Search found 109 matches
- Sun Mar 15, 2020 6:27 am
- Forum: Administrative Questions and Class Announcements
- Topic: final exam 2020
- Replies: 2
- Views: 359
Re: final exam 2020
Dr. Lavelle has been great with updating us on all the adjustments he has been working hard to make, so check your email and you'll find all the info from him. Everything has been moved to online and we will be taking the final exam through CCLE.
- Sun Mar 15, 2020 6:25 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 7B.3
- Replies: 2
- Views: 301
Re: 7B.3
It tells you that it is a first order reaction, and therefore you know to use the equation ln[A] = -kt + ln[A]0
Make sure that you molarity is in relation to the reactant, and then you just plug in what you know to solve for k, which is the constant.
Make sure that you molarity is in relation to the reactant, and then you just plug in what you know to solve for k, which is the constant.
- Sun Mar 15, 2020 6:21 am
- Forum: Balancing Redox Reactions
- Topic: Pt in Cell Diagram
- Replies: 14
- Views: 949
Re: Pt in Cell Diagram
whenever there is no other conducting solid, Pt is usually used.
- Sun Mar 15, 2020 6:16 am
- Forum: Balancing Redox Reactions
- Topic: oh
- Replies: 11
- Views: 828
Re: oh
The main reason OH would be in a chemical equation would be for basic solutions. In general always use H2O to add oxygen. If it is a basic solution, what I usually do is wait until everything is balanced according to an acidic solution, then I add an OH for every H+ on both sides, and cancel out the...
- Sun Mar 15, 2020 6:07 am
- Forum: Administrative Questions and Class Announcements
- Topic: Printing for Final
- Replies: 9
- Views: 744
Re: Printing for Final
No printing needed! Just log on to CCLE and everything will be accessible there, starting at 11:15 am.
- Sun Mar 15, 2020 6:06 am
- Forum: Balancing Redox Reactions
- Topic: Test 2 Return
- Replies: 20
- Views: 1226
Re: Test 2 Return
Depends on the TA, some might leave them in mailboxes for pickup or schedule a time when you can get it from them.
- Sun Mar 15, 2020 6:01 am
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: What was your favorite chem topic?
- Replies: 137
- Views: 11638
Re: What was your favorite chem topic?
Probably Electrochemistry, initially I thought cell diagrams were impossible to understand but after learning them I finally began to enjoy it lol
- Sun Mar 15, 2020 5:58 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Catalysts
- Replies: 3
- Views: 297
Re: Catalysts
Dr. Lavelle also states during lecture that catalysts are not formed, but used up. Therefore, a reactant would be a good example of this since it is not the product of the reaction.
- Fri Mar 13, 2020 11:51 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Reaction Rate
- Replies: 2
- Views: 209
Reaction Rate
Are reaction rates only determined by the reactants of the slow step?
- Thu Mar 12, 2020 9:06 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Methods
- Replies: 3
- Views: 259
Methods
Which method did Dr. Lavelle say we should be using? Pre-equilibrium or Steady-state?
- Tue Mar 10, 2020 8:43 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Chemistry 14B Final
- Replies: 9
- Views: 745
Re: Chemistry 14B Final
If lectures are supposed to be online, where will we find it? Will they be Bruincasted?
- Mon Mar 09, 2020 3:34 pm
- Forum: General Rate Laws
- Topic: 7B9 part a
- Replies: 2
- Views: 300
7B9 part a
7B.9 For the first-order reaction A -> 3B + C, when [A]0 = 0.015 mol.L-1, the concentration of B increases to 0.018 mol.L-1 in 3.0 min. (a) What is the rate constant for the reaction expressed as the rate of loss of A? For part a, I did .018/3 = .006 to get the amount that C would increase as well, ...
- Sat Mar 07, 2020 10:55 pm
- Forum: General Rate Laws
- Topic: 7A.17 part C
- Replies: 3
- Views: 323
Re: 5A.17 part C
Hi, could you please include the entire problem in your posts, both this and future posts? My copy of the textbook does not appear to have a 5A.17 Sorry! I meant 7A. 17 The following data were obtained for the reaction A + B + C -> products, with A in first order and B and C in second order. It giv...
- Sat Mar 07, 2020 1:21 pm
- Forum: General Rate Laws
- Topic: 7A.17 part C
- Replies: 3
- Views: 323
7A.17 part C
For 5A.17, I used the formula to calculate for k. However, my answer is 2.85 while the book says it is 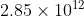
Is there some conversion that I am missing in this problem?
Is there some conversion that I am missing in this problem?
- Sat Mar 07, 2020 11:44 am
- Forum: General Rate Laws
- Topic: 7A. 7 Homework Problem
- Replies: 2
- Views: 287
Re: 7A. 7 Homework Problem
Nevermind! I just watched this video
https://www.youtube.com/watch?v=1g-vDSWYins
which really helped me understand, we are supposed to use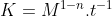
https://www.youtube.com/watch?v=1g-vDSWYins
which really helped me understand, we are supposed to use
- Sat Mar 07, 2020 11:37 am
- Forum: General Rate Laws
- Topic: 7A. 7 Homework Problem
- Replies: 2
- Views: 287
7A. 7 Homework Problem
7A.7 says Express the units for rate constants when the concentrations are in moles per liter and time is in seconds for (a) zeroth-order reactions; (b) first-order reactions; (c) second-order reactions.
Is this just memorization? Or do we use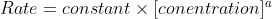 ?
?
Is this just memorization? Or do we use
- Wed Mar 04, 2020 6:03 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 6N 13
- Replies: 4
- Views: 766
6N 13
6N.13 Calculate the reaction quotient, Q, for the following cell reactions, given the measured values of the cell potential. Balance the chemical equations by using the smallest whole-number coefficients. (a) Pt(s) Sn4+(aq),Sn2+(aq) Pb4+(aq),Pb2+(aq) C(gr), Ecell = +1.33 V. I'm having a little troub...
- Wed Mar 04, 2020 12:36 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 6N5 part a
- Replies: 1
- Views: 184
6N5 part a
6N5.a asks for the PH in the equation (a) Pt(s) H2(g, 1.0 bar) H+(pH ?) (salt bridge) Cl2(aq, 1.0 mol.L-1) Hg2Cl2(s) Hg(l), Ecell = +0.33 V. Using the Nerst equation, I came up with 0.33 = .27 - (.05916/2)(log(1/ x^{2} )) After solving I found the pH to be 10, yet the answer is 1. What am I doing wr...
- Tue Mar 03, 2020 11:20 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 6N.1.b)
- Replies: 4
- Views: 311
Re: 6N.1.b)
Justin Sarquiz 2F wrote:I think the book made a mistake because I got K=107. The book said that 2 electrons were transferred, but it should be that one electron was transferred.
I also got K=107
- Mon Mar 02, 2020 11:40 pm
- Forum: Van't Hoff Equation
- Topic: Hw question 5J.15
- Replies: 2
- Views: 337
Re: Hw question 5J.15
I was looking at this question in other chemistry posts, and someone said we have to use the equation ln(K2/K1) = (deltaH/R)[(1/T1) - (1/T2)] Though I am not really sure how to apply it.
- Mon Mar 02, 2020 10:36 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6M13 part d
- Replies: 1
- Views: 172
6M13 part d
part d of 6M.13 asks us to calculate the cell potential for 2 NO3-(aq) + 4H+(aq) + Zn(s) --> Zn2+(aq) + 2 NO2(g) + 2H2O(l) To calculate this, I found the reduction potential of NO3- (0.96) and added it to the oxidation potention of Zn (0.76). However, that gives me 1.72V which is different from the ...
- Sun Mar 01, 2020 8:18 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6M.5
- Replies: 4
- Views: 365
Re: 6M.5
To determine which one is the cathode or anode, look at the cell potentials of each half-reaction from appendix 2b. The one with the higher cell potential is the cathode. In this case, NO3 -/NO is the cathode because .96 > .79.
- Fri Feb 28, 2020 10:25 am
- Forum: Administrative Questions and Class Announcements
- Topic: Test 2
- Replies: 5
- Views: 376
Test 2
Will content from 5G be on Test 2?
- Tue Feb 25, 2020 12:36 pm
- Forum: Balancing Redox Reactions
- Topic: Redox rxns- differences for solving?
- Replies: 2
- Views: 194
Redox rxns- differences for solving?
Are there different techniques we should use for balancing a redox reaction in bronsted neutralization and solubility?
- Tue Feb 25, 2020 12:26 pm
- Forum: Balancing Redox Reactions
- Topic: 6L. 7 Part C
- Replies: 1
- Views: 128
6L. 7 Part C
6L.7 Part C asks to balance the redox reaction in a nickel-cadmium cell as follows:
Cd(s) + 2 Ni(OH)3(s) -> Cd(OH)2(s) + 2 Ni(OH)2(s)
How do we know to assume that it is occurring in a basic solution?
Cd(s) + 2 Ni(OH)3(s) -> Cd(OH)2(s) + 2 Ni(OH)2(s)
How do we know to assume that it is occurring in a basic solution?
- Thu Feb 20, 2020 6:42 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Gibbs free energy equation [ENDORSED]
- Replies: 1
- Views: 96
Gibbs free energy equation [ENDORSED]
For G = -nFE, how should we calculate n? Is it just the change in moles (n final - n initial)? Thanks in advance!
- Thu Feb 20, 2020 4:46 pm
- Forum: Balancing Redox Reactions
- Topic: 6K3, part d
- Replies: 2
- Views: 136
6K3, part d
Part d asks to balance the redox reaction of Cl2(g) -> HClO(aq) + Cl2(g) . In the answer key, the product is Cl- . When would we know that it is okay to change the products?
- Tue Feb 11, 2020 9:42 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Enthalpy and Heat
- Replies: 4
- Views: 440
Enthalpy and Heat
What is the difference between H and q? When would they be equal?
- Sun Feb 09, 2020 8:10 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Midterm Content
- Replies: 6
- Views: 384
Midterm Content
Will the midterm include questions and problems from topic 4J?
- Sun Feb 09, 2020 7:20 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: 4I.3: Negative Entropy?
- Replies: 1
- Views: 128
4I.3: Negative Entropy?
4I.3 states The standard entropy of vaporization of benzene is approximately 85 J.K-1.mol-1 at its boiling point. (b) What is the standard entropy change of the surroundings when 10. g of benzene, C6H6, vaporizes at its boiling point?
Why is the answer to part b negative?
Why is the answer to part b negative?
- Fri Feb 07, 2020 11:40 pm
- Forum: Phase Changes & Related Calculations
- Topic: 4C.13 phase change
- Replies: 2
- Views: 161
4C.13 phase change
4C.13 says, "An ice cube of mass 50.0 g at 0.0 8C is added to a glass containing 400.0 g of water at 45.0 8C. What is the final temperature of the system?" I was wondering why, when calculating the heat absorbed, do we add (50g)(4.184J.°C-1.g-1)(Tfinal) to (n)(deltaH) ? I was solving the p...
- Fri Feb 07, 2020 8:23 pm
- Forum: Calculating Work of Expansion
- Topic: Units for Work
- Replies: 5
- Views: 274
Re: Units for Work
I just looked it up and there is a chart in the textbook which explains that the pressure should be in Pa, and volume in m^3, so no conversions need to be done for this this equation.
- Fri Feb 07, 2020 7:46 pm
- Forum: Calculating Work of Expansion
- Topic: Units for Work
- Replies: 5
- Views: 274
Units for Work
For 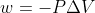 , are we expected to memorize that there are 101.325 J in 1L.atm ?
, are we expected to memorize that there are 101.325 J in 1L.atm ?
- Fri Feb 07, 2020 3:23 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Entropy Surroundings
- Replies: 4
- Views: 237
Entropy Surroundings
When would the change in entropy of the surroundings be 0?
- Fri Feb 07, 2020 3:11 pm
- Forum: Phase Changes & Related Calculations
- Topic: Negative Work
- Replies: 18
- Views: 1498
Negative Work
In the homework problem 4B.7, the answer for work is -1626. However, I got 1626. When should I know to make work negative?
- Thu Jan 30, 2020 12:05 pm
- Forum: Phase Changes & Related Calculations
- Topic: 4A.3
- Replies: 3
- Views: 171
Re: 4A.3
The purpose of using 101.325 is to convert atm to Pa. In SI units, Pa is J.m^-3 . The equation used is w=PAD, and work is in joules, so the units would essential cancel out to joules because w=(J.m^3)(m^2)(m) .
- Thu Jan 30, 2020 12:01 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Celcius and Kelvin
- Replies: 11
- Views: 458
Re: Celcius and Kelvin
No need to memorize, the number is on the constants and equations sheet.
- Thu Jan 30, 2020 12:00 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Qv vs Qp
- Replies: 7
- Views: 161
Re: Qv vs Qp
qv implies constant volume, whereas qp is constant pressure.
- Thu Jan 30, 2020 10:21 am
- Forum: Calculating Work of Expansion
- Topic: 4A.3
- Replies: 8
- Views: 443
Re: 4A.3
Should we always convert to J (using the 101325 Pa value) on exams? Correct me if I'm wrong, but I don't recall hearing that in lecture? I didn't hear him talk about it either in lecture, but it makes sense because work is equal to J and you have to make sure the units work out. Pa is equal to J.m^...
- Thu Jan 30, 2020 10:14 am
- Forum: Calculating Work of Expansion
- Topic: 4A.3
- Replies: 8
- Views: 443
Re: 4A.3
I did this problem yet I keep getting my final answer as 28.6, which would round up to 29. The answer says it' supposed to be 28. Has anyone else been getting the same thing?
- Tue Jan 28, 2020 10:27 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Types of Systems
- Replies: 3
- Views: 148
Types of Systems
In homework problem 4A part c, the answer key states that a bomb calorimeter in which benzene is burned is an isolated system. Can someone explain this to me?
- Mon Jan 20, 2020 8:20 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: 5.39 HW
- Replies: 2
- Views: 175
5.39 HW
5.39 states, In an experiment, 0.020 mol NO2 was introduced into a flask of volume 1.00 L and the reaction 2 NO2(g) ∆ N2O4(g) was allowed to come to equilibrium at 298 K. (a) Using information in Table 5E.2, calculate the equilibrium concentrations of the two gases. (b) The volume of the flask is re...
- Thu Jan 16, 2020 11:30 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Factos effecting Equilibrium
- Replies: 6
- Views: 261
Re: Factos effecting Equilibrium
Only the stoichiometric coefficients and temperature can change K. Concentrations do not effect K, because a change in concentration only mean that the reaction has to adjust to move back to equilibrium.
- Thu Jan 16, 2020 11:25 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kc vs Kp
- Replies: 5
- Views: 215
Re: Kc vs Kp
Kc can also be used with gases, I think in the problem they will specifically ask for Kc/Kp, otherwise, Lyndon told us in a workshop that Kc is usually used.
- Thu Jan 16, 2020 11:20 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6A19 part c
- Replies: 5
- Views: 195
Re: 6A19 part c
005391550 wrote:Do you mean (1.0x10^-14)/3.1? Because I'm not sure where you got the 3.2x10^-14
Thanks! Yeah I meant that, I just fixed the post.
- Wed Jan 15, 2020 5:50 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6A19 part c
- Replies: 5
- Views: 195
6A19 part c
I was trying to solve part c of 6A19, however, I got \div (3.1)=3.2\times 10^{-15}) The book says the answer is
The book says the answer is 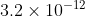 . Is anyone also getting the same thing? Or are my calculations wrong? Thanks in advance!
. Is anyone also getting the same thing? Or are my calculations wrong? Thanks in advance!
- Fri Jan 10, 2020 2:55 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Le Chatelier's Principle
- Replies: 7
- Views: 313
Re: Le Chatelier's Principle
Yes, it basically shows that a chemical reaction can adjust in order to minimize the effect and go back to equilibrium.
- Fri Jan 10, 2020 2:53 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Why Q would be greater than K
- Replies: 5
- Views: 148
Re: Why Q would be greater than K
Q in itself is the reaction quotient at any time, so initially, a large amount of product was added the reaction would continue in the reverse direction because Q > K.
- Fri Jan 10, 2020 2:51 pm
- Forum: Ideal Gases
- Topic: pressure [ENDORSED]
- Replies: 13
- Views: 1119
Re: pressure [ENDORSED]
PV=nRT is a good formula that shows the relationship between pressure, volume, temperature, concentration, and moles. Increasing moles and temperature will increase the pressure.
- Tue Jan 07, 2020 5:13 pm
- Forum: Ideal Gases
- Topic: Review Ideal Gases
- Replies: 6
- Views: 336
Review Ideal Gases
On the syllabus, it says we should review ideal gases. Does any have any tips on how we should do that? Thanks in advance!
- Tue Jan 07, 2020 4:19 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Concentrations at Equilibrium
- Replies: 6
- Views: 250
Concentrations at Equilibrium
At equilibrium, are the concentrations of reactants and products the same?
- Thu Dec 05, 2019 9:06 pm
- Forum: Polyprotic Acids & Bases
- Topic: Strength of Acids
- Replies: 3
- Views: 254
Strength of Acids
So on homework problem 6C 19 part c it asks for thee stronger acid between HBrO2 and HClO2. Can someone explain to me why the answer is HClO2? I thought that because bromium's electronegativity is lower is would be more willing to give up H and therefore be a stronger acid.
- Wed Dec 04, 2019 6:12 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: 6B 1
- Replies: 2
- Views: 176
6B 1
The question asks for change in pH when HCL concentration is reduced by 12%. I saw a previous chem community post where they plugged in -log(.12) to find the answer, but why don't we plug in H+ (pH= -log([.88])) since it is asking for the value of the change?
- Wed Dec 04, 2019 4:24 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: 6B.1
- Replies: 3
- Views: 359
Re: 6B.1
The pH equation is -log([H+]). The equation for change is -log(change in concentration / original concentration). In this case: -log(0.12*[HCl] / [HCl]). We don't need the actual value of [HCl], we can just assume it's 1 to represent the original value. Thus, we have -log(0.12) = 0.92 Why don't we ...
- Wed Dec 04, 2019 2:30 pm
- Forum: Naming
- Topic: Naming Acids/ Bases
- Replies: 4
- Views: 414
Re: Naming Acids/ Bases
Is there a difference between nitric acid and nitrous acid?
- Wed Dec 04, 2019 2:28 pm
- Forum: Naming
- Topic: Naming Acids/ Bases
- Replies: 4
- Views: 414
Naming Acids/ Bases
Should we know how to name acids/ bases for the final? For example when to use -ic versus -ous in an acid?
- Wed Dec 04, 2019 12:04 pm
- Forum: Hybridization
- Topic: Hybridization
- Replies: 2
- Views: 229
Hybridization
In question 2F 13, it gives the molecule CH2CHCN and asks us to find the hybridization of each carbon atom. To what extent do we take into account double bonds when determining hybridization? Are the shared electrons with double bonds spin paired?
- Tue Dec 03, 2019 5:39 pm
- Forum: Bronsted Acids & Bases
- Topic: Memorization
- Replies: 1
- Views: 147
Memorization
What are common acids and bases that we should memorize in order to come up with reactions?
- Tue Dec 03, 2019 11:38 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Coordination Number
- Replies: 6
- Views: 202
Coordination Number
How can I determine the coordination number without drawing the Lewis Structure?
- Mon Dec 02, 2019 11:52 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Polydentate
- Replies: 3
- Views: 286
Polydentate
How can one determine the number of sites to which a ligand can combine to create a polydentate?
- Mon Dec 02, 2019 11:27 pm
- Forum: Naming
- Topic: Cyano vs. Cyanido
- Replies: 2
- Views: 167
Cyano vs. Cyanido
What is the difference between cyano and cyanido?
- Mon Dec 02, 2019 11:18 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: SO4
- Replies: 3
- Views: 372
SO4
If we are given SO4, do we automatically assume the need to create octets, even if it means adding a charge?
- Mon Dec 02, 2019 8:13 am
- Forum: Naming
- Topic: Oxidation States
- Replies: 3
- Views: 327
Oxidation States
How do I find the oxidation state of a molecule? Does it require drawing out the Lewis structure? Thanks!
Naming
Can someone walk me through how to name _{6}]^{4-}) ?
?
I know that you start with naming the ligand before the TM, so I got to hexacyano, but how did iron become ferrate?
I know that you start with naming the ligand before the TM, so I got to hexacyano, but how did iron become ferrate?
- Sat Nov 30, 2019 9:35 pm
- Forum: Lewis Acids & Bases
- Topic: Nitrate
- Replies: 4
- Views: 258
Re: Nitrate
So if nitrate is NO3-, then why is Zinc Nitrate Zn(NO3)2
- Sat Nov 30, 2019 8:38 pm
- Forum: Lewis Acids & Bases
- Topic: Nitrate
- Replies: 4
- Views: 258
Nitrate
How do we know what nitrate is by just looking at the name? Is it just memorization?
- Sat Nov 30, 2019 8:17 pm
- Forum: Bronsted Acids & Bases
- Topic: HBr
- Replies: 3
- Views: 247
HBr
Is the reaction for HBr in water HBr(aq) + H2O(aq) ---> Br-(aq) + H3O+(aq)
In general, how do we know what to write for the reaction?
In general, how do we know what to write for the reaction?
- Fri Nov 29, 2019 5:58 pm
- Forum: Bronsted Acids & Bases
- Topic: Bronsted base
- Replies: 3
- Views: 100
Bronsted base
Why is NH3 a bronsted base? Doesn't it provide protons?
- Mon Nov 25, 2019 6:56 am
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3678540
Re: Post All Chemistry Jokes Here
Two chemists walk into a bar. One tells the bartender, "I'll have an H2O." The other says, "I'll have an H2O too!" The second chemist dies.
Found this one online and it made me laugh a little bit harder than I should’ve.
Found this one online and it made me laugh a little bit harder than I should’ve.
- Mon Nov 25, 2019 6:53 am
- Forum: Sigma & Pi Bonds
- Topic: sigma and pi bonds
- Replies: 27
- Views: 1701
Re: sigma and pi bonds
The first bond is always a sigma bond, all bonds following that would be a pi bond.
- Mon Nov 25, 2019 6:51 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Molecular Shape vs. Molecular Geometry
- Replies: 3
- Views: 214
Re: Molecular Shape vs. Molecular Geometry
After looking a bit more into it I find that it also can be the difference between electron geometry and molecular geometry. They are essentially the same thing, but this naming is easier for me.
- Thu Nov 21, 2019 12:15 am
- Forum: Dipole Moments
- Topic: Intermolecular forces
- Replies: 2
- Views: 272
Re: Intermolecular forces
SBr4 is a nonpolar molecule because the central atom (S) is surrounded by 4 identical (Br) molecules that are equivalent in their electronegativity, so the dipoles cancel each other out, and as a result, there is no dipole-dipole interaction. The individual bonds can still be polar but the whole mo...
- Wed Nov 20, 2019 11:49 pm
- Forum: Dipole Moments
- Topic: Intermolecular forces
- Replies: 2
- Views: 272
Intermolecular forces
Why does SBR4 not have a dipole-dipole interaction?
- Wed Nov 20, 2019 8:47 pm
- Forum: Sigma & Pi Bonds
- Topic: Resonance Structures
- Replies: 2
- Views: 226
Resonance Structures
If a molecule has resonance structures that would change the number of sigma and pi bonds, which one should we choose? An example where I found this was in SO2.
- Mon Nov 18, 2019 8:13 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Molecular Shape vs. Molecular Geometry
- Replies: 3
- Views: 214
Molecular Shape vs. Molecular Geometry
What’s the difference between molecular shape and geometry? We talked a bit about it during discussion but I’m just confused on what extent we need to know for the exam. Thank you!
- Wed Nov 13, 2019 10:17 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSEPR formula
- Replies: 1
- Views: 77
VSEPR formula
Will we be going over the VSEPR formulas in class?
- Wed Nov 13, 2019 10:03 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Problem 2E 7
- Replies: 5
- Views: 344
Problem 2E 7
Why are all the bond lengths identical in SOCL2? Doesn't Sulfur have a lone pair so that would repel the other atoms and create a difference in bond length?
- Tue Nov 05, 2019 9:34 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Midterm
- Replies: 3
- Views: 363
Midterm
Do we have to memorize the nodal planes for each orbital?
- Tue Nov 05, 2019 9:23 pm
- Forum: Dipole Moments
- Topic: Drawing Unpaired Electrons
- Replies: 7
- Views: 353
Drawing Unpaired Electrons
If a question asks for us to find a dipole moment for a lewis structure, should we draw the unpaired electrons?
For example, in the Dino Nuggets mini supplemental questions for 2a, the answer key does not draw the unpaired electrons.
For example, in the Dino Nuggets mini supplemental questions for 2a, the answer key does not draw the unpaired electrons.
- Tue Nov 05, 2019 8:53 pm
- Forum: Photoelectric Effect
- Topic: Light acts as a wave
- Replies: 4
- Views: 421
Light acts as a wave
Why does the photoelectric effect tell us that light can act as wave when in the experiment frequency/ intensity of light did not play a role in removing electrons from the metal?
- Tue Nov 05, 2019 8:47 pm
- Forum: Photoelectric Effect
- Topic: Photoelectric Effect vs. De Broglie
- Replies: 8
- Views: 778
Photoelectric Effect vs. De Broglie
How do you know when do differentiate if you should use the speed of light equation or De Broglie?
- Tue Nov 05, 2019 8:11 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Solving with Heisenberg
- Replies: 4
- Views: 439
Solving with Heisenberg
Why do we sometimes multiply \Delta v by an integer? For example, in the Dino nuggets problem, it states that "Lyndon gets so angry at Matt's mistake that he yeets a rock at him that weighs 2.8g with a whopping speed of 373.23 \frac{+}{-} .34 m/s. What is the indeterminancy of position of the r...
- Tue Nov 05, 2019 7:33 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Quantum Number Sequence
- Replies: 1
- Views: 101
Quantum Number Sequence
Why is the quantum number sequence {5,0,-1,+1/2} not possible? Also, how do I calculate 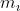 to the electron's actual position?
to the electron's actual position?
- Mon Nov 04, 2019 9:13 am
- Forum: Octet Exceptions
- Topic: Octet Exceptions
- Replies: 2
- Views: 137
Octet Exceptions
What are the different elements that do not require an octet?
- Thu Oct 31, 2019 10:35 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: HW question 1B.25
- Replies: 3
- Views: 151
Re: HW question 1B.25
Hi! So in this problem, you know that you should use the Heisenberg equation in order to find uncertainty of speed of the electron. Since Delta p * delta x = h/4pi, and delta p can be substituted by m * delta v (velocity), you can rearrange and solve for the minimum uncertainty in a particle's velo...
- Thu Oct 31, 2019 10:27 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Bohr Frequency Condition, Electrons
- Replies: 2
- Views: 232
Bohr Frequency Condition, Electrons
What does it mean that an electron drops between levels in the context of the Bohr Frequency condition?
- Thu Oct 31, 2019 5:09 pm
- Forum: Photoelectric Effect
- Topic: HW 1.3
- Replies: 1
- Views: 119
HW 1.3
For this problem, why does the answer key give it in J when the question asks for watts?
- Thu Oct 31, 2019 5:05 pm
- Forum: Octet Exceptions
- Topic: Half-Full Shell Stability
- Replies: 4
- Views: 155
Re: Half-Full Shell Stability
Yes. For example, Chromium has the electron configuration [Ar]3d 5 4s 1 instead of the expected configuration [Ar]3d 4 4s 2 . This fact came mainly from experimental observation so it's not entirely known why it is more stable, but since the 3d orbital is at a lower energy level than the 4s orbital...
- Thu Oct 31, 2019 4:52 pm
- Forum: Octet Exceptions
- Topic: Half-Full Shell Stability
- Replies: 4
- Views: 155
Half-Full Shell Stability
Why are half-filled shells more stable? Can 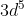 an example of this because an electron will move from the 4s subshell to make the th 3d subshell half-filled?
an example of this because an electron will move from the 4s subshell to make the th 3d subshell half-filled?
- Thu Oct 31, 2019 12:52 pm
- Forum: Properties of Light
- Topic: HW #1.31
- Replies: 1
- Views: 125
HW #1.31
I was wondering what equation I should use for part b of 1.31? I tried to use K_{max}= hv-\phi but the value I get for (hv) is so small that my answer just ends up being the work function. This is the problem: ( and I used the 405 nm wavelength ) The agents want to use a handheld laser to illuminate...
- Thu Oct 31, 2019 12:30 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Midterm
- Replies: 2
- Views: 169
Midterm
Will our midterm include chemistry bonds?
- Wed Oct 30, 2019 8:33 pm
- Forum: Ionic & Covalent Bonds
- Topic: Midterm Review
- Replies: 1
- Views: 198
Re: Midterm Review
I went to the fundamentals review session today, and most of what we did is go over Test 1. So if you are not able to attend another fundamentals review session looking over the concepts covered on Test 1 would be a sufficient substitute.
- Wed Oct 30, 2019 6:53 pm
- Forum: Lewis Structures
- Topic: 4s and 3d
- Replies: 4
- Views: 193
4s and 3d
I'm not sure how to write the electron configuration when it comes to 4s and 3d orbitals. For example, why does 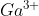 end with
end with 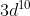 rather than
rather than 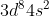 ?
?
- Wed Oct 30, 2019 4:17 pm
- Forum: Lewis Structures
- Topic: Valence Electrons from Periodic Table
- Replies: 10
- Views: 692
Valence Electrons from Periodic Table
How do you tell the number of valence electrons by just looking at the periodic table?
- Sun Oct 20, 2019 9:06 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Z
- Replies: 5
- Views: 319
Z
What is the significance of the number Z?
- Sun Oct 20, 2019 9:03 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Why is 4s before 3d?
- Replies: 9
- Views: 997
Why is 4s before 3d?
Hello, I was wondering why electrons fill 4s before 3d? Is this an important topic to know for the midterm?
- Sun Oct 20, 2019 8:58 pm
- Forum: Trends in The Periodic Table
- Topic: Study Buddies?
- Replies: 8
- Views: 248
Re: Study Buddies?
that would be so helpful! my email is rbishay140@gmail.com
- Thu Oct 17, 2019 3:38 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Wavelength to Series/ Wave type
- Replies: 2
- Views: 197
Wavelength to Series/ Wave type
I know professor Lavelle wrote out easier numbers for us to remember wavelength as it relates to a certain type of wave. If someone could include it below that would be very much appreciated! Also, how do we know to use Balmer or Lymer series based on wavelength?
- Wed Oct 16, 2019 4:46 pm
- Forum: Properties of Electrons
- Topic: Relationship between Speed and Frequency
- Replies: 3
- Views: 166
Relationship between Speed and Frequency
On homework problem 1A #3, why does speed not also decrease when frequency decreases? I combined equations to obtain \frac{c}{f}=\frac{h}{mv} , with f being frequency and v as velocity. Is it incorrect to combine the de broglie equation with speed of light equation? If so, what is the relationship b...
- Sun Oct 13, 2019 9:15 pm
- Forum: Properties of Light
- Topic: Frequency, Wavelength, Amplitude, and Velocity
- Replies: 4
- Views: 182
Re: Frequency, Wavelength, Amplitude, and Velocity
According to the photoelectric effect experiment, light can act as a wave.
Wavelength and frequency have an inverse relationship. Amplitude is the height of a wave. Velocity in terms of light is c, the speed of light.
c= λ(wavelength) multiplied by v(frequency).
Wavelength and frequency have an inverse relationship. Amplitude is the height of a wave. Velocity in terms of light is c, the speed of light.
c= λ(wavelength) multiplied by v(frequency).

