
- Views: 104
Re: ∝ notation
Yes ,if instead b had said the wavelength increases it would have been correct because c= 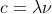 so wavelength and frequency have an inverse relationship so when one increased the other has to decrease.
so wavelength and frequency have an inverse relationship so when one increased the other has to decrease.
- Sat Oct 12, 2019 5:50 pm
- Forum: Properties of Light
- Topic: Homework 1A3
- Replies: 2
- Views: 124
Homework 1A3
Why is the speed of the radiation constant when the frequency of EM radiation decreases?
- Fri Oct 04, 2019 11:13 am
- Forum: Molarity, Solutions, Dilutions
- Topic: Homework Question G11
- Replies: 2
- Views: 175
Re: Homework Question G11
Using the fact that n = MV, by rearranging the equation you get V = n/M. 4.50 mmol = 4.5 x 10^-3 m so V = (4.5 x 10^-3 m)/ (.278 M) = 16.2 mL
- Fri Oct 04, 2019 10:46 am
- Forum: SI Units, Unit Conversions
- Topic: What is a t
- Replies: 2
- Views: 186
What is a t
In homework L35 it asks you to use a mass of 2.50 t. What is the conversion from t to grams?
- Fri Oct 04, 2019 10:45 am
- Forum: SI Units, Unit Conversions
- Topic: Homework: L35
- Replies: 2
- Views: 126
Re: Homework: L35
Also the last equation that you balanced isn't balanced because the textbook has an error and the last equation should be Fe3Br8 + Na2CO3 forms NaBr + CO2 + Fe3O4.
- Fri Oct 04, 2019 10:42 am
- Forum: Balancing Chemical Reactions
- Topic: Homework L35
- Replies: 1
- Views: 89
Re: Homework L35
Nevermind, the textbook has an error on problem L35. The last equation should be Fe3Br8 + Na2CO3 forms NaBr + CO2 + Fe3O4.
- Fri Oct 04, 2019 10:32 am
- Forum: Balancing Chemical Reactions
- Topic: Homework L35
- Replies: 1
- Views: 89
Homework L35
How do you balance this equation?
FeBr2 + Na2CO3 goes to form NaBr + CO2 + Fe3O4
FeBr2 + Na2CO3 goes to form NaBr + CO2 + Fe3O4

