Search found 90 matches
- Wed Feb 19, 2020 1:31 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Decreasing pressure
- Replies: 7
- Views: 606
Re: Decreasing pressure
a decrease in pressure means an increase in volume.
- Wed Feb 19, 2020 1:30 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: midterm question// Concentration ratio [ENDORSED]
- Replies: 9
- Views: 726
Re: midterm question// Concentration ratio [ENDORSED]
was the equation given on the equation sheet on the midterm? I dont recall it being given
- Wed Feb 19, 2020 1:29 am
- Forum: Ideal Gases
- Topic: pv=nrt
- Replies: 19
- Views: 1211
Re: pv=nrt
at STP, you simply input the standard temperature and pressure into the equation: 1 atm and 273 degrees kelvin. Then you may proceed with the problem as normal
- Tue Feb 11, 2020 4:00 pm
- Forum: Phase Changes & Related Calculations
- Topic: Homework 8.27
- Replies: 6
- Views: 2766
Re: Homework 8.27
Using pv=nrt, I determined that n=0.003mol. However, when I put that into the equation w=-nRTln(v2/v1), I get -3.18J, which is not possible since an isothermal reversible expansion always does more work than an irreversible one. Can anyone determine what I did wrong? I'm having trouble figuring it o...
- Tue Feb 11, 2020 3:47 pm
- Forum: Phase Changes & Related Calculations
- Topic: Homework 8.27
- Replies: 6
- Views: 2766
Re: Homework 8.27
I'm a bit confused as to where the given temperature comes in
- Sun Feb 09, 2020 9:02 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: problem 4A9
- Replies: 2
- Views: 260
problem 4A9
A piece of copper of mass 20.0 g at 100.0 8C is placed in a vessel of negligible heat capacity but containing 50.7 g of water at 22.0 8C. Calculate the final temperature of the water. Assume that no energy is lost to the surroundings. Im a bit confused on how to proceed with this problem. I used q=M...
- Sun Feb 09, 2020 9:00 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Partial Pressure
- Replies: 7
- Views: 442
Re: Partial Pressure
partial pressure is specifically the pressure of a specific gas in a mixture or reaction, whereas pressure is the pressure of the entire reaction.
- Sun Feb 09, 2020 8:55 pm
- Forum: Phase Changes & Related Calculations
- Topic: irreversible equations
- Replies: 2
- Views: 258
Re: irreversible equations
no, not for calculating work.
- Sun Feb 09, 2020 8:55 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: HW 4c.3
- Replies: 2
- Views: 283
Re: HW 4c.3
you need to plug the equation into PV=nRT in order to proceed with this problem since it is stated in the problem that it is an ideal gas. Thus, for constant pressure you solve for the P and for constant volume you solve for the V.
- Sun Feb 09, 2020 8:53 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: including phase changes
- Replies: 2
- Views: 243
Re: including phase changes
usually the phase change will be given in the question and you just apply it as needed
- Thu Feb 06, 2020 6:51 pm
- Forum: Calculating Work of Expansion
- Topic: Calculating required heat
- Replies: 5
- Views: 7413
Re: Calculating required heat
why do we need to add the specific heat capacities? and how are we supposed to assume this is a composite system? im working on this question right now and im also wondering if we add the specific heat capacities of water and copper in one equation or do two separate equations?
- Tue Jan 28, 2020 3:10 am
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Heat Supplied to a system
- Replies: 6
- Views: 474
Heat Supplied to a system
(a) Calculate the heat that must be supplied to a copper kettle of mass 400.0 g containing 300.0 g of water to raise its tem- perature from 20.0 8C to the boiling point of water, 100.0 8C. (b) What percentage of the heat is used to raise the temperature of the water? I am slightly confused on how to...
- Tue Jan 28, 2020 3:08 am
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: homework question 4A.3
- Replies: 6
- Views: 323
Re: homework question 4A.3
how do you go about the conversions for this problem? the answer is given using L times atm.
- Thu Jan 23, 2020 9:37 am
- Forum: Phase Changes & Related Calculations
- Topic: Enthalpy
- Replies: 4
- Views: 357
Re: Enthalpy
Jasmine Fendi 1D wrote:Enthalpy is just the measure of heat! Temperature and enthalpy are related but are different, as seen in heat curves. The x-axis is heat supplied and the y-axis is temperature
How are heat and enthalpy different?
- Thu Jan 23, 2020 9:36 am
- Forum: Phase Changes & Related Calculations
- Topic: Adding and subtracting properties
- Replies: 6
- Views: 399
Adding and subtracting properties
Can someone please explain why we do final minus initial when doing state properties?
- Thu Jan 23, 2020 9:34 am
- Forum: Phase Changes & Related Calculations
- Topic: heating curves
- Replies: 4
- Views: 1506
Re: heating curves
No. The heating curve introduced in lecture is specific to only water
- Thu Jan 23, 2020 9:28 am
- Forum: Phase Changes & Related Calculations
- Topic: Heating curve
- Replies: 2
- Views: 246
Heating curve
In lecture, Professor Lavelle asked why steam causes severe burns, and proceeded to show the heat curve. Can someone explain the heating curve and what it means?
- Wed Jan 22, 2020 10:05 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: exothermic reactions
- Replies: 19
- Views: 2073
Re: exothermic reactions
yes, cooling will favor the products!
- Thu Jan 16, 2020 12:16 pm
- Forum: Ideal Gases
- Topic: Kp given instead of Kc
- Replies: 8
- Views: 504
Kp given instead of Kc
If Kp is given rather than Kc in a problem but the product is given in moles or grams rather than bars, how do you proceed with the problem? how can you convert to pressure?
- Wed Jan 15, 2020 10:29 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5I29
- Replies: 2
- Views: 209
5I29
At 25 8C, K 5 3.2 3 10234 for the reaction 2 HCl(g) ∆ H2(g) 1 Cl2(g). If a reaction vessel of volume 1.0 L is filled with HCl at 0.22 bar, what are the equilibrium partial pressures of HCl, H2, and Cl2? Do we need to set up an ice box with the partial pressure of HCl although it is given in partial ...
- Mon Jan 13, 2020 10:35 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: HW 5I23
- Replies: 1
- Views: 894
HW 5I23
A reaction mixture consisting of 2.00 mol CO and 3.00 mol H2 is placed in a reaction vessel of volume 10.0 L and heated to 1200. K. At equilibrium, 0.478 mol CH4 was present in the system. Determine the value of Kc for the reaction CO(g) 1 3 H2(g) ∆ CH4(g) 1 H2O(g) at 1200. K. Can someone explain ho...
- Thu Jan 09, 2020 12:27 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: units of K
- Replies: 10
- Views: 529
Re: units of K
K does not have units. because it is a ratio of products to reactants, and is a constant in the reaction. Also, if you worked out the math in an equilibrium problem K = [P]/[R], and so the molarities cancel out.
- Thu Jan 09, 2020 12:25 pm
- Forum: Ideal Gases
- Topic: Solids and Liquids
- Replies: 7
- Views: 458
Re: Solids and Liquids
The reason solids are not used in equilibrium reactions is because their concentrations stay constant throughout the reaction
- Thu Jan 09, 2020 12:23 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5G3
- Replies: 8
- Views: 348
Re: 5G3
Yes, gases are included in the gas phase. H20 in the liquid phase is not used because they do not affect the reactant amount at equilibrium.
- Thu Jan 09, 2020 12:21 pm
- Forum: Ideal Gases
- Topic: kc vs kp
- Replies: 19
- Views: 3506
Re: kc vs kp
Kp and Kc are both calculated in the same way. However, Kc is denoted when a problem is given using molar concentrations, and Kp is denoted when a problem is given using pressures of a gas
- Thu Jan 09, 2020 12:20 pm
- Forum: Ideal Gases
- Topic: K and Q
- Replies: 13
- Views: 376
Re: K and Q
Q can be used whether or not the reaction is at equilibrium, and is used to determine whether or not a reaction is in equilibrium. K can only be used when a reaction is in equilibrium. Both are calculated in the same way.
- Thu Jan 09, 2020 12:18 pm
- Forum: Ideal Gases
- Topic: 5G.9 -- Partial Pressures?
- Replies: 2
- Views: 127
Re: 5G.9 -- Partial Pressures?
Because more moles of O3 are placed in a container of the same volume in the second experiment, the partial pressure of O2 will be higher. This is because the Kp must be the same, so the pressure of O2 of the second experiment must be higher to maintain the same Product to reactant ratio as the firs...
- Sat Dec 07, 2019 9:50 pm
- Forum: Conjugate Acids & Bases
- Topic: how to figure out?
- Replies: 12
- Views: 729
Re: how to figure out?
the conjugate base is the product you have after your acid gives off a proton; the conjugate acid is what product you have after your base accepts a proton.
- Sat Dec 07, 2019 9:48 pm
- Forum: Calculating the pH of Salt Solutions
- Topic: Sig Figs for logarithmic funcitons
- Replies: 6
- Views: 456
Re: Sig Figs for logarithmic funcitons
count the sig figs AFTER your decimal :)
- Sat Dec 07, 2019 9:46 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Strong Acids
- Replies: 3
- Views: 295
Re: Strong Acids
are these memorized or should we be able to explain these?
- Sat Dec 07, 2019 9:44 pm
- Forum: Biological Examples
- Topic: Final
- Replies: 6
- Views: 512
Re: Final
The hydrogen bonding od DNA Base pairs, heme as a coordination compound, cisplatin for chemotherapy, vitamins as acceptors of dangerous radicals, the role of carbon dioxide in blood ph These are the examples i can think of can you elaborate on the vitamins? So as you already know, radicals contain ...
- Sat Dec 07, 2019 9:43 pm
- Forum: Biological Examples
- Topic: Final
- Replies: 6
- Views: 512
Re: Final
the hydrogen bonding of base pairs, edta, hemoglobin and myoglobin, cisplatin v translation as an effective chemotherapy drug.
- Sat Dec 07, 2019 9:40 pm
- Forum: Biological Examples
- Topic: Polydentate
- Replies: 3
- Views: 143
Re: Polydentate
They are the same things, however hexadentate is a more specific and accurate response especially for edta since we focused on it so much!
- Sat Dec 07, 2019 9:38 pm
- Forum: Biological Examples
- Topic: chemotherapy drugs
- Replies: 13
- Views: 621
Re: chemotherapy drugs
Its not chemotherapy's structure, it's the structure of the chemotherapy drug known as cisplatin. Cisplatin has two chlorines on the same side that attach to the guanine base pairs of a DNA molecule, allowing for a stronger bond to block DNA polymerase from replicating a cancerous DNA strand. Transp...
- Sat Dec 07, 2019 9:31 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: chelating ligands [ENDORSED]
- Replies: 51
- Views: 98652
Re: chelating ligands [ENDORSED]
sigma bonds are able to rotate already!
- Tue Dec 03, 2019 11:07 pm
- Forum: Polyprotic Acids & Bases
- Topic: How can you tell
- Replies: 18
- Views: 1036
Re: How can you tell
make sure that the anion has more than one hydrogen!
- Tue Dec 03, 2019 11:06 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: atomic spectra
- Replies: 1
- Views: 215
Re: atomic spectra
it would make the emission of energy negative, as it is emitting more than it already has when a photon is emitted.
- Tue Dec 03, 2019 11:04 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: transition of orbitals
- Replies: 2
- Views: 326
transition of orbitals
Which of the following increase when the electron in a hydrogen atom undergoes a transition from the 1s-orbital to a 2p-orbital? (a) Energy of the electron. (b) Value of n. (c) Value of l. (d) Radius of the atom. Can someone explain part d of this problem? what does the radius of the atom have to do...
- Tue Dec 03, 2019 11:42 am
- Forum: Lewis Acids & Bases
- Topic: Lewis vs. Bronsted
- Replies: 3
- Views: 201
Re: Lewis vs. Bronsted
A molecule that is a Lewis acid can also be considered a Bronsted acid (and vice versa) since their definitions do line up. In order for a molecule to accept an electron pair (be a Bronsted acid), it donates a proton (is a Lewis acid). Typically you can use both definitions to define an acid unless...
- Tue Dec 03, 2019 11:38 am
- Forum: Lewis Acids & Bases
- Topic: Bronsted v Lewis acids and bases
- Replies: 1
- Views: 142
Bronsted v Lewis acids and bases
Can someone explain the difference between bronsted and Lewis acids and bases, and also conjugate? I dont understand the differences between them
- Tue Dec 03, 2019 11:37 am
- Forum: Lewis Acids & Bases
- Topic: Bronsted v Lewis acids and bases
- Replies: 1
- Views: 184
Bronsted v Lewis acids and bases
Can someone explain the difference between bronsted and Lewis acids and bases, and also conjugate? I dont understand the differences between them
- Sun Dec 01, 2019 1:38 pm
- Forum: Lewis Structures
- Topic: lewis symbols
- Replies: 1
- Views: 221
lewis symbols
On the basis of the expected charges of the monatomic ions, draw the formula unit of each of the following compounds using Lewis symbols: (a) thallium(III) chloride; (b) aluminum sulfide; (c) barium oxide.
- Sun Dec 01, 2019 1:38 pm
- Forum: Dipole Moments
- Topic: Hydrogen Bonding
- Replies: 20
- Views: 1172
Re: Hydrogen Bonding
Yes, with a nitrogen, oxygen, or fluorine
- Sun Dec 01, 2019 1:37 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: shapes
- Replies: 1
- Views: 168
shapes
The following species have the same number of electrons: 1 21 Cd, In , and Sn . (a) Write the electron configurations for each species. Explain any differences. (b) How many unpaired elec- trons, if any, are present in each species? (c) What neutral atom, if 31 any, has the same electron configurati...
- Sun Dec 01, 2019 1:35 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Determining Polarity
- Replies: 10
- Views: 609
Re: Determining Polarity
memorization in this situation will help - the chart in the textbook provides the shapes along with their polarities.
- Sun Dec 01, 2019 1:33 pm
- Forum: Lewis Acids & Bases
- Topic: acid v. base?
- Replies: 16
- Views: 962
Re: acid v. base?
Lewis bases will typically contain the lone pair to donate to the Lewis acid
- Fri Nov 22, 2019 7:30 pm
- Forum: Electronegativity
- Topic: 2D.3
- Replies: 3
- Views: 407
Re: 2D.3
You can determine ionic bonds by their electronegativity differences: if the difference is over 2, it is considered ionic.
- Fri Nov 22, 2019 7:29 pm
- Forum: Dipole Moments
- Topic: boiling point
- Replies: 6
- Views: 651
Re: boiling point
The number of lone pairs (more electrons) increases polarizability thus a higher melting point.
- Fri Nov 22, 2019 7:28 pm
- Forum: Hybridization
- Topic: 2F.1
- Replies: 3
- Views: 184
Re: 2F.1
The orientation of the orbitals will be facing outwards, so 120 degrees
- Fri Nov 22, 2019 7:27 pm
- Forum: Dipole Moments
- Topic: Polar or Nonpolar
- Replies: 5
- Views: 482
Re: Polar or Nonpolar
Always look at the symmetry of a molecule first. If a molecule is symmetrical, it is non polar. If not, find the Mose electronegative atom and that is the slightly negative end of a polar molecule
- Fri Nov 22, 2019 7:23 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: polarizability
- Replies: 9
- Views: 826
Re: polarizability
A higher polarizability indicates a higher melting point since it has higher intermolecular forces
- Fri Nov 15, 2019 12:24 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: T shape
- Replies: 7
- Views: 511
T shape
Can someone explain T shape and give an example of a molecule with the T shape?
- Fri Nov 15, 2019 12:23 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: bond angles
- Replies: 10
- Views: 509
Re: bond angles
Simply look at the. lone pairs and bonds in the molecule top determine your answer
- Fri Nov 15, 2019 12:23 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Octahedral
- Replies: 5
- Views: 406
Re: Octahedral
The shape of the orbitals is octahedral. One orbital contains a lone pair of electrons so the remaining five atoms connected to the central atom gives the molecule a square pyramidal shape
- Fri Nov 15, 2019 12:22 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: T-shaped
- Replies: 3
- Views: 279
Re: T-shaped
A t shaped has three bonds and two lone pairs, as discussed in section 2E of the textbook.
- Fri Nov 15, 2019 12:21 am
- Forum: Octet Exceptions
- Topic: incomplete octet
- Replies: 6
- Views: 495
Re: incomplete octet
Incomplete octets can occur when elements do not have enough electrons to complete an octet around each element, even with double or triple bonds.
- Fri Nov 15, 2019 12:21 am
- Forum: Octet Exceptions
- Topic: incomplete octet
- Replies: 6
- Views: 495
Re: incomplete octet
Incomplete octets can occur when elements do not have enough electrons to complete an octet around each element, even with double or triple bonds.
- Sun Nov 10, 2019 11:19 pm
- Forum: Dipole Moments
- Topic: H Bonding
- Replies: 4
- Views: 285
Re: H Bonding
H bonding is not as strong because it is less of a bond and more of an attraction between the slightly negative and slightly positive sides of molecules.
- Sun Nov 10, 2019 11:09 pm
- Forum: Administrative Questions and Class Announcements
- Topic: 14BL
- Replies: 5
- Views: 295
Re: 14BL
Its a lot of commitment, with the studying from 14B and the hours spent in lab. Its suggested you take them separate quarters, or even better, take the lab during summer if you can!
- Sun Nov 10, 2019 11:08 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Polarizability
- Replies: 4
- Views: 359
Re: Polarizability
Larger atoms means more electrons meaning more shielding can occur
- Sun Nov 10, 2019 11:07 pm
- Forum: Octet Exceptions
- Topic: Octet Rule exceptions
- Replies: 14
- Views: 871
Re: Octet Rule exceptions
The first four elements will never have an octet. P,Cl, and S will typically break the octet
- Sun Nov 10, 2019 11:06 pm
- Forum: Resonance Structures
- Topic: octet v. expanded octet
- Replies: 5
- Views: 349
Re: octet v. expanded octet
Its always one or the other. Elements like P,CL, and S typically break the octet rule
- Sun Nov 10, 2019 11:05 pm
- Forum: Octet Exceptions
- Topic: Antioxidants
- Replies: 9
- Views: 717
Re: Antioxidants
Antioxidants give an e- to a radical to stabilize it. Foods like dark berries contain a lot of these and are critical in a balanced diet to stabilize free radicals in the body.
- Sun Nov 10, 2019 11:04 pm
- Forum: Properties of Light
- Topic: How to find the longest wavelength?
- Replies: 6
- Views: 4685
Re: How to find the longest wavelength?
find the wavelength of light equal to the work function
- Sun Nov 10, 2019 11:03 pm
- Forum: Ionic & Covalent Bonds
- Topic: Hydrogen Bond Strength
- Replies: 8
- Views: 481
Re: Hydrogen Bond Strength
Hydrogen bonds are the weakest amongst the bonds. Ionic is next strongest, and covalent bonds are the strongest bonds
- Tue Nov 05, 2019 11:19 am
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: +/- speed
- Replies: 3
- Views: 165
+/- speed
A bowling ball of mass 8.00 kg is rolled down a bowling alley lane at 5.00+/- 5.00m/s. What is the minimum uncertainty of its position?
When given a +/- in speed, how do we proceed with the problem?
When given a +/- in speed, how do we proceed with the problem?
- Thu Oct 31, 2019 10:25 pm
- Forum: Resonance Structures
- Topic: Resonance Structures
- Replies: 5
- Views: 272
Re: Resonance Structures
The most stable structure is that with the lowest formal charge. So while multiple structures may be drawn, there is typically one with the lowest formal charge that is accurate.
- Thu Oct 31, 2019 10:23 pm
- Forum: Ionic & Covalent Bonds
- Topic: Midterm
- Replies: 28
- Views: 1331
Re: Midterm
The midterm covers everything from fundamentals to 2D in the homework assignments and readings.
- Thu Oct 31, 2019 10:22 pm
- Forum: Octet Exceptions
- Topic: Octet Rule Exceptions
- Replies: 4
- Views: 200
Re: Octet Rule Exceptions
The octet rule mainly only applies to atoms with less electrons. As the atomic number increases, the atom is more capable of having more than an octet.
- Thu Oct 31, 2019 10:20 pm
- Forum: Electronegativity
- Topic: 2D.1
- Replies: 4
- Views: 325
Re: 2D.1
Electronegativity decreases down a group and across a period, so just look at the positions of the elements on the periodic table.
- Thu Oct 31, 2019 10:19 pm
- Forum: Lewis Structures
- Topic: Exceptions to the Octet
- Replies: 5
- Views: 269
Exceptions to the Octet
How does P violate the octet rule? does it have a valence shell of 10?
- Thu Oct 31, 2019 10:18 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: D orbital
- Replies: 5
- Views: 231
Re: D orbital
Since each orbital holds two electrons, the d-orbital has 5, allowing for ten total electrons.
- Thu Oct 31, 2019 10:16 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Heisenberg
- Replies: 3
- Views: 142
Re: Heisenberg
If the uncertainty was too large, the equation would essentially be useless
- Sun Oct 27, 2019 11:18 pm
- Forum: Electronegativity
- Topic: Electronegativity
- Replies: 6
- Views: 947
Re: Electronegativity
since oxygen has a smaller radius, its electronegativity is higher!
- Sun Oct 27, 2019 11:15 pm
- Forum: Trends in The Periodic Table
- Topic: Ionizatiom Energy
- Replies: 1
- Views: 117
Ionizatiom Energy
Why does ionization energy decrease as shells fill up?
- Sun Oct 27, 2019 11:14 pm
- Forum: Ionic & Covalent Bonds
- Topic: 4s before 3d
- Replies: 4
- Views: 199
4s before 3d
I'm still confused on why the 4s group comes before the 3d group from Dr. Lavelle's lecture, can someone explain why and for what situations this applies?
- Sun Oct 27, 2019 11:12 pm
- Forum: Ionic & Covalent Bonds
- Topic: Ground State
- Replies: 11
- Views: 557
Ground State
What exactly does ground state electron configuration mean? What is it asking me to find?
- Sun Oct 20, 2019 5:22 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Orbitals
- Replies: 3
- Views: 310
Re: Orbitals
i believe this is how you do it: a) 103 because ml can be any value between [-l, l] so there are 51*2+1 possible orbitals bc) 1 because they give you a ml value so there's only one possible orbital d) if n=57 then there are 57 possible l values and for each of them they'd have l*2+1 orbitals (or ju...
- Fri Oct 18, 2019 11:38 am
- Forum: Photoelectric Effect
- Topic: When energy is equal to work function
- Replies: 9
- Views: 999
Re: When energy is equal to work function
when the energy of the photon of the energy required to eject the electron from the metal, the electron will be ejected from the metal!
- Fri Oct 18, 2019 11:37 am
- Forum: Photoelectric Effect
- Topic: HW 1B.5
- Replies: 8
- Views: 370
Re: HW 1B.5
you must multiply by 10^3 to convert to the correct units
- Fri Oct 18, 2019 11:35 am
- Forum: DeBroglie Equation
- Topic: De Broglie's Equation
- Replies: 17
- Views: 626
Re: De Broglie's Equation
since wavelength=h/mv, the object must have a mass. While Light has a velocity, it does not have a mass to calculate
- Fri Oct 18, 2019 11:33 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Orbitals
- Replies: 3
- Views: 310
Orbitals
Can anyone help with this problem?
How many orbitals can have the following quantum numbersinanatom:(a)n53,l51;(b)n55,l53,ml 521; (c)n52,l51,ml 50;(d)n57?
How many orbitals can have the following quantum numbersinanatom:(a)n53,l51;(b)n55,l53,ml 521; (c)n52,l51,ml 50;(d)n57?
- Fri Oct 18, 2019 11:30 am
- Forum: Photoelectric Effect
- Topic: Spectrum of light
- Replies: 6
- Views: 324
Re: Spectrum of light
Gamma Ray, X ray, Ultraviolet, Visible spectrum, Infared, Microwave, Radio
- Fri Oct 18, 2019 11:29 am
- Forum: Photoelectric Effect
- Topic: Spectrum of light
- Replies: 6
- Views: 324
Re: Spectrum of light
Gamma Ray, X ray, Ultraviolet, Visible spectrum, Infared, Microwave, Radio
- Thu Oct 03, 2019 11:12 pm
- Forum: Empirical & Molecular Formulas
- Topic: 100 gram Method?
- Replies: 9
- Views: 1169
Re: 100 gram Method?
The 100-gram trick essentially simplifies calculations when given percent compositions of elements in a molecule. What you do, is convert the mass percentages to grams (as if out of 100) and divide by the molar masses in order to find the moles of each element and be able to determine your ratios fr...
- Thu Oct 03, 2019 11:06 pm
- Forum: Limiting Reactant Calculations
- Topic: Clarification
- Replies: 4
- Views: 387
Re: Clarification
Yes, since the limiting reactant is the product that is not in excess, it is the product that will be completely used up and therefore determines the maximum (theoretical) yield that can be produced.
- Thu Oct 03, 2019 11:04 pm
- Forum: SI Units, Unit Conversions
- Topic: which unit to use
- Replies: 9
- Views: 526
Re: which unit to use
Always stay consistent with the units given to you in the problem, but always remember that you may have to convert some units in order to fit into the problem (such as converting milliliters to liters to find molarity.) However if an answer seems too small or too large for a given unit, feel free t...
- Thu Oct 03, 2019 6:59 pm
- Forum: Properties of Light
- Topic: What does μm mean?
- Replies: 4
- Views: 478
Re: What does μm mean?
the 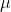 represents the prefix micro-, thus the term meaning micrometer, which is
represents the prefix micro-, thus the term meaning micrometer, which is 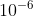 meters
meters
- Thu Oct 03, 2019 6:56 pm
- Forum: Significant Figures
- Topic: Sig Fig Rules
- Replies: 7
- Views: 422
Re: Sig Fig Rules
With sig figs, use the exact values you calculate until you reach your final answer. Then, round to the same number of sig figs as the values given in the problem. If the value is below five, round down. If the value is five or above, round up to the net nearest integer.
- Thu Oct 03, 2019 6:55 pm
- Forum: Significant Figures
- Topic: Sig Fig Rules
- Replies: 7
- Views: 422
Re: Sig Fig Rules
With sig figs, use the exact values you calculate until you reach your final answer. Then, round to the same number of sig figs as the values given in the problem. If the value is below five, round down. If the value is five or above, round up to the net nearest integer.

