Search found 111 matches
- Mon Mar 16, 2020 2:20 am
- Forum: First Order Reactions
- Topic: How to solve
- Replies: 3
- Views: 335
Re: How to solve
For the most part, it is just plugging in your values and solving. Sometimes you will need to do some intermediate calculations in order to obtain all the necessary values to calculate the desired answer.
- Mon Mar 16, 2020 2:19 am
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: k
- Replies: 36
- Views: 1643
Re: k
Since k is the rate constant, if everything else is constant, if you increase the value of k, the reaction rate will increase.
- Mon Mar 16, 2020 2:18 am
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: graphs and order
- Replies: 19
- Views: 951
Re: graphs and order
Zero order: [A] vs time (linear)
First order: ln[A] vs time (linear)
Second order: 1/[A] vs time (linear)
First order: ln[A] vs time (linear)
Second order: 1/[A] vs time (linear)
- Mon Mar 16, 2020 2:17 am
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Reverse rate Laws
- Replies: 3
- Views: 338
Re: Reverse rate Laws
If you know the K for the forward reaction and if you also have the overall K, you can then determine the reverse K and therefore the rate for the reverse reaction.
- Mon Mar 16, 2020 2:15 am
- Forum: Zero Order Reactions
- Topic: Half life
- Replies: 20
- Views: 1039
Re: Half life
t1/2=[A]0/2k
- Sun Mar 08, 2020 9:40 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Application
- Replies: 6
- Views: 505
Re: Application
I think we will usually be using:
G=-nFE
G=-RTlnQ
G=-nFE
G=-RTlnQ
- Sun Mar 08, 2020 9:29 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Vertical lines vs commas
- Replies: 7
- Views: 469
Re: Vertical lines vs commas
If they are in the same state, then use commas.
Ex: Fe3+(aq),Fe2+(aq)
If they are different states, then use the vertical lines to indicate that.
Ex: Fe3+(aq),Fe2+(aq)
If they are different states, then use the vertical lines to indicate that.
- Sun Mar 08, 2020 9:28 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Platinum
- Replies: 10
- Views: 676
Re: Platinum
If you have an ion-gas reaction or if you do not have a solid state metal/conductor on that part of the galvanic cell, then you need to include some kind of conductor like Pt(s)! Platinum is only one of them, but it's probably the most common one to use.
- Sun Mar 08, 2020 9:25 pm
- Forum: Balancing Redox Reactions
- Topic: e- amount
- Replies: 9
- Views: 663
Re: e- amount
You just need to multiply the reaction(s) to balance out the number of electrons, and then you should be able to cancel them out since they will then be equal.
- Mon Mar 02, 2020 11:18 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6L.7
- Replies: 3
- Views: 332
Re: 6L.7
Hi there! I think for this problem, we just have to look in the Appendix for half reactions that, when we combine them, will give us the resulting equation. Hope this helps!
- Sun Mar 01, 2020 9:41 pm
- Forum: Balancing Redox Reactions
- Topic: 6K.3d
- Replies: 5
- Views: 404
Re: 6K.3d
We know that Cl2 both oxidizes and reduces, so 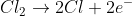
- Sun Mar 01, 2020 9:33 pm
- Forum: Balancing Redox Reactions
- Topic: anode/cathod reversible
- Replies: 4
- Views: 366
Re: anode/cathod reversible
My guess is, same as Nicholas, that we know that electrochem reactions proceed forward to give a positive Ecell value.
- Sun Mar 01, 2020 9:32 pm
- Forum: Balancing Redox Reactions
- Topic: basic solution
- Replies: 4
- Views: 329
Re: basic solution
In a basic solution, make sure whatever is being oxidized or reduced is balanced first, then begin to balance out the oxygens and later hydrogens with water. Then balance out the inequality that the H2O creates with water on the other side and then the same number of OH- ions on the side you origina...
- Sun Mar 01, 2020 9:30 pm
- Forum: Balancing Redox Reactions
- Topic: homework topic 6K
- Replies: 4
- Views: 380
Re: homework topic 6K
This is to ensure that both sides of the equation are balanced, based on whether the solution is acidic or basic. In this case, it appears to be acidic.
- Sun Mar 01, 2020 9:29 pm
- Forum: Balancing Redox Reactions
- Topic: 6K 1 part d
- Replies: 2
- Views: 261
Re: 6K 1 part d
The 14H+ becomes 8H+ because it cancels out with the 6H+ on the other side when you are combining the two half reactions. In addition, you get the 3 as the coefficient to the molecules with carbon because you have to multiply the oxidation half reaction, which has 2 electrons by 3 in order to cancel...
- Sun Feb 23, 2020 9:52 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: 3rd law of thermodynamics
- Replies: 3
- Views: 342
Re: 3rd law of thermodynamics
Yes, since at 0K atoms can't move, so there is no disorder since there is no other possible state/position that the atoms could be in, which means zero entropy.
- Sun Feb 23, 2020 9:42 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Limiting reactant and heat
- Replies: 4
- Views: 600
Re: Limiting reactant and heat
Endothermic reactions require heat, so if there is not enough heat provided to the system, then the reaction cannot continue. In this sense, heat can be thought of as the "limiting reactant".
- Sun Feb 23, 2020 9:41 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Standard Enthalpy of Formation
- Replies: 3
- Views: 356
Re: Standard Enthalpy of Formation
If you're asked for the enthalpy change of some reaction or whatever, you'll typically be provided with values that correspond to the standard enthalpies of formation for certain components of the reaction that you can then use in your calculations to determine enthalpy change of a reaction, etc.
- Sun Feb 23, 2020 9:38 pm
- Forum: Balancing Redox Reactions
- Topic: Oxidation Numbers/States
- Replies: 8
- Views: 547
Re: Oxidation Numbers/States
Oxidation state is the charge of the atom with ionic chemical bonds. Oxidation number is the Roman numerals that we use to represent the oxidation state of the atom.
- Sun Feb 23, 2020 9:35 pm
- Forum: Balancing Redox Reactions
- Topic: Polyatomic Ions
- Replies: 4
- Views: 322
Re: Polyatomic Ions
I think it's good practice to know a few of them, like SO42-, or CO32-... stuff like that.
- Sun Feb 16, 2020 10:03 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: q=n*delta H
- Replies: 5
- Views: 573
Re: q=n*delta H
Whether you use mass or the number of moles for these calculations just depends on what units the values you're given use, or if you're expected to have an answer with certain units.
- Sun Feb 16, 2020 9:59 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: change in enthalpy
- Replies: 2
- Views: 151
Re: change in enthalpy
Enthalpy is the heat transfer at a constant pressure... so keeping that in mind, that is why we use Cp.
- Sun Feb 16, 2020 9:58 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Work
- Replies: 14
- Views: 1023
Re: Work
Matthew Chan 1B wrote:When there is work done on a system, the work is negative. However, when the system itself does work, then work is positive.
Sorry, I meant it the other way around haha! Work is negative when the system does work. When the system has work done on it, then work is positive. Sorry about the typo!
- Sun Feb 16, 2020 9:56 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Negative work
- Replies: 14
- Views: 956
Re: Negative work
Work is negative when the system does work itself, which makes sense since the system is "losing" work/energy, which makes sense why it would be negative.
- Sun Feb 16, 2020 9:54 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Enthalpy and Heat
- Replies: 3
- Views: 312
Re: Enthalpy and Heat
Heat is the form of energy transfer from a one temperature to another, like from hot to cold. Enthalpy is the heat transfer at a constant pressure. So the heat added or lost by the system is the enthalpy change.
- Sun Feb 16, 2020 9:53 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Work
- Replies: 14
- Views: 1023
Re: Work
When there is work done on a system, the work is negative. However, when the system itself does work, then work is positive.
- Tue Feb 11, 2020 2:01 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: 4C.3
- Replies: 7
- Views: 298
Re: 4C.3
DHavo_1E wrote:Hello,
Can I ask why for constant volume we do not get an answer for the change in enthalpy? Thank you
This is because at a constant volume, qv =
- Sun Feb 09, 2020 6:33 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: 4B.13a
- Replies: 3
- Views: 143
Re: 4B.13a
Hey! The 101.325 J value is just a conversion that we should know. I think it's just good to memorize it as it's definitely important, since we measure work in joules and not L.atm!
- Sun Feb 09, 2020 6:31 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Textbook question 4B.3
- Replies: 3
- Views: 132
Re: Textbook question 4B.3
If this offers any reassurance, I also got 490 Joules for my answer. Either we're somehow all wrong or the solutions manual is wrong. Chem_Mod?
- Sun Feb 09, 2020 6:29 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Specific Heat Capacity
- Replies: 3
- Views: 267
Re: Specific Heat Capacity
Remember that \Delta H is always q, but q is not always \Delta H . We also have to keep in mind that \Delta H is only equal to q at a constant pressure. If we have constant volume, then q is equal to \Delta U . That being said, when we have a system that is being held at a constant pressure, we gene...
- Sun Feb 09, 2020 6:25 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Intensive vs Extensive
- Replies: 7
- Views: 367
Re: Intensive vs Extensive
I believe that extensive properties depend on how much of a sample you have, while intensive properties don't really depend on quantity of the sample or the size of the sample, etc.
- Sun Feb 09, 2020 6:23 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: irreversible and reversible
- Replies: 3
- Views: 293
Re: irreversible and reversible
When we have an irreversible reaction, there is a sudden change, since everything happens right away. It's like pulling the pin out of a piston and so the piston will immediately extend if there is some pressure within it after the pin is removed. When we have a reversible reaction, we have work whi...
- Sun Feb 02, 2020 3:42 pm
- Forum: Phase Changes & Related Calculations
- Topic: phase change heat supplied
- Replies: 3
- Views: 157
Re: phase change heat supplied
Transitioning from a liquid to a gas requires the breaking of bonds, which is why so much more energy is needed to make this change in comparison to the transition from solid to liquid.
- Sun Feb 02, 2020 3:41 pm
- Forum: Phase Changes & Related Calculations
- Topic: phase change from liquid to vapor
- Replies: 8
- Views: 370
Re: phase change from liquid to vapor
If you look at the heating curve of water, there is a much bigger "flat line" going from liquid to vapor than from solid to liquid, which basically tells us that there is more energy required to vaporize. With this higher amount of energy required to vaporize compared to transition from so...
- Sun Feb 02, 2020 3:38 pm
- Forum: Phase Changes & Related Calculations
- Topic: Internal Energy, U
- Replies: 6
- Views: 316
Re: Internal Energy, U
Only at constant pressure and volume! :)
- Sun Feb 02, 2020 3:37 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Value of q
- Replies: 11
- Views: 593
Re: Value of q
If there was a system where no energy was lost or gained/with all perfect conditions, q (of the system) = -q (of the surroundings). This is so that if there is any change in one, the other will inversely adapt/change, since if there is heat lost by the system, the surroundings will gain that heat.
- Sun Feb 02, 2020 3:34 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Calorimeter
- Replies: 3
- Views: 193
Re: Calorimeter
I think that the negative sign indicates that the reaction is exothermic, so the system itself is losing heat, while the surroundings are gaining heat.
- Mon Jan 27, 2020 12:01 am
- Forum: Phase Changes & Related Calculations
- Topic: Test 1
- Replies: 6
- Views: 234
Re: Test 1
Wait, they'll be returned in this week's discussions?
- Mon Jan 27, 2020 12:00 am
- Forum: Phase Changes & Related Calculations
- Topic: State Property
- Replies: 6
- Views: 206
Re: State Property
A state property is a quantity that is independent of how the substance was prepared
- Sun Jan 26, 2020 11:59 pm
- Forum: Phase Changes & Related Calculations
- Topic: Chem 14A Final Pickup
- Replies: 8
- Views: 372
Re: Chem 14A Final Pickup
You will still be able to pick them up thius week
- Sun Jan 26, 2020 11:59 pm
- Forum: Phase Changes & Related Calculations
- Topic: Hess's Law
- Replies: 5
- Views: 173
Re: Hess's Law
Hess's Law states that regardless of the multiple stages or steps of a reaction, the total enthalpy change for the reaction is the sum of all changes
- Sun Jan 26, 2020 11:58 pm
- Forum: Phase Changes & Related Calculations
- Topic: Steam vs water
- Replies: 5
- Views: 206
Steam vs water
Could someone explain the example that Lavelle went over with steam and why it has much more severe burns than liquid water?
- Sun Jan 19, 2020 5:59 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: lewis structure
- Replies: 11
- Views: 471
Re: lewis structure
Knowing how to draw the lewis structures won't specifically be tested (I think), but it will just help with being able to understand what happens during a reaction when doing problems with acids/bases, salts, etc. so that you can see the proton/electron transfers.
- Sun Jan 19, 2020 5:54 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: ICE Tables
- Replies: 13
- Views: 519
Re: ICE Tables
It's usually because H2O is in excess, which means that we cancel it out, since there's no real change to it. I think Lavelle gave the analogy: If someone has one million dollars, and they give ten dollars to someone else, we don't say that they have $999,990. We just say that they have about a mill...
- Sun Jan 19, 2020 5:51 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: small Ka
- Replies: 5
- Views: 207
Re: small Ka
As mentioned above, we only account for the Ka values for the weak acids, since we know that the strong acids basically dissociate 100%. Also, when x is 5% of the initial concentration, we can get rid of the x since it would be such a small negligible amount anyway.
- Sat Jan 18, 2020 5:54 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: K and Kc
- Replies: 3
- Views: 158
K and Kc
I was just wondering if we have to know how to convert between K and K c . It's done in Example 5H.1 Suppose you are a scientist studying the reactions of SO2 and O2. If you wish to use gas molar concentrations, you must first convert the equilibrium constant K to Kc. At 400 Celsius, the equilibrium...
- Wed Jan 15, 2020 2:00 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Endothermic and Exothermic Reactions Class Example
- Replies: 5
- Views: 209
Endothermic and Exothermic Reactions Class Example
I was wondering if someone could help explain the example that Dr. Lavelle gave in class when discussing the reaction from N2 to 2N. I understand that N2 is more stable and therefore it is favored, but then how does that make the reaction endothermic? Thanks!
- Sun Jan 12, 2020 3:07 pm
- Forum: Ideal Gases
- Topic: Units of Pressure
- Replies: 8
- Views: 299
Re: Units of Pressure
I think that we will be given these values on a constants sheet or something like that. Just make sure that when dealing with units of pressure, pick one unit and be consistent with it throughout the problem. Be sure to not interchange them.
- Sun Jan 12, 2020 3:04 pm
- Forum: Ideal Gases
- Topic: Bars vs atmospheres
- Replies: 13
- Views: 391
Re: Bars vs atmospheres
Both are units for measuring pressure. They are both okay to use, just make sure that you stay consistent with whichever unit you choose or with whatever is given in the problem.
- Sun Jan 12, 2020 3:03 pm
- Forum: Ideal Gases
- Topic: Test 1
- Replies: 7
- Views: 396
Re: Test 1
I think if we just look at the homework problems that he has assigned and look at the difficulty level of those, that would give us a pretty good idea of what the test would look like. Also I assume whatever has been mentioned in lecture up to Test 1 would be fair game as well.
- Sun Jan 12, 2020 3:02 pm
- Forum: Ideal Gases
- Topic: Partial Pressure
- Replies: 4
- Views: 160
Re: Partial Pressure
Yeah, as Tanmay said above, I don't think that we have to know how to calculate it yet.
- Sun Jan 12, 2020 2:59 pm
- Forum: Ideal Gases
- Topic: R in PV=nRT
- Replies: 34
- Views: 6717
Re: R in PV=nRT
R just represents the gas constant, which is 8.314 J.mol-1.K-1
- Sun Dec 08, 2019 4:13 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Oxalate
- Replies: 2
- Views: 261
Re: Oxalate
Oxalate follows the polydentate "template" that Dr. Lavelle mentioned: it has atom--spacer atom--spacer atom--atom. We can see that as O--C--C--O and the O's are the atoms that bind to the metal. Therefore, it's bidentate.
- Sun Dec 08, 2019 4:11 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Hydrogen Bonding
- Replies: 2
- Views: 300
Hydrogen Bonding
In water, can SO3 (sulfur trioxide) form hydrogen bonds with the water molecules?
- Sat Dec 07, 2019 1:07 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: chelating ligands [ENDORSED]
- Replies: 51
- Views: 98913
Re: chelating ligands [ENDORSED]
Elizabeth Harty 3A wrote:How do you know if there are sigma bonds available for rotation?
dont sigma bonds already have the ability to rotate? so if its just a single sigma bond then it can rotate but if theres pi bonds then you cant rotate. did i answer your question?
- Sat Dec 07, 2019 1:33 am
- Forum: Administrative Questions and Class Announcements
- Topic: MARSHMALLOW- FINAL REVIEW SESSION [ENDORSED]
- Replies: 115
- Views: 13303
Re: MARSHMALLOW- FINAL REVIEW SESSION [ENDORSED]
For the Mini marshmallow worksheet, for problem 2, we are asked to write the name/formula for the coordination compound, etc. in problem 2a, the coordination compound is [Ni(NH 3 ) 2 O 2 ]Br 2 . However, in the answer key, the compound is something completely different. I just wanted to check to see...
- Fri Dec 06, 2019 1:11 am
- Forum: Sigma & Pi Bonds
- Topic: Marshmallow 41.C
- Replies: 3
- Views: 424
Re: Marshmallow 41.C
the C=C bond is the strongest because atoms that share the same/similar energies that overlap will have the strongest bonds. Thus, the homonuclear bond between C=C is the strongest out of the other options. I think this is correct... but not entirely sure
- Sat Nov 30, 2019 3:41 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: chelating ligands [ENDORSED]
- Replies: 51
- Views: 98913
chelating ligands [ENDORSED]
How would you determine whether a ligand can bind at multiple sites (or be chelating?) Is there a certain angle threshold? For example like in 9C.7 (I've attached an image of the isomers of diaminobenzene that the book uses). Note that B and C should be switched around to represent what the textbook...
Re: 9C3d
The OH2 is just to draw attention to the fact that the oxygen is what is contributing the electrons to the central metal atom
- Sat Nov 30, 2019 2:05 am
- Forum: Naming
- Topic: Ligand Order
- Replies: 5
- Views: 368
Re: Ligand Order
Do we arrange on the basis of the element's letters or the names of element/compounds?
- Sat Nov 30, 2019 2:02 am
- Forum: Naming
- Topic: -ido vs -o
- Replies: 5
- Views: 345
Re: -ido vs -o
Are we still allowed to use -o like fluoro and chloro rather than -ido?
- Sat Nov 30, 2019 2:00 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Ligands
- Replies: 6
- Views: 456
Re: Ligands
Yes, ligands are considered lewis bases because they are donating an electron pair.
- Sun Nov 24, 2019 11:36 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: IMF [ENDORSED]
- Replies: 6
- Views: 949
Re: IMF [ENDORSED]
Induced dipole is when there is a molecule that is not polar or has no dipole moment, but then it experiences a temporary dipole moment because of the dipole moment of another molecule. A dipole dipole moment is where there are two molecules that experience attraction between the partially positive...
- Sun Nov 24, 2019 8:42 pm
- Forum: Hybridization
- Topic: 2.61 Radicals
- Replies: 1
- Views: 163
2.61 Radicals
In Focus 2.61, it asks you to draw the Lewis structure for HOCO, and to identify whether or not it is a radical. Evidently, the lewis structure for HOCO results in: H-O-C=O, with the Carbon having a single electron on it, or a radical. How do we know that carbon is the atom that will have the single...
- Sun Nov 24, 2019 2:40 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: IMF [ENDORSED]
- Replies: 6
- Views: 949
Re: IMF [ENDORSED]
Induced dipole is when there is a molecule that is not polar or has no dipole moment, but then it experiences a temporary dipole moment because of the dipole moment of another molecule. A dipole dipole moment is where there are two molecules that experience attraction between the partially positive ...
- Sun Nov 24, 2019 2:36 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Bronsted Acids/ Lewis Acids
- Replies: 2
- Views: 252
Re: Bronsted Acids/ Lewis Acids
Lewis acids and bases are defined as being able to accept or donate electron pairs, while Bronsted Lowry acids and bases are defined as being able to accept or donate hydrogen ions (H+).
- Sun Nov 24, 2019 1:47 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Chelating complexes
- Replies: 4
- Views: 248
Re: Chelating complexes
A chelating complex is a complex that contains a ligand that forms a ring of atoms that includes the central metal atom.
- Sun Nov 24, 2019 1:41 pm
- Forum: Biological Examples
- Topic: Heme complex
- Replies: 8
- Views: 682
Heme complex
Can someone explain what the Heme complex is and what significance/applications it has? Thanks.
- Sun Nov 24, 2019 1:39 pm
- Forum: Hybridization
- Topic: Knowing when hybridization occurs
- Replies: 6
- Views: 430
Re: Knowing when hybridization occurs
Orbitals hybridize whenever the resulting molecule will be lower in energy.
- Sun Nov 17, 2019 10:00 pm
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: Simpler Terms
- Replies: 4
- Views: 763
Re: Simpler Terms
Paramagnetic compounds have unpaired electrons while diamagnetic compounds the electrons all have paired spins.
- Sun Nov 17, 2019 9:57 pm
- Forum: Hybridization
- Topic: Test 2
- Replies: 11
- Views: 605
Test 2
If I have discussion on Tuesday, will Hybridization be on Test 2? Is it something that's going to be discussed Monday lecture?
- Sun Nov 17, 2019 9:56 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bond Angles
- Replies: 4
- Views: 264
Re: Bond Angles
With enough practice and being able to determine if something is bent or linear, etc. you can likely determine the correct bond angles because after lots of practice, it should be second-nature to remember what bonds correlate with what shapes.
- Sun Nov 17, 2019 9:53 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: london forces
- Replies: 5
- Views: 247
Re: london forces
Due to electrons always moving around and never staying in one set place, they sometimes create temporary dipole moments, which is why there are london forces in every bond.
- Sun Nov 17, 2019 9:51 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: dipole moments
- Replies: 11
- Views: 653
Re: dipole moments
Dipole moments cancel when there are moments facing in opposite directions, effectively canceling each other out.
- Sun Nov 17, 2019 9:51 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: vsepr angles
- Replies: 10
- Views: 492
vsepr angles
This is kind of a dumb question, but do we have to memorize the bond angles?
- Wed Nov 06, 2019 10:44 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Large Anion + Small Cation
- Replies: 2
- Views: 450
Re: Large Anion + Small Cation
When there is a large difference in the electronegativity between two elements, the electrons from the large electron dense regions of the large anion are pulled away from that region and (more electrons = more shielding, so electrons are held less tightly) closer to the small cation and the electro...
- Wed Nov 06, 2019 10:34 pm
- Forum: Dipole Moments
- Topic: Hydrogen Dipoles
- Replies: 3
- Views: 159
Re: Hydrogen Dipoles
We need to draw dipole moments for these bonds between the atoms, as there will inevitably be some pull of electrons one direction or the other between two atoms. Then, looking at the broader picture, we can eventually determine if the moments cancel each other out or overall point in a certain dire...
- Wed Nov 06, 2019 10:27 pm
- Forum: Resonance Structures
- Topic: resonance importance?
- Replies: 7
- Views: 460
Re: resonance importance?
Electrons don't actually stay in place like how they look in Lewis structures. They are not stationary. They are constantly moving around and thus, the resonance structures allow us to get a better picture of what the actual molecule looks like, since resonance depicts how the electrons may move aro...
- Wed Nov 06, 2019 10:25 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Central atom: formal charge v electronegativity
- Replies: 3
- Views: 242
Re: Central atom: formal charge v electronegativity
You should put the more electronegative element in the center, and then try and minimize formal charges of that atom and the atoms that surround it.
- Wed Nov 06, 2019 10:20 pm
- Forum: Dipole Moments
- Topic: Dipole moments
- Replies: 5
- Views: 428
Re: Dipole moments
Dipole moments arise from differences in electronegativity in a covalent bond between two atoms. The arrow of the dipole moment is generally pointed towards the element that is more electronegative. They represent the direction in which the electrons are pulled toward, given the differences in elect...
- Sat Nov 02, 2019 8:21 pm
- Forum: Bond Lengths & Energies
- Topic: Electron Density and Bond Length
- Replies: 2
- Views: 395
Electron Density and Bond Length
Can someone explain why a bigger electron density region means that there will be a longer bond?
- Sat Nov 02, 2019 8:02 pm
- Forum: Electronegativity
- Topic: electronegativity
- Replies: 3
- Views: 158
Re: electronegativity
On the periodic table that we got for test 1, we didn't get electronegativity values. However, if we're just comparing values, we have to know the trends of electronegativity across the periodic table. The trend is that electronegativity increases from left to right, and increases from bottom to top...
- Sat Nov 02, 2019 7:57 pm
- Forum: Sigma & Pi Bonds
- Topic: sigma and pi bonds question
- Replies: 3
- Views: 205
sigma and pi bonds question
Do we have to know about sigma and pi bonds for the upcoming midterm? I don't remember anything being said in class, but I see this topic thread, so I'm not sure now. Does anyone know? Thanks.
- Sat Nov 02, 2019 7:52 pm
- Forum: Lewis Structures
- Topic: Drawing Structures for Ionic Bonds
- Replies: 2
- Views: 121
Re: Drawing Structures for Ionic Bonds
You would draw the lewis structure of each ion and draw them next to each other.
- Sat Nov 02, 2019 7:51 pm
- Forum: Lewis Structures
- Topic: Formal Charge for Lewis Structures
- Replies: 4
- Views: 275
Re: Formal Charge for Lewis Structures
My TA told me whenever drawing Lewis structures, we should always try and calculate formal charges. We should do this to make sure we're drawing the most accurate structure that we can.
- Sat Oct 26, 2019 3:37 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: ionization energy and electron affinty
- Replies: 3
- Views: 181
Re: ionization energy and electron affinty
Ionization Energy: the energy required to remove an electron from a gas-phase atom
Electron Affinity: the energy released when an electron is added to a gas-phase atom
Electron Affinity: the energy released when an electron is added to a gas-phase atom
- Sat Oct 26, 2019 3:31 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Radial Distribution Function
- Replies: 4
- Views: 245
Re: Radial Distribution Function
I don't think that it was explicitly mentioned in the outline for the Quantum World unit, and I never heard him state it in class.
I could be wrong, though.
I could be wrong, though.
- Sat Oct 26, 2019 3:30 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Electron Configuration Exceptions
- Replies: 2
- Views: 337
Re: Electron Configuration Exceptions
Chromium: [Ar]3d 5 4s 1 Takes the electron from the 4s subshell and adds it to the 3d subshell because 3d will be more stable with that fifth electron, since it will now be half filled. Copper: [Ar]3d 10 4s 1 Takes the electron from the 4s subshell and adds it to the 3d subshell because 3d will be m...
- Sat Oct 26, 2019 3:23 pm
- Forum: Trends in The Periodic Table
- Topic: First vs. Second Ionization Energies
- Replies: 2
- Views: 136
Re: First vs. Second Ionization Energies
The second ionization energy is higher than the first ionization energy because, in short, it takes more energy to remove an electron from a positively-charged atom (cation) than it does to remove an electron from a neutral atom. When an electron is removed, that means there's more protons than elec...
- Sat Oct 26, 2019 3:20 pm
- Forum: Trends in The Periodic Table
- Topic: Effective Nuclear Charge and Ionization Energy
- Replies: 2
- Views: 164
Re: Effective Nuclear Charge and Ionization Energy
Effective nuclear charge means the amount of force that the electrons in the valence shell experience. And so, as the effective nuclear charge increases, that means the electrons are being held on to more tightly, which means that it will require more energy to remove the electrons from the valence ...
- Sat Oct 19, 2019 5:49 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Determining # of Subshells in an Orbital
- Replies: 3
- Views: 263
Re: Determining # of Subshells in an Orbital
Subshells, aka the angular momentum quantum number, are represented by the letter l . We calculate l by taking our principle quantum number n , and subtracting one from it. l = n -1. Then, to calculate how many orbitals are in the subshell, we take the value of l and all numbers equal to and in betw...
- Sat Oct 19, 2019 11:56 am
- Forum: *Shrodinger Equation
- Topic: Psi ^2
- Replies: 2
- Views: 100
Re: Psi ^2
I think that it's something we just have to accept as fact. It's a pretty complicated mathematical equation, but basically it's said that the probability of obtaining any possible measurement outcome is equal to the square of the corresponding amplitude. Not sure, but hopefully this helps. Also, Tif...
- Sat Oct 19, 2019 11:45 am
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: HW 1B.27
- Replies: 2
- Views: 154
Re: HW 1B.27
There is an error in the solution manual. Check Dr. Lavelle's solution manual errors PDF on his website; he addresses that mistake.
https://lavelle.chem.ucla.edu/wp-conten ... rs_7Ed.pdf
https://lavelle.chem.ucla.edu/wp-conten ... rs_7Ed.pdf
- Sat Oct 19, 2019 11:43 am
- Forum: *Particle in a Box
- Topic: Shape of Wavefunction
- Replies: 4
- Views: 1419
Re: Shape of Wavefunction
- Sat Oct 19, 2019 11:39 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Shell/Orbital Energies
- Replies: 2
- Views: 92
Re: Shell/Orbital Energies
Higher numbered shells (like n=2 to n=1) have higher energy because of the force that it takes for the nucleus to hold onto the electron. When the electron is closer, it takes less energy to keep the electron. However, as the electrons are further out/further away from the nucleus, it takes more ene...
- Thu Oct 17, 2019 11:44 pm
- Forum: Photoelectric Effect
- Topic: Quick Question
- Replies: 3
- Views: 139
Quick Question
I was wondering if ionization energy and threshold energy are related. Are they the same? Or is there a difference? The textbook gives a definition that seems pretty similar to what threshold energy is. Thanks in advance for clarifying.
- Sun Oct 13, 2019 11:33 pm
- Forum: Photoelectric Effect
- Topic: Threshold Energy
- Replies: 4
- Views: 251
Re: Threshold Energy
Yes, different elements will hold onto their electrons with different amounts of strength. Thus, we can say that the threshold energy does indeed vary between elements.
- Sun Oct 13, 2019 11:31 pm
- Forum: Photoelectric Effect
- Topic: Intensity vs. Length of Waves
- Replies: 4
- Views: 244
Re: Intensity vs. Length of Waves
A shorter wavelength means that the energy of the radiation increases. Fluctuation in the wavelength of the radiation affects the energy. Intensity is not affected by changing the frequency/wavelength. You can think of the intensity as the amplitude of the wave. Hope this helps.
- Sun Oct 13, 2019 11:26 pm
- Forum: Photoelectric Effect
- Topic: Photoelectric Effect
- Replies: 3
- Views: 223
Re: Photoelectric Effect
Non-metals will not exhibit the photoelectric effect due to the fact that they do not have any 'spare' electrons in their outer shells. However, metals do contain these 'spare' electrons. These electrons are the ones that can be dislodged/removed by a photon of light that has sufficient energy to do...
- Sun Oct 13, 2019 11:22 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Question about 1A.15
- Replies: 2
- Views: 182
Question about 1A.15
While I was working through this problem, I wasn't sure how I was supposed to already know that n 1 =1. The problem reads: In the ultraviolet spectrum of atomic hydrogen, a line is observed at 102.6 nm. Determine the values of n for the initial and final energy levels of the electron during the emis...

