Search found 101 matches
- Thu Mar 05, 2020 11:23 pm
- Forum: Student Social/Study Group
- Topic: Midterm and Final Question
- Replies: 18
- Views: 1123
Re: Midterm and Final Question
Look out for Lyndon's study packets, they are very helpful and he usually has a big review session where he explains the answers. In my experience, they have helped a lot and are sometimes harder than the actual midterm/final, so they definitely prepare you well.
- Thu Mar 05, 2020 11:21 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Concentration Cells
- Replies: 5
- Views: 437
Re: Concentration Cells
Because the reactions are the same on both sides, just going in opposite directions. This means that Ecathode=Eanode, and since Ecell=Ecathode-Eanode, Ecell=0.
- Thu Mar 05, 2020 11:20 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: units of k
- Replies: 9
- Views: 716
Re: units of k
For first order, the units are just s^-1
For second order, the units are L x mol^-1 x s^-1
For third order, the units are L^2 x mol^-2 x s^-2
The pattern continues in this way.
For second order, the units are L x mol^-1 x s^-1
For third order, the units are L^2 x mol^-2 x s^-2
The pattern continues in this way.
- Thu Mar 05, 2020 11:13 pm
- Forum: General Rate Laws
- Topic: Determining Order
- Replies: 6
- Views: 479
Re: Determining Order
Katie Bart 1I wrote:Can the order keep increasing? Or do they stop at two?
Order can be higher than two, but they become less common the higher order you go. It is harder for three molecules to collide than for two molecules to collide. The same goes for higher orders.
- Thu Mar 05, 2020 11:10 pm
- Forum: General Rate Laws
- Topic: units
- Replies: 9
- Views: 560
Re: units
are the units for rate different for reactions of different orders? The units for the rate are always going to be moles per liter per second, but the units for the rate constant k will change according to the overall order. k changes so that the proper units cancel so that the rate stays at moles p...
- Thu Mar 05, 2020 11:09 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: reduction and oxidation
- Replies: 4
- Views: 304
Re: reduction and oxidation
It's the other way around. Since Ecell = Ecathode-Eanode, you want the Eanode to be the most negative so that the overall cell potential is positive. At the anode, oxidation occurs.
- Thu Mar 05, 2020 11:06 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Standard Delta G vs Non-Standard Delta G
- Replies: 4
- Views: 353
Re: Standard Delta G vs Non-Standard Delta G
- Thu Mar 05, 2020 11:03 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: different units
- Replies: 4
- Views: 390
Re: different units
It is because you want the right units in the end when you multiply everything to find the rate using the differential or integrative rate law. In a reaction with an overall order of 4 for example, you will have the unit of mol/L multiplied by itself 4 times, so the units of k must be different so t...
- Thu Mar 05, 2020 10:59 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6M5
- Replies: 2
- Views: 200
Re: 6M5
Liquid mercury can conduct electricity, so it is one of the few exceptions were a solid electrode is not needed.
- Thu Mar 05, 2020 10:58 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Electromotive force (emf)
- Replies: 9
- Views: 653
Re: Electromotive force (emf)
And also, maximum nonexpansion work is the same as Gibbs free energy, so emf directly relates to 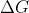
- Tue Feb 25, 2020 8:10 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6L - Where do you find Estandard values?
- Replies: 2
- Views: 142
Re: 6L - Where do you find Estandard values?
You can find the standard reduction potentials in Appendix 2B of the book.
- Tue Feb 25, 2020 8:08 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6L.3b
- Replies: 3
- Views: 267
Re: 6L.3b
C(gr) is used as an electrode because the anode has Hydrogen gas and hydrogen aqueous, so a solid must be included to allow electron transfer. Graphite and Platinum are common inert solids used as electrodes.
- Tue Feb 25, 2020 3:07 pm
- Forum: Balancing Redox Reactions
- Topic: Which equation do we flip?
- Replies: 6
- Views: 406
Re: Which equation do we flip?
You should flip the reaction that makes the standard reduction potential of the overall cell positive.
- Tue Feb 25, 2020 11:33 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Concentration Cells
- Replies: 2
- Views: 206
Concentration Cells
Can concentration cells be made with a salt bridge? Or can they only be made with a porous disk?
- Sun Feb 23, 2020 10:23 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Nernst Equation
- Replies: 4
- Views: 286
Re: Nernst Equation
We use the Nernst equation to find the cell reduction potential when we know the concentrations of the products and reactants, as well as the moles of electrons transferred and the standard reduction potential.
- Sun Feb 23, 2020 10:13 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: | divider in cell diagram
- Replies: 5
- Views: 358
Re: | divider in cell diagram
Yes, it is because they are both aqueous.
- Wed Feb 19, 2020 8:01 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: E as intensive property
- Replies: 5
- Views: 386
Re: E as intensive property
It means that it doesn't matter how many times the reaction occurs, E will remain the same as long as it is at standard conditions. Unlike in something like enthalpy or Gibb's free energy where the standard formation value gets multiplied by the moles of the reaction, E will remain the same for one ...
- Wed Feb 19, 2020 7:55 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Answer is different for 5G.15
- Replies: 5
- Views: 360
Re: Answer is different for 5G.15
I also keep getting -2.7kJ. Not sure how it is supposed to be -27 honestly.
- Wed Feb 19, 2020 7:45 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Salt Bridge and function
- Replies: 4
- Views: 326
Re: Salt Bridge and function
The salt bridge allows the charges between the anode and cathode to be the same. As the cathode gains electrons from the anode, the buildup of electrons on the cathode would eventually stop the flow of electrons.
- Wed Feb 19, 2020 7:43 pm
- Forum: Balancing Redox Reactions
- Topic: Homework 6K.3 d
- Replies: 1
- Views: 200
Homework 6K.3 d
The question asks you to balance the reaction of chlorine in water:
Cl2(g)-->HClO(aq) + Cl2(g)
How would you set up the half-reactions for this problem? Would one of them be just Cl2-->Cl2? I do not see how electrons can be transferred between two of the same species.
Cl2(g)-->HClO(aq) + Cl2(g)
How would you set up the half-reactions for this problem? Would one of them be just Cl2-->Cl2? I do not see how electrons can be transferred between two of the same species.
- Sun Feb 09, 2020 10:08 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: heat capacities and enthalpy of phase changes
- Replies: 2
- Views: 200
Re: heat capacities and enthalpy of phase changes
I don't think there is a way to calculate enthalpy of phase changes based on the heat capacity equation. The only way I know if calculating the enthalpy of phase changes is by adding and subtracting other enthalpies of phase changes because enthalpy is a state function, for example: Enthalpy of vapo...
- Sun Feb 09, 2020 10:01 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Difference between Cv and Cp?
- Replies: 8
- Views: 530
Re: Difference between Cv and Cp?
Cv is the specific heat of a substance under constant pressure, while Cp is the specific heat of a substance under constant pressure. Generally, Cv is lower because a system at constant volume does no work, so all of the energy inputed into the system will be used toward increasing the temperature.
- Sun Feb 09, 2020 9:55 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Calorimeters
- Replies: 17
- Views: 994
Re: Calorimeters
A bomb calorimeter has rigid walls that do not allow for expansion, therefore it is constant volume. A system with constant pressure would be open to the environment.
- Sun Feb 09, 2020 9:53 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: ICE BOX Reverse ?
- Replies: 4
- Views: 384
Re: ICE BOX Reverse ?
You cannot assume the concentration of a species is 1 if it is not given to you. If you are trying to solve for initial concentrations and you are given the change in concentration after reaching equilibrium, then you substitute that change into x in your ICE table and you can solve for concentratio...
- Sun Feb 09, 2020 9:51 pm
- Forum: Calculating Work of Expansion
- Topic: Vacuum
- Replies: 3
- Views: 129
Re: Vacuum
The system will be under free expansion or it will be stated in the problem.
- Sun Feb 09, 2020 9:51 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: conditions for free expansion?
- Replies: 3
- Views: 136
Re: conditions for free expansion?
If I remember correctly, free expansion occurs when a gas is being heated in a vacuum. It is called free expansion because there is no external pressure, so the gas can expand without opposing a force (no work is done), so it is said to expand freely.
- Sun Feb 09, 2020 9:50 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Hess's Law
- Replies: 10
- Views: 298
Re: Hess's Law
We can use Hess's law because enthalpy is a state property, so it doesn't matter the path you take to get from an initial condition to a final condition, the change in enthalpy will always be the same.
- Sun Feb 09, 2020 9:48 pm
- Forum: Calculating Work of Expansion
- Topic: 4A.9
- Replies: 2
- Views: 191
Re: 4A.9
In problems like these, it is important to recognize that whatever heat is released by the copper is absorbed by the water. -q copper =q water Also, it is important to know that the final temperature of the copper and water will be the same. We know this because once the water and copper reach the s...
- Sun Feb 09, 2020 9:44 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: 4A.3
- Replies: 2
- Views: 153
Re: 4A.3
The change in internal energy is deltaU=q+w, since there is no mention of heat, we assume q to be 0. Now, deltaU=w, so change in internal energy is just change in work you calculated previously.
- Sun Feb 09, 2020 9:41 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Isolated// Energy
- Replies: 11
- Views: 609
Re: Isolated// Energy
No, because the definition of an isolated system is that no energy is exchanged with the environment. This includes heat and work. Consider it like a thermally-insulated and rigid bomb calorimeter.
- Fri Jan 31, 2020 10:35 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: accuracy of bond enthalpies
- Replies: 8
- Views: 412
Re: accuracy of bond enthalpies
Because they are not tailor-made to every type of bond in every molecule, they are an average of the energy required to break bonds across many different molecules. For example, the O-H bond in CCl3COOH is different than the O-H bond in CCH3COOH because we know that the Cl pulls electrons more than ...
- Fri Jan 31, 2020 10:27 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Test #1// #5
- Replies: 5
- Views: 258
Re: Test #1// #5
I'm assuming you meant to say the Pkb of F- and that the reaction given to you was the dissociation of HF in water. To do this problem, you have to first convert the Pkb of F- into the Pka of HF. This can be done using 14-10.2 because F- and HF are conjugates. Once you find the Pka, you convert it i...
- Fri Jan 31, 2020 10:14 am
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: 4B.9
- Replies: 3
- Views: 166
Re: 4B.9
When q=0 and deltaU=q+w, then
deltaU=(0)+w
deltaU=w
d states deltaU=q or deltaU=0. For this to be true according to the derivation from before, w must equal 0.
deltaU=(0)+w
deltaU=w
d states deltaU=q or deltaU=0. For this to be true according to the derivation from before, w must equal 0.
- Fri Jan 31, 2020 10:09 am
- Forum: Phase Changes & Related Calculations
- Topic: standard reaction enthalpy vs. standard enthalpy of formation
- Replies: 7
- Views: 1080
Re: standard reaction enthalpy vs. standard enthalpy of formation
Specifically, standard enthalpy of formation is the enthalpy required to form one mole of a substance from its elements in their most stable form. The standard enthalpy of formation for elements in their most stable form is 0.
- Fri Jan 31, 2020 10:05 am
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Isolated vs Closed [ENDORSED]
- Replies: 34
- Views: 4337
Re: Isolated vs Closed [ENDORSED]
If something is specified as being insulated and rigid, that means it doesn't exchange heat or work of expansion with the surroundings. This makes the system isolated. If these are not specified, but the system doesn't allow the exchange of matter with the surroundings, then the system of closed.
- Tue Jan 21, 2020 9:10 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Increase in Pressure
- Replies: 1
- Views: 71
Re: Increase in Pressure
An increase in pressure by compression of the system will favor the side of the reaction with fewer moles of gas. This is because when you reduce the volume of the system (via compression) then the concentrations are increased, and the reaction quotient shifts above or below K. This shift in Q resul...
- Tue Jan 21, 2020 9:05 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Second Ionization in Polyprotic Acid Solutions
- Replies: 2
- Views: 75
Re: Second Ionization in Polyprotic Acid Solutions
For most polyprotic acids except sulfuric acid, the second ionization contributes so few H3O+ that its effect on pH is negligible.
- Mon Jan 20, 2020 9:28 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Pressure goes to less moles of gas explaination
- Replies: 4
- Views: 213
Re: Pressure goes to less moles of gas explaination
He explained that if pressure is increased by decreasing volume (if a piston pushed down on the reaction, for example) then the concentration of each of the gases in the reaction would increase. We calculate concentration by using \frac{n}{V} where n is moles of gas and V is the volume of the contai...
- Mon Jan 20, 2020 9:19 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Inert Gas
- Replies: 6
- Views: 215
Re: Inert Gas
Although adding an inert gas affects the total volume of the reaction, they do not interact with anything, thus causing there to be no change in concentration. I thought the whole point was that adding a inert gas doesn't affect the volume of the container, which was why it has no affect on concent...
- Mon Jan 20, 2020 9:15 pm
- Forum: Polyprotic Acids & Bases
- Topic: Deprotonation
- Replies: 3
- Views: 211
Re: Deprotonation
Typically the Ka for the second deprotonation is extremely small. Apart from sulfuric acid, for most acids it doesn't have a meaningful effect on pH.
- Thu Jan 16, 2020 6:07 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Negative pH
- Replies: 6
- Views: 260
Re: Negative pH
Yes, pH can be negative and it just means that the molarity of H+ is greater than 1. Google tells me that some acids have been observed at pH -18.
- Thu Jan 16, 2020 11:41 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Units in ICE Table
- Replies: 8
- Views: 261
Re: Units in ICE Table
The values of the ICE table must be in concentration because they will be calculated using Kc, which deals with concentrations.
- Thu Jan 16, 2020 11:37 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Partial Pressure (5J.1)
- Replies: 3
- Views: 157
Re: Partial Pressure (5J.1)
I have another similar question with 5J.3 - can someone explain why adding/removing a certain amount of a compound might affect its equilbrium? Adding or removing a certain amount of compound will not affect the equilibrium constant K because only temperature will do that. It will affect the equili...
- Thu Jan 16, 2020 11:34 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Partial Pressure (5J.1)
- Replies: 3
- Views: 157
Re: Partial Pressure (5J.1)
When looking at problems like this, it is a good idea to keep the equation for K in mind. According to that equation, if one product increases the other products must decrease to keep the same ratio of K. Additionally, if one reactant increases then the products must increase to keep the same ratio ...
- Thu Jan 16, 2020 11:27 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Ka and Kb
- Replies: 5
- Views: 170
Re: Ka and Kb
K B and K A are calculated the same way. From what I gathered from the lecture, K B has to do with the equilibrium constant of bases and has [OH - ] as a product. Conversely, K A has to do with the equilibrium constant of acids and has [H 3 O + ] as a product. That's the extent to how much they diff...
- Thu Jan 16, 2020 11:21 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Factos effecting Equilibrium
- Replies: 6
- Views: 261
Re: Factos effecting Equilibrium
Temperature is the only thing that affects the equilibrium constant k. Factors such as concentrations of species, partial pressures of species, and volume of the container can all affect the reaction quotient, q, and drive the reaction in a certain direction in order to achieve equilibrium again.
- Thu Jan 09, 2020 8:26 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Solutions Manual
- Replies: 4
- Views: 248
Re: Solutions Manual
You can find the solutions manual at Powell Library. It is on hold for this class, and every time I check it out it has been available. Just ask the help desk and have your Bruin Card on hand.
- Thu Jan 09, 2020 8:25 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kc and Kp
- Replies: 4
- Views: 203
Re: Kc and Kp
Kc is used for concentration in general, it applies to aqueous solutions and gases. Kp applies to partial pressures of gases.
- Thu Jan 09, 2020 8:22 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Catalyst and Equilibrium
- Replies: 2
- Views: 95
Re: Catalyst and Equilibrium
A catalyst speeds up a reaction but is not used during it, it is neither a product nor a reactant. It doesn't affect the concentrations of product or reactant at equilibrium.
- Thu Jan 09, 2020 8:17 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Units for K
- Replies: 10
- Views: 294
Re: Units for K
K is technically calculated using an attribute called the activity of a substance, and activity is unitless.
- Thu Jan 09, 2020 8:16 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Calculating K
- Replies: 6
- Views: 287
Calculating K
In a reaction where the only substance that can be inputed into K is on the reactant side (the rest of the reactants and products are either solids or pure liquids), is K calculated as just the concentration of that substance? Or is it the inverse of the concentration?
- Fri Nov 29, 2019 9:15 pm
- Forum: Naming
- Topic: Polydentate Ligands
- Replies: 3
- Views: 263
Re: Polydentate Ligands
It's tough to say but generally the bonding sites should be on the same side of the molecule. For example, cisplatin works because both of the Chlorines are on the same side and can bind to DNA at two points. If the Chlorines were in an orientation where they were opposite each other, the resulting ...
- Fri Nov 29, 2019 9:08 pm
- Forum: Bronsted Acids & Bases
- Topic: Strong acids and bases
- Replies: 2
- Views: 100
Re: Strong acids and bases
I would study the strong acids and bases, as well as the trends in what makes an acid/base strong or weak.
- Fri Nov 29, 2019 6:18 pm
- Forum: Bronsted Acids & Bases
- Topic: Bronsted base
- Replies: 3
- Views: 100
Re: Bronsted base
In the following reaction:
HCl(aq)+NH3(aq)→NH+4(aq)+Cl−(aq)
The NH3 accepts H+ ions donated from the HCl. That makes it a bronsted base.
HCl(aq)+NH3(aq)→NH+4(aq)+Cl−(aq)
The NH3 accepts H+ ions donated from the HCl. That makes it a bronsted base.
- Fri Nov 29, 2019 6:08 pm
- Forum: Hybridization
- Topic: Writing the hydrization
- Replies: 10
- Views: 768
Re: Writing the hydrization
I think it wouldn't hurt to specify the principle quantum number when you know what the central atom is. If you only know the areas of electron density around the central atom, then you can only discern the hybridization without the principle quantum number.
- Fri Nov 29, 2019 5:54 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Rydberg
- Replies: 4
- Views: 342
Re: Rydberg
A photon is absorbed when the energy of an electron increases and its principle quantum number increases. The energy is positive when a photon is absorbed.
- Tue Nov 19, 2019 2:20 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: h bonding and dipole dipole
- Replies: 4
- Views: 290
Re: h bonding and dipole dipole
Yes, a molecule with hydrogen bonding must also have dipole-dipole interactions.
- Tue Nov 19, 2019 1:21 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Linear Shape
- Replies: 6
- Views: 371
Re: Linear Shape
Only if the bonded atoms on either side of the central atom are the same will the molecule be nonpolar. If the bonded atoms are different, there will be a net dipole moment.
- Tue Nov 19, 2019 1:19 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bond Angles
- Replies: 4
- Views: 230
Re: Bond Angles
In most cases when there is a lone pair, the actual bond angles are less than the expected for the electron arrangement. This is because lone pairs are more diffuse than bonding atoms, in other words they take up more space. As a result, they push the bonding atoms away more than a bonding atom woul...
- Tue Nov 19, 2019 1:14 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Types of forces
- Replies: 4
- Views: 207
Re: Types of forces
It does not mention that there are other ions around, therefore there are no ion-dipole forces. Also, the question does not mention that there are other nonpolar molecules around, so there are no dipole-induced dipole forces.
- Tue Nov 19, 2019 1:10 pm
- Forum: Dipole Moments
- Topic: Elongated vs. Spherical Molecules
- Replies: 3
- Views: 293
Re: Elongated vs. Spherical Molecules
The molecular formulas of organic molecules will typically be written in sections. If a molecule has many sections, such as CH3(CH2)3CH3, then it will be elongated. A molecule written like C5H12, is spherical. Notice how they have the same amounts of C and H atoms, but they are written in a way that...
- Tue Nov 19, 2019 1:05 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Trigonal Bipyramidal
- Replies: 1
- Views: 124
Re: Trigonal Bipyramidal
If all the equatorial atoms are the same, you can break the dipole vectors into their component vectors and you will see that they all cancel. The axial atoms are pointed 180 degrees apart, so their dipoles cancel.
- Tue Nov 19, 2019 1:01 pm
- Forum: Lewis Structures
- Topic: H2SeO4
- Replies: 2
- Views: 359
Re: H2SeO4
The lewis structure where Se is surrounded by four oxygens allows for all the formal charges to be zero when two of the oxygens double-bond with Se and the other two oxygens single bond with Se and H. The lewis structure where the two hydrogens bond with the central Se atom doesn't allow for all for...
- Tue Nov 19, 2019 12:56 pm
- Forum: Dipole Moments
- Topic: Polar/Nonpolar Molecule Question
- Replies: 2
- Views: 191
Re: Polar/Nonpolar Molecule Question
If you draw the molecule out, it will look like 2 C 2 H 5 groups bonded on either side of a central oxygen atom. The oxygen can only single bond with both of the carbons next to it because the carbons already have three bonds. This leaves the central oxygen atom with two bonding pairs and two lone p...
- Tue Nov 19, 2019 12:51 pm
- Forum: Bond Lengths & Energies
- Topic: Boiling point
- Replies: 11
- Views: 1212
Re: Boiling point
If a problem strictly mentions molar mass, or states that there are no dipole-dipole interactions or hydrogen bonding, then increasing molar mass increases the Van de Waal forces because larger molecules can have larger instantaneous dipole moments. Larger Van de Waal forces means more attraction be...
- Tue Nov 19, 2019 12:49 pm
- Forum: Hybridization
- Topic: How to do 2.F.7?
- Replies: 1
- Views: 98
Re: How to do 2.F.7?
You determine the hybridization of orbitals by the number of areas of electron density around a central atom. Areas of electron density include bonding pairs and lone pairs. If there are n number of areas of electron density, you need n number of hybridized orbitals.
- Tue Nov 19, 2019 12:45 pm
- Forum: Hybridization
- Topic: hybridization
- Replies: 11
- Views: 576
Re: hybridization
Look at the lewis structure to help figure out the hybridization of an atom. If a central atom has four areas of electron density around it, then you need a hybridization that corresponds to those four areas. In this case, sp 3 . If an atom has three areas of electron density, the hybridization is s...
- Tue Nov 19, 2019 12:42 pm
- Forum: Dipole Moments
- Topic: NH2OH
- Replies: 2
- Views: 302
Re: NH2OH
The overall shape of the molecule is not not symmetrical, there is a net dipole moment because the dipoles are not equal in magnitude and in opposite directions.
- Tue Nov 19, 2019 12:39 pm
- Forum: Hybridization
- Topic: test 2
- Replies: 6
- Views: 389
Re: test 2
According to my TA hybridization won't be on the test, but it wouldn't hurt to know since it explains the behavior of some of the topics mentioned on the test.
- Tue Nov 19, 2019 12:37 pm
- Forum: Dipole Moments
- Topic: Polar or nonpolar
- Replies: 8
- Views: 579
Re: Polar or nonpolar
O2 is nonpolar because it is two of the same atom, there is no net dipole moment because both oxygens "pull" on electrons the same amount in opposite directions.
- Tue Nov 19, 2019 12:34 pm
- Forum: Hybridization
- Topic: What are pi bonds in relation to sigma?
- Replies: 2
- Views: 189
Re: What are pi bonds in relation to sigma?
Pi bonds form in cases where there is one or more p orbitals that have not been hybridized into an spn orbital. These leftover p orbitals are able to form a pi bond by bonding perpendicular to the plane of the sigma bond.
- Sun Nov 10, 2019 11:43 pm
- Forum: Electronegativity
- Topic: Noble Gases
- Replies: 40
- Views: 12808
Re: Noble Gases
I like to consider electronegativity as how much an atom will hog an electron when it is bonded. Since Neon already has an octet, it won't want any more electrons so, hypothetically, it will have very low electronegativity.
- Sun Nov 10, 2019 11:40 pm
- Forum: Dipole Moments
- Topic: Intermolecular Forces
- Replies: 2
- Views: 131
Re: Intermolecular Forces
Although nonpolar molecules may have symmetrical electron distributions, there are instances when one area of the molecule is more negative than the other. This fluctuating electron distribution results in fluctuating dipoles. Van der Waals forces are the results of those instantaneous dipoles that ...
- Sun Nov 10, 2019 11:31 pm
- Forum: Dipole Moments
- Topic: Representation
- Replies: 1
- Views: 144
Re: Representation
I think it is represented with an arrow with its tail beginning at the positive atom and pointing toward the negative atom.
- Sun Nov 10, 2019 11:30 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Polar vs covalent
- Replies: 7
- Views: 426
Re: Polar vs covalent
Covalent bonds occur between two nonmentals, while ionic bonds occur between a metal and a nonmetal.
- Sun Nov 10, 2019 11:27 pm
- Forum: Dipole Moments
- Topic: H Bonding
- Replies: 4
- Views: 285
Re: H Bonding
H bonding occurs with N, O, and F because those elements have high electronegativity. When they bond covalently with Hydrogen, they will hog the electrons more and make that part of the molecule partially negative and the hydrogen atom partially positive. While this can occur with other elements, th...
- Tue Oct 29, 2019 12:38 pm
- Forum: Ionic & Covalent Bonds
- Topic: Study For midterm
- Replies: 7
- Views: 367
Re: Study For midterm
I would look thoroughly through the topics discussed in the syllabus and try to do a problem pertaining to each one. Bring up questions you have with your TA and spread the studying out over several days.
- Tue Oct 29, 2019 12:33 pm
- Forum: Lewis Structures
- Topic: Homework problem 2C.3
- Replies: 5
- Views: 160
Re: Homework problem 2C.3
I asked my TA and he said that we aren't expected to know nomenclature right now, but I would try to memorize the important polyatomic ions like phosphate.
- Tue Oct 29, 2019 12:28 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: HW Question 2A5
- Replies: 3
- Views: 131
Re: HW Question 2A5
It is energetically favorable for Cu to have a ground state electron configuration of [Ar]3d 10 4s 1 . This is because when the 4s shell is half-filled and the 3d shell is completely filled, the atom is more stable. When you take away an electron, you take it away from the 4s shell because it is fur...
- Tue Oct 29, 2019 12:16 pm
- Forum: Resonance Structures
- Topic: stability
- Replies: 2
- Views: 98
Re: stability
Resonance allows for delocalization, in which one or more electrons are spread out evenly across all bonds. This lowers the overall energy of the molecule.
- Tue Oct 29, 2019 12:07 pm
- Forum: Resonance Structures
- Topic: hybrid structure
- Replies: 4
- Views: 173
Re: hybrid structure
No, resonance happens when you have the same arrangement of atoms, but different arrangements of electrons. For example, in ClO- the single bond could be in four different locations.
- Sun Oct 20, 2019 9:49 pm
- Forum: DeBroglie Equation
- Topic: Unit Conversion
- Replies: 5
- Views: 177
Re: Unit Conversion
The prefix Mega (M) in SI units always means 10^6. So 1 MHz is 1*10^6 Hz.
- Sun Oct 20, 2019 9:45 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Electron configurations of ions
- Replies: 4
- Views: 183
Re: Electron configurations of ions
Elements that are closer to the left side of the periodic table generally give up electrons and elements on the right side of the periodic table generally take electrons. This is reflected in the increasing ionization energy going right to left across a period.
- Sun Oct 20, 2019 9:40 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Exceptions within P.t.
- Replies: 2
- Views: 93
Re: Exceptions within P.t.
In addition to the half-filled 4s orbital lending more stability, a completely full 3d orbital is more stable than one that is partially filled. If I remember correctly, Dr. Lavalle also said something about the 3d orbital having slightly less energy than the 4s orbital, but I'm not sure if that has...
- Sun Oct 20, 2019 9:23 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Noble Gases in Electron Configurations
- Replies: 6
- Views: 411
Re: Noble Gases in Electron Configurations
In addition to what everyone else said, I think it's because the noble gasses don't react much and are very stable, much like the innermost electrons that they represent.
- Sun Oct 20, 2019 9:20 pm
- Forum: Properties of Electrons
- Topic: Absorption and Emission Spectra of Electrons
- Replies: 1
- Views: 108
Absorption and Emission Spectra of Electrons
When a wavelength of light is absorbed in the UV (Lyman) region, then can we assume that n i is 1? And for wavelengths of light emitted that correspond to the Lyman series, can we assume that n f is 1? Or do we always assume that n f is 1 for regardless of whether the electron emits or absorbs a pho...
- Wed Oct 16, 2019 7:53 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Homework Problem Help
- Replies: 5
- Views: 243
Re: Homework Problem Help
An orbital must have 3 quantum numbers, and no two orbitals can have the same quantum numbers. Knowing this, b and d both indicate only 1 orbital because they list 3 quantum numbers. For a and c, since all 3 quantum numbers are not given we must list the other possible numbers to find the number of ...
- Wed Oct 16, 2019 5:41 pm
- Forum: DeBroglie Equation
- Topic: De Broglie Wavelength
- Replies: 7
- Views: 327
Re: De Broglie Wavelength
I think it just means to use the De Broglie equation because the De Broglie equation is derived from the equations E=pc, c= wavelength*frequency, and E=hv which we have learned before. However, this equation only works for any particle with a rest mass, momentum, and wavelength so if it asks for th...
- Wed Oct 16, 2019 5:36 pm
- Forum: Limiting Reactant Calculations
- Topic: Fundamentals Problem Question
- Replies: 1
- Views: 294
Re: Fundamentals Problem Question
This is a multistep reaction. You have to first balance both reaction equations. Next, you have to convert the amounts of phosphorus and oxygen into moles. Then, you use the mole ratio to find out which reactant will run out first. Phosphorus is the limiting reactant because it will run out first. O...
- Wed Oct 16, 2019 5:26 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: 1D.25
- Replies: 3
- Views: 184
Re: 1D.25
When a question asks whether a certain orbital can exist, it is asking if the quantum numbers are possible. If we look at 6f as an example, Because: n=6 The possible values of l are: l=(0 to n-1) or 0,1,2,3,4,5 We know that the f subshell occurs when l=3. Since 3 is listed in the possible values of ...
- Wed Oct 16, 2019 5:20 pm
- Forum: Properties of Electrons
- Topic: Relationship between Speed and Frequency
- Replies: 3
- Views: 166
Re: Relationship between Speed and Frequency
We assume the speed of light to be constant, so any changes in frequency won't impact the speed of the wave. Also, 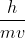 only applies to particles with resting mass like electrons, not electromagnetic radiation.
only applies to particles with resting mass like electrons, not electromagnetic radiation.
- Wed Oct 09, 2019 1:02 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Light energy
- Replies: 3
- Views: 241
Re: Light energy
I think you just need to memorize the fact that visible light occurs between 400 and 700 nm.
- Wed Oct 09, 2019 12:58 pm
- Forum: Properties of Light
- Topic: Homework Problems
- Replies: 3
- Views: 266
Re: Homework Problems
The questions for 1A begin on page 9. The last question for 1F is on page 63.
- Wed Oct 09, 2019 12:53 pm
- Forum: SI Units, Unit Conversions
- Topic: Sig Fig Help! [ENDORSED]
- Replies: 6
- Views: 482
Re: Sig Fig Help! [ENDORSED]
1.0003 has 5 significant figures because the zeroes come between nonzero digits. 0.0003 only has 1 significant figure. This is because you can rewrite it as 3x10^-4 (which only has one sigfig). The leading zeroes are not significant even if they come after a decimal point.
- Wed Oct 09, 2019 12:49 pm
- Forum: Empirical & Molecular Formulas
- Topic: Writing Empirical Formulas
- Replies: 8
- Views: 897
Re: Writing Empirical Formulas
Generally, the order the elements are given in the problem will be the same order you can write them in your answer. In organic molecules, that normally means C, H, then everything else.
- Wed Oct 09, 2019 12:45 pm
- Forum: Photoelectric Effect
- Topic: Threshold energy
- Replies: 6
- Views: 163
Re: Threshold energy
Different elements have different threshold energies. If it's not given, you can always determine the threshold energy by using
Threshold energy=Energy of photon-kinetic energy of ejected electron
Threshold energy=Energy of photon-kinetic energy of ejected electron
- Tue Oct 01, 2019 8:54 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: Question on E27
- Replies: 4
- Views: 123
Re: Question on E27
You use the molar masses of oxygen and hydrogen to find the mass of one mole of water molecules. Next, divide that number by avogadro's constant to find the mass of one water molecule.
- Tue Oct 01, 2019 8:49 pm
- Forum: Balancing Chemical Reactions
- Topic: Question H7a
- Replies: 6
- Views: 324
Re: Question H7a
Calcium is in the second group of the periodic table so calcium ions are Ca2+. Hydroxide ions have a -1 charge, so you need 2 OH to make the overall charge neutral. So Calcium hydroxide is Ca(OH)2.
- Tue Oct 01, 2019 4:31 pm
- Forum: Significant Figures
- Topic: Sig Figs for Tests/Quizzes
- Replies: 7
- Views: 432
Re: Sig Figs
For sig figs, do we round to the correct number of sig figs at the end or do we constantly round to the correct number as we go? Generally, it is better to try to use exact values throughout the calculations and then round at the end. If your calculator has it, it could be helpful to save the exact...
- Tue Oct 01, 2019 4:23 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Accuracy and Precision [ENDORSED]
- Replies: 4
- Views: 265
Re: Accuracy and Precision [ENDORSED]
I don't think we need to calculate the level of precision and accuracy, we just need to identify situations and experimental results that are precise, accurate, both and neither.

