Search found 103 matches
- Tue Mar 10, 2020 10:08 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: 7b.3c - where did the ln come from?
- Replies: 6
- Views: 399
Re: 7b.3c - where did the ln come from?
It is a first order rate law so use lnA = -kt + lnA
- Tue Mar 10, 2020 10:05 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Calculating K
- Replies: 6
- Views: 428
Re: Calculating K
K has no units because it is a ration.
- Tue Mar 10, 2020 10:00 pm
- Forum: General Rate Laws
- Topic: Determining Rate Laws
- Replies: 4
- Views: 337
Re: Determining Rate Laws
You can also look at K's units to find which order it is. M/S is 0th order. 1/S is 1st order. 1/SM is 2nd order.
- Tue Mar 10, 2020 9:58 pm
- Forum: Phase Changes & Related Calculations
- Topic: delta G0 versus delta G
- Replies: 15
- Views: 2618
Re: delta G0 versus delta G
Anything with a o means that it is standard. So  o is standard and
o is standard and  is regular.
is regular.
- Tue Mar 10, 2020 9:54 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Equations
- Replies: 8
- Views: 571
Re: Equations
∆G° = ∆H° - T∆S°and ∆G° = - RT ln K and G=-nFE are essential, but are also on your equation sheet.
- Thu Mar 05, 2020 4:51 pm
- Forum: Balancing Redox Reactions
- Topic: Acidic and Basic Redox Reactions
- Replies: 9
- Views: 565
Re: Acidic and Basic Redox Reactions
It should specify if it is Basic or Acidic. Acidic meaning balance with H+ and Basic meaning balance with OH-.
- Thu Mar 05, 2020 4:45 pm
- Forum: Balancing Redox Reactions
- Topic: oxidizing and reducing agents
- Replies: 10
- Views: 826
Re: oxidizing and reducing agents
Look at the oxidation numbers of the different elements.
- Thu Mar 05, 2020 4:41 pm
- Forum: Balancing Redox Reactions
- Topic: Half reactions
- Replies: 6
- Views: 481
Re: Half reactions
Add H2O fist to balance the oxygen and then the H+ to balance the hydrogen.
- Thu Mar 05, 2020 4:38 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagram Order
- Replies: 8
- Views: 627
Re: Cell Diagram Order
The anode is on the left and the cathode is on the right. Make sure you put lines in between the solid, gas and aq.
- Thu Mar 05, 2020 4:35 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Inert Metals
- Replies: 4
- Views: 353
Re: Inert Metals
I would use Pt(s) as it is more common.
- Mon Mar 02, 2020 12:12 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Notation commas
- Replies: 4
- Views: 344
Re: Cell Notation commas
The comas are used in a cell diagram to separate two of the same states in a half reaction.
- Mon Mar 02, 2020 12:03 pm
- Forum: Balancing Redox Reactions
- Topic: Oxidation/Reduction
- Replies: 17
- Views: 1173
Re: Oxidation/Reduction
Find the change in oxidation number for an atom. If the oxidation number decreases from reactants to products then it is reduction. If the oxidation number increases from reactants to products then it is oxidized.
- Mon Mar 02, 2020 12:01 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Order of compounds in cell diagram?
- Replies: 5
- Views: 409
Re: Order of compounds in cell diagram?
anode is the left and cathode is the right. Also remember aq us closest to the center of the cell diagram than gas and lastly solid.
- Mon Mar 02, 2020 11:59 am
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: k
- Replies: 10
- Views: 615
Re: k
k is the constant rate so it has no units.
- Mon Mar 02, 2020 11:58 am
- Forum: Balancing Redox Reactions
- Topic: Anode and Xathod
- Replies: 9
- Views: 552
Re: Anode and Xathod
Cathode is were reduction occurs and anode is were oxidation occurs.
- Wed Feb 19, 2020 10:28 pm
- Forum: Balancing Redox Reactions
- Topic: Understanding Half-Reactions
- Replies: 11
- Views: 717
Re: Understanding Half-Reactions
It helps you separate what is being reduced and what is being oxidized in the reaction. After balancing the reduction and oxidation separately we can then add them together to make the overall balanced redox reaction. This would be a lot more difficult to do if we did not separate them first.
- Wed Feb 19, 2020 10:18 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Anode and Cathode
- Replies: 14
- Views: 1038
Re: Anode and Cathode
CNourian2H wrote:how do the terms anode and cathode relate to oxidation and reduction. He used the latter pair of terms to define anode and cathode, but can someone explain this further?
Cathode is the reduction reaction and the anode is the oxidation reaction.
- Wed Feb 19, 2020 10:15 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Anode and Cathode position
- Replies: 5
- Views: 346
Re: Anode and Cathode position
It is usually written in that order, but I would always double check to see where the oxidation and reduction reactions are placed in correspondence to the left and the right of the galvanic cell.
- Wed Feb 19, 2020 10:12 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Lavelle's Office Hours
- Replies: 5
- Views: 470
Re: Lavelle's Office Hours
Does anyone know what Lavelle's office hours are like? Is it going over general HW problems and information, or do we need to ask him questions and bring our own HW problems in? It's similar to the TA office hours where you are able to ask questions. When I went it was mostly questions regarding th...
- Wed Feb 19, 2020 10:09 pm
- Forum: Balancing Redox Reactions
- Topic: Balancing Redox Reactions
- Replies: 5
- Views: 316
Re: Balancing Redox Reactions
To write the half reactions you need find out what is being oxidized and reduced which you do by finding the oxidation number for each species present.
- Fri Feb 14, 2020 10:13 am
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Why is delta U = 0 for isothermal reactions?
- Replies: 11
- Views: 4362
Re: Why is delta U = 0 for isothermal reactions?
U is equal to 0 because it is an isolated system so there is no energy energy leaving or going into the system.
- Fri Feb 14, 2020 10:09 am
- Forum: Student Social/Study Group
- Topic: Midterm
- Replies: 8
- Views: 710
Re: Midterm
Question 8 was problem 4I.9 on our homework problems, that is what my TA said.
- Fri Feb 14, 2020 10:02 am
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: PV=nRT
- Replies: 5
- Views: 315
Re: PV=nRT
In that case I would use P1V1=P2V2. PV=nRT is usually used when you have three values and the constant and only need to solve for the last value.
- Fri Feb 14, 2020 10:00 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Cv vs Cp
- Replies: 17
- Views: 990
Re: Cv vs Cp
Cv is constant volume and Cp is constant pressure.
- Fri Feb 14, 2020 9:59 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Delta S
- Replies: 8
- Views: 1464
Re: Delta S
delta S is the total between delta S of the surroundings and the system.
- Fri Feb 07, 2020 9:28 pm
- Forum: Phase Changes & Related Calculations
- Topic: -w vs w
- Replies: 15
- Views: 671
Re: -w vs w
w is when work is being done one the system. -w is when the system is doing work.
- Fri Feb 07, 2020 9:25 pm
- Forum: General Science Questions
- Topic: Midterm
- Replies: 17
- Views: 1003
Re: Midterm
There are many review sessions for the midterm. The one on Sunday should cover everything with practice problems.
- Fri Feb 07, 2020 9:21 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Isolated vs Closed [ENDORSED]
- Replies: 34
- Views: 4313
Re: Isolated vs Closed [ENDORSED]
Closed system can exchange heat while an isolated system has no effect from the surroundings.
- Fri Feb 07, 2020 9:19 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: closed vs isolated
- Replies: 14
- Views: 474
Re: closed vs isolated
Isolated systems do not interact with the environment around it. A closed system can transfer energy.
- Fri Feb 07, 2020 9:11 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: isolated system
- Replies: 8
- Views: 505
Re: isolated system
It is so tightly sealed that the volume can not change and there is no effect from the outside system.
- Tue Jan 28, 2020 4:31 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Adding Enthalpies
- Replies: 5
- Views: 225
Re: Adding Enthalpies
Because they are state functions the enthalpies should be added.
- Tue Jan 28, 2020 4:15 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: qp vs qv
- Replies: 6
- Views: 361
Re: qp vs qv
There are really no other differences other then what you said qv occurs under constant volume and qp under constant pressure.
- Tue Jan 28, 2020 4:09 pm
- Forum: Ideal Gases
- Topic: Kc vs Kp
- Replies: 109
- Views: 4941
Re: Kc vs Kp
Kp is when you deal with partial pressure so it is used for gasses. Use Kc when you see aq.
- Tue Jan 28, 2020 4:05 pm
- Forum: Ideal Gases
- Topic: Chemical Equilibrium
- Replies: 4
- Views: 224
Re: Chemical Equilibrium
Catalyst speeds up the reaction.
- Tue Jan 28, 2020 4:04 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Gas Constant
- Replies: 13
- Views: 573
Re: Gas Constant
The gas constant is given on the formula sheet, make sure you chose the one with the units that apply to the equation.
- Tue Jan 21, 2020 8:13 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Acids and Bases
- Replies: 7
- Views: 194
Re: Acids and Bases
Acids donate protons and bases accept protons.
- Tue Jan 21, 2020 8:08 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: partial pressure
- Replies: 5
- Views: 282
Re: partial pressure
They both work, check what units are given in the problem and use the same units.
- Tue Jan 21, 2020 8:06 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: ice box approximation
- Replies: 9
- Views: 344
Re: ice box approximation
If the K value is less than 10^-3 then you can approximate and there is not need for the quadratic formula.
- Tue Jan 21, 2020 8:03 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Calculator
- Replies: 4
- Views: 192
Re: Calculator
Yes, scientific calculators are permitted, however graphing calculators are not.
- Tue Jan 21, 2020 8:00 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Test One Content
- Replies: 6
- Views: 321
Re: Test One Content
It is on chemical equilibrium as well as acids and bases
- Wed Jan 15, 2020 3:31 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Endothermic and Exothermic Reactions Class Example
- Replies: 5
- Views: 209
Re: Endothermic and Exothermic Reactions Class Example
The N2 bonds are broken which requires heat making it an endothermic reaction.
- Wed Jan 15, 2020 3:27 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: x is small approximation
- Replies: 6
- Views: 546
Re: x is small approximation
We can approximate if x is less then 1.0 x 10^-4.
- Wed Jan 15, 2020 3:21 pm
- Forum: Ideal Gases
- Topic: mole fraction
- Replies: 4
- Views: 193
Re: mole fraction
Yes, you multiply the mole fraction with the total pressure to get the partial pressure.
- Wed Jan 15, 2020 3:17 pm
- Forum: Ideal Gases
- Topic: equilibrium constant
- Replies: 5
- Views: 197
Re: equilibrium constant
K represents the ratio of products to reactants so P/R.
- Wed Jan 15, 2020 3:14 pm
- Forum: Ideal Gases
- Topic: bars vs atm
- Replies: 8
- Views: 296
Re: bars vs atm
They are two different units, but they do the same thing:measure pressure. As long as you are constant with what unit you use for a single problem you should be ok.
- Thu Jan 09, 2020 4:56 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: solid/liquid
- Replies: 7
- Views: 202
Re: solid/liquid
Yes the solids and liquids have no effect on the calculation.
- Thu Jan 09, 2020 4:55 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Using Kc Vs Kp
- Replies: 22
- Views: 1064
Re: Using Kc Vs Kp
The units in the question should help you find out when to use Kc and Kp. Kc is in M and Kp is in Bar.
- Thu Jan 09, 2020 4:52 pm
- Forum: Ideal Gases
- Topic: K
- Replies: 10
- Views: 525
Re: K
Yes it will still be the same, but Kc is in terms of Moles and Kp is in terms of Bars.
- Thu Jan 09, 2020 4:48 pm
- Forum: Ideal Gases
- Topic: Understanding Q
- Replies: 19
- Views: 751
Re: Understanding Q
Yes you can solve Q the same way as you would solve K.
- Thu Jan 09, 2020 4:47 pm
- Forum: Ideal Gases
- Topic: K value
- Replies: 14
- Views: 1173
Re: K value
When K is a large value > 10^3 then there are more products at equilibrium. When K is a small value <10^-3 then there are more reactants.
- Fri Dec 06, 2019 11:51 am
- Forum: Air Pollution & Acid Rain
- Topic: Acid Rain
- Replies: 6
- Views: 725
Re: Acid Rain
Acid rain is when CO2 reacts in the atmosphere which results in acid rain. This rain, like stated in the name, is Acidic. When there is an excessive amount of CO2 in the air there will be a lot of acid rain. This contributes to the greenhouse effect and global warming.
- Fri Dec 06, 2019 11:48 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Ligand Definition
- Replies: 4
- Views: 359
Re: Ligand Definition
Ligands are ions or molecules that are attached to the central atom, they are lewis acids.
- Fri Dec 06, 2019 11:45 am
- Forum: Bond Lengths & Energies
- Topic: Hydrogen Bonding
- Replies: 13
- Views: 1112
Re: Hydrogen Bonding
Hydrogen bonding occurs when the hydrogen bonds with N,O or F
- Fri Dec 06, 2019 11:41 am
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: pKa and Ka
- Replies: 10
- Views: 636
Re: pKa and Ka
One thing to know that Dr. Lavelle mentioned in class is that if a pKa value is given it is highly likely that that acid is a weak acid. Strong acids strongly favor the products and, thus, have an astronomically large pKa. Does that mean strong acids have a high conc of H+? Yes, the pKa is so large...
- Fri Dec 06, 2019 11:35 am
- Forum: Coordinate Covalent Bonds
- Topic: Hemoglobin and Myoglobin
- Replies: 1
- Views: 213
Re: Hemoglobin and Myoglobin
know how to draw the lewis structure. Hemoglobin transports oxygen thought the bloodstream because the oxygens are weakly attached, bonds to 4 oxygens. Myoglobin stores oxygen in muscles because the single oxygen is strongly attached.
- Sun Dec 01, 2019 4:32 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: When VSEPR doesn't work
- Replies: 4
- Views: 202
Re: When VSEPR doesn't work
For Lavell's class I think we should stick to using VSEPR theory to determine the shape of a molecule as he taught in lecture.
- Sun Dec 01, 2019 4:27 pm
- Forum: Sigma & Pi Bonds
- Topic: Drawing Sigma and Pi bonds
- Replies: 7
- Views: 773
Re: Drawing Sigma and Pi bonds
You should name the bonds individually and draw the sigma next to the first bond and the pi next to the second and/or third bond, depending on the number of bonds.
- Sun Dec 01, 2019 4:26 pm
- Forum: Naming
- Topic: Roman numerals
- Replies: 6
- Views: 502
Re: Roman numerals
The roman numeral tells us the charge on the metal ion.
- Sun Dec 01, 2019 4:24 pm
- Forum: Hybridization
- Topic: Hybridization of Hydrogen
- Replies: 3
- Views: 305
Re: Hybridization of Hydrogen
It can only from one bond because it only has one electron, which is in the 1s orbital so it can only be hybridized in the 1s orbital.
- Sun Dec 01, 2019 4:23 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Determining Polarity
- Replies: 10
- Views: 609
Re: Determining Polarity
Check the net dipoles of the molecule. If you see lone pairs the molecule will always be polar.
- Sat Nov 23, 2019 6:43 pm
- Forum: Resonance Structures
- Topic: Resonance and Naming
- Replies: 11
- Views: 888
Re: Resonance and Naming
No, it is the same compound.
- Sat Nov 23, 2019 6:40 pm
- Forum: Sigma & Pi Bonds
- Topic: How to Find Sigma Bonds and Pi Bonds
- Replies: 15
- Views: 1214
Re: How to Find Sigma Bonds and Pi Bonds
Look at the lewis structure and the bonds formed between the atoms. single bond is 1 sigma bond, double bond is 1 sigma and 1 pi bond, triple bond is 1 sigma and 2 pi bonds. So the fist bond is always sigma and anything after is a pi bond.
- Sat Nov 23, 2019 6:36 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: What are Ligands?
- Replies: 6
- Views: 221
Re: What are Ligands?
A ligan is a ion or a molecule bonding to a metal atom.
- Sat Nov 23, 2019 6:30 pm
- Forum: Naming
- Topic: Naming Order
- Replies: 6
- Views: 363
Re: Naming Order
The naming of the molecule should be in alphabetical order.
- Sat Nov 23, 2019 6:28 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Seesaw
- Replies: 23
- Views: 1042
Re: Seesaw
Yes, it would be less because the lone pairs push on the other bonds.
- Sat Nov 23, 2019 6:24 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSEPER shape for IF4-
- Replies: 5
- Views: 1948
Re: VSEPER shape for IF4-
The electron geometry is octahedral and the molecular geometry is square planar.
- Wed Nov 13, 2019 9:03 pm
- Forum: Hybridization
- Topic: 2F.5
- Replies: 4
- Views: 315
Re: 2F.5
You add the number of lone pars of the atom to the number of atoms the atom is bonded to.
- Wed Nov 13, 2019 8:54 pm
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: bond order
- Replies: 4
- Views: 489
Re: bond order
805097738 wrote:can someone explain what bond order is?
Bond order is measure of the number of electrons involved in bonds between atoms in the molecule. The higher the bond order means the stronger the chemical bond.
- Wed Nov 13, 2019 8:48 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSEPER shape for IF4-
- Replies: 5
- Views: 1948
Re: VSEPER shape for IF4-
It is octahedral because there are 6 bonding pars and 2 lone pairs. Square planar is when there are 4 bonding pairs and two lone pairs.
- Wed Nov 13, 2019 8:42 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: 2E.5 b
- Replies: 4
- Views: 133
Re: 2E.5 b
The are asking for the ClO2 cation so there are 18 electrons. The shape is trigonal planar and the bond angle is 120 degrees.
- Wed Nov 13, 2019 8:38 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: 2E.15
- Replies: 3
- Views: 215
Re: 2E.15
TeCl4 is See-saw shaped so the bond angles have to be 120 and 90.
- Fri Nov 08, 2019 3:44 pm
- Forum: Lewis Structures
- Topic: Drawing Lewis Structures
- Replies: 18
- Views: 698
Re: Drawing Lewis Structures
No, not every atom has to have a formal charge of 0 but they all have to add up to 0 to make the formal charge of the molecule 0.
- Fri Nov 08, 2019 3:37 pm
- Forum: Resonance Structures
- Topic: Bond lengths
- Replies: 11
- Views: 414
Re: Bond lengths
The bond lengths are all the same because the actual structure is a mix of all the resonance structures.
- Fri Nov 08, 2019 3:31 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: central atom
- Replies: 21
- Views: 1041
Re: central atom
Yes, the optimal structure would be the middle atom having a 0 charge.
- Fri Nov 08, 2019 3:26 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: central atom
- Replies: 21
- Views: 1041
Re: central atom
APatel_4A wrote:How do we know what the central atom should be?
It is the least electronegative atom
- Fri Nov 08, 2019 2:57 pm
- Forum: Resonance Structures
- Topic: Formal Charge Question
- Replies: 16
- Views: 933
Re: Formal Charge Question
They have to add up to the same formal charge.
- Fri Nov 08, 2019 2:55 pm
- Forum: Lewis Structures
- Topic: Formal Charges
- Replies: 15
- Views: 974
Re: Formal Charges
505106414 wrote:Is it okay to break the octet rule in order to minimize formal charge?
No unless the atom itself is able to break the octet rule.
- Fri Nov 08, 2019 2:53 pm
- Forum: Hybridization
- Topic: Electrons
- Replies: 13
- Views: 1280
Re: Electrons
It means the electron is exited
- Sat Nov 02, 2019 2:16 pm
- Forum: Octet Exceptions
- Topic: Exceptions?
- Replies: 5
- Views: 261
Re: Exceptions?
The elements in group 3 can break the octet rule.
- Wed Oct 30, 2019 5:00 pm
- Forum: Lewis Structures
- Topic: Valence Electrons from Periodic Table
- Replies: 10
- Views: 692
Re: Valence Electrons from Periodic Table
To find the valence electron from the periodic table look at the group number.
- Wed Oct 30, 2019 4:57 pm
- Forum: Ionic & Covalent Bonds
- Topic: Partial charge
- Replies: 4
- Views: 138
Re: Partial charge
They are found in ionic bonds as-well.
- Wed Oct 30, 2019 4:51 pm
- Forum: Octet Exceptions
- Topic: expanded valence shells
- Replies: 2
- Views: 148
Re: expanded valence shells
Some atoms are more stable with expanded valence shells like phosphorus when it bonds with Chlorine.
- Wed Oct 30, 2019 4:45 pm
- Forum: Lewis Structures
- Topic: 2B 19
- Replies: 3
- Views: 231
Re: 2B 19
It means each atom has to have an octet.
- Wed Oct 23, 2019 4:09 pm
- Forum: Ionic & Covalent Bonds
- Topic: Ionic vs covalent
- Replies: 8
- Views: 890
Re: Ionic vs covalent
An ionic bond is the transfer of electrons from a metal to a non metal atom.
A covalent bond is the sharing of electrons of non metals.
A covalent bond is the sharing of electrons of non metals.
- Wed Oct 23, 2019 4:03 pm
- Forum: Octet Exceptions
- Topic: Octet Rule
- Replies: 6
- Views: 278
Re: Octet Rule
There are a few exceptions, but generally yes they need to follow the octet rule.
- Wed Oct 23, 2019 3:57 pm
- Forum: Lewis Structures
- Topic: Molecule Formulas
- Replies: 5
- Views: 800
Re: Molecule Formulas
They should all be given, however I assume we would have to know simple molecules like water.
- Wed Oct 23, 2019 3:54 pm
- Forum: Lewis Structures
- Topic: Finding Valence Electrons
- Replies: 8
- Views: 432
Re: Finding Valence Electrons
To find the number of valence electrons look at the group number, however this does not work for d block elements.
- Wed Oct 23, 2019 3:51 pm
- Forum: Lewis Structures
- Topic: Electronegativity
- Replies: 5
- Views: 256
Re: Electronegativity
Electronegativity decreases down a group and increases across a period.
- Wed Oct 16, 2019 5:10 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: X Y and Z
- Replies: 7
- Views: 281
Re: X Y and Z
In his lecture today, Lavelle emphasized that the plane the electron is found in is arbitrary and different textbooks associate different l values with different x,y, and z planes. So, because he said this we can assign the x,y,z variables to the values we have correct? That confused me as well. I ...
- Wed Oct 16, 2019 5:05 pm
- Forum: Trends in The Periodic Table
- Topic: Ionic radii
- Replies: 11
- Views: 373
Re: Ionic radii
As you go down the group the number of electrons increases for each atom. You then get more shells and the valence electrons in the outermost shell get farther away from the nucleus.
- Wed Oct 16, 2019 5:02 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Magnetic Quantum
- Replies: 2
- Views: 107
Re: Magnetic Quantum
No, I think as long as you list them you should be fine.
- Wed Oct 16, 2019 3:53 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: X Y and Z
- Replies: 7
- Views: 281
Re: X Y and Z
I think this is related to the model that was shown during the lecture today. Depending on the  number we can use the table to find out if it is x, y, or z.
number we can use the table to find out if it is x, y, or z.
- Wed Oct 16, 2019 3:48 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Electron spin
- Replies: 8
- Views: 301
Re: Electron spin
Is it only possible for an electron to have a +1/2 or -1/2 spin?
- Wed Oct 09, 2019 12:25 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Spectral Lines
- Replies: 3
- Views: 156
Re: Spectral Lines
Location on the electromagnetic spectrum doesn't exactly determine which series a line belongs to. Each line represents energy released by the movement of a hydrogen electron from one energy level to a lower energy level. Each energy level is represented by what's called the principal quantum numbe...
- Wed Oct 09, 2019 12:22 pm
- Forum: Properties of Electrons
- Topic: Changes in Orbitals
- Replies: 3
- Views: 99
Re: Changes in Orbitals
Im pretty sure you would use the equation 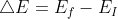
Correct me if I'm wrong.
Correct me if I'm wrong.
- Wed Oct 09, 2019 12:15 pm
- Forum: *Black Body Radiation
- Topic: Black Body
- Replies: 5
- Views: 317
Re: Black Body
I think professor mentioned in class that the perfect black body that can absorb all frequencies of light does not currently exist. Cuz if we can still see it, it must has reflected some sort of frequencies which are able to be detected by our eyes, or it will just be invisible. Plz correct me if I...
- Wed Oct 09, 2019 12:10 pm
- Forum: Properties of Light
- Topic: Photons
- Replies: 7
- Views: 333
Re: Photons
A photon is just the name for a quantum of light, right?
- Wed Oct 09, 2019 12:05 pm
- Forum: Properties of Light
- Topic: Self-test 1A.1B
- Replies: 4
- Views: 145
Re: Self-test 1A.1B
Joseph Saba wrote:I believe since we use the equation c = w*f, then 3e8= 98.6e6x. to solve for x (wavelength) then divide by the frequency. I got 3.05 meters.
Are c and w*f constant values in the equation you used?
- Thu Oct 03, 2019 1:33 pm
- Forum: Limiting Reactant Calculations
- Topic: Percentage Yields
- Replies: 8
- Views: 586
Re: Percentage Yields
Percent yield means the actual yield divided by the theoretical yield. You want the percent yield to be as close to 100% as possible to get the maximum amount of yield form the product you created.
- Thu Oct 03, 2019 1:28 pm
- Forum: Balancing Chemical Reactions
- Topic: "Combustion" Term
- Replies: 8
- Views: 396
Re: "Combustion" Term
You can use the term Metabolism which is a general term for biological processes.
Or you can use the term Oxidation which means the compound reacts with oxygen.
Or you can use the term Oxidation which means the compound reacts with oxygen.

