This is a helpful link if you're confused!
https://www.khanacademy.org/science/che ... r-small-kc
Search found 104 matches
- Thu Mar 12, 2020 10:07 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Approximating X
- Replies: 13
- Views: 854
- Thu Mar 12, 2020 10:05 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: midterm q3c
- Replies: 3
- Views: 370
Re: midterm q3c
This was a bit of a tricky question - much due to the fact that we did not know what buffers were! Buffers have a region where the pH does not change as much despite adding acids or bases. The way to make a solution decrease in pH is by adding more acidic compounds, so one would need to identify the...
- Thu Mar 12, 2020 9:58 am
- Forum: Balancing Redox Reactions
- Topic: Test 2
- Replies: 19
- Views: 982
Re: Test 2
Test 2 is a good review for Electrochem. I was pretty surprised about the last question! I didn't know that the electrode size in the anode doesn't matter!
- Thu Mar 12, 2020 9:48 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Just a reminder about pH and pKa!
- Replies: 2
- Views: 349
Just a reminder about pH and pKa!
Comparing pKa/pKb with pH/pOH will be important to know how to do on the final - I think! See...pKa = -log(Ka), so the higher the pKa, the lower the Ka. Ka = \frac{[A-][H+]}{[HA]} Lower Ka means higher products - and lower H+ pH = -log(H+), which means the higher the pH, the lower the H+ Therefore, ...
- Thu Mar 12, 2020 9:38 am
- Forum: *Making Buffers & Calculating Buffer pH (Henderson-Hasselbalch Equation)
- Topic: pH of a buffer solution calculation
- Replies: 4
- Views: 1520
Re: pH of a buffer solution calculation
How do we calculate the pH of a buffer solution? Are there any good videos explaining this? We only have to calculate the pH based on the ice table example used in the notes. Exactly! But the Henderson-Hasselbach Equation is a shortcut that lets us skip the ICE table entirely! The equation can only...
- Sun Mar 08, 2020 3:52 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Nernst
- Replies: 10
- Views: 641
Re: Nernst
If you are struggling with the Nernst equation, I suggest watching this video:
https://www.khanacademy.org/science/che ... t-equation
It's pretty helpful!
https://www.khanacademy.org/science/che ... t-equation
It's pretty helpful!
- Sun Mar 08, 2020 3:50 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: concentration cells
- Replies: 5
- Views: 420
Re: concentration cells
You will need to use the Nernst equation for most concentration cell questions, especially for finding Ecell or a concentration of a half-cell. Just like the comment before this one says, E°cell = 0, and we have to apply this information to get more information.
- Sun Mar 08, 2020 3:47 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: When to add Platinum
- Replies: 8
- Views: 485
Re: When to add Platinum
Platinum is usually included when there is no electrode available to drive the reaction.
- Sun Mar 08, 2020 3:46 pm
- Forum: Balancing Redox Reactions
- Topic: spontaneous redox reactions
- Replies: 4
- Views: 379
Re: spontaneous redox reactions
In any sort of chemistry, a reaction is spontaneous when it has enough energy to go to completion and to be released (or when the energy required to break bonds is less than the energy required to form bonds). This is also present in redox reactions.
- Tue Mar 03, 2020 12:17 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: A Reactivity Epiphany
- Replies: 2
- Views: 538
A Reactivity Epiphany
This is not a question, but a mere epiphany I had while studying for the second chem test! Does anyone remember the activity series from high school? I sure didn't until a few hours ago. For those of you who don't remember, or have never seen one, here is a link to one: https://s2.studylib.net/store...
- Sat Feb 29, 2020 11:48 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Le Chateliers with Ecell
- Replies: 2
- Views: 258
Re: Le Chateliers with Ecell
I would say that Le Chat applies to E-cell. For example, if you increase the concentrations of reactants, more products will form, and more reactants will be oxidized. In a cell ... I'm not sure if the reverse can occur.
- Sat Feb 29, 2020 11:45 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: platinum
- Replies: 5
- Views: 404
Re: platinum
You don't have to use platinum, but platinum is the best solid conductor to use for this class. There are other solid conductors out there that can be used in these reactions, and if the question wants you to use them, they will provide the data for them.
- Sat Feb 29, 2020 11:43 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: potential vs voltage
- Replies: 2
- Views: 251
Re: potential vs voltage
Also, the max potential is when no voltage has passed.
- Sat Feb 29, 2020 11:42 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Anode vs Cathode
- Replies: 15
- Views: 869
Re: Anode vs Cathode
There is no left or right in determining what an anode or cathode is. You should know that the anode is the part where things get oxidized and the cathode is the part where things get reduced. They may try to trick you on the test by switching the positions of the anode and cathode.
- Sat Feb 29, 2020 11:40 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: No Salt Bridge
- Replies: 7
- Views: 557
Re: No Salt Bridge
I found this site helpful when learning about the function of a salt bridge, which is to make sure that only electrons provide the energy to the system.
https://chemistry.stackexchange.com/que ... re-be-used
I hope this helps!
https://chemistry.stackexchange.com/que ... re-be-used
I hope this helps!
- Sun Feb 23, 2020 12:21 pm
- Forum: Balancing Redox Reactions
- Topic: Oxidizing/Reducing Agents
- Replies: 11
- Views: 832
Re: Oxidizing/Reducing Agents
Oxidizing agents are reduced.
Reducing agents are oxidized.
These get tricky because what they do is opposite of what their names imply. It almost got me on my recent LS7A midterm, haha.
Reducing agents are oxidized.
These get tricky because what they do is opposite of what their names imply. It almost got me on my recent LS7A midterm, haha.
- Sun Feb 23, 2020 12:20 pm
- Forum: Balancing Redox Reactions
- Topic: Oxidation Numbers
- Replies: 9
- Views: 661
Re: Oxidation Numbers
The oxidation numbers for metals in their natural states are their charges, I believe.
- Sun Feb 23, 2020 12:16 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Autoprotolysis
- Replies: 6
- Views: 1176
Re: Autoprotolysis
Autoprotolysis is simply the proton transfer between two identical compounds, such as water, weak acids, etc.
- Sun Feb 23, 2020 12:15 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Cubic to quadratic function
- Replies: 4
- Views: 440
Re: Cubic to quadratic function
If you are referring to problems with ka, then there will usually be a step where you can ignore the x, so that a cubic will not be needed to be solved. We cannot solve cubics without a graphic calculator.
- Sun Feb 23, 2020 12:13 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Adding Inert Gas
- Replies: 20
- Views: 1129
Re: Adding Inert Gas
Additional inert Gases don't cause a shift in equilibrium, as they are not necessarily part of the reaction.
- Sun Feb 16, 2020 10:33 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: memorize
- Replies: 14
- Views: 859
Re: memorize
We are given the entropy values we need to know for the exam on the equation sheet as they did for the midterm. We will not know or use the entropy values that are not proved on the equation sheet.
- Sun Feb 16, 2020 10:30 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Delta H
- Replies: 10
- Views: 926
Re: Delta H
Delta H can usually be written as q. This is derived from the equation 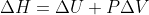 .
. 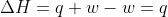 , so
, so 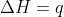 .
.
- Sun Feb 16, 2020 10:25 am
- Forum: Phase Changes & Related Calculations
- Topic: Delta H Fusion
- Replies: 9
- Views: 702
Re: Delta H Fusion
You've got to remember that temperature does not increase when the heat is added during a phase change. Therefore, we cannot include it in the regular  equation, which is why we multiply the heat inputted by the moles and use that to calculate the delta H Fusion.
equation, which is why we multiply the heat inputted by the moles and use that to calculate the delta H Fusion.
- Sun Feb 16, 2020 10:19 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Extensive and Intensive Properties
- Replies: 12
- Views: 1049
Re: Extensive and Intensive Properties
I used https://www.thoughtco.com/intensive-vs- ... ies-604133 when I was studying for my exam.
- Sun Feb 16, 2020 10:18 am
- Forum: General Science Questions
- Topic: 50 post grade
- Replies: 25
- Views: 1865
Re: 50 post grade
I thought that the posts were monitored via MyUCLA since we have to sign-on every time we post on Chem community. Therefore, as long as you have posted at least 5 times by Sunday 11:59 PM, you should get the five points of participation credit for said week.
- Sun Feb 09, 2020 5:23 pm
- Forum: Phase Changes & Related Calculations
- Topic: Heating Curve Phase Changes
- Replies: 11
- Views: 622
Re: Heating Curve Phase Changes
I believe that the heat energy inputted into the system at that time is there to break or form the bonds, transitioning the state of the sample.
- Sun Feb 09, 2020 5:21 pm
- Forum: Phase Changes & Related Calculations
- Topic: phase changes, temperature constant?
- Replies: 11
- Views: 632
Re: phase changes, temperature constant?
An increase in heat energy is not the same thing as an increase in temperature (delta H is different than T). Therefore it is possible for heat energy to be introduced to the system and for the temperature to not increase.
- Sun Feb 09, 2020 5:18 pm
- Forum: Phase Changes & Related Calculations
- Topic: Environment
- Replies: 4
- Views: 235
Re: Environment
Burning fossil fuels leads to an increase in carbon emissions, leading to effects such as global warming. Is this important to know for this course?
- Sun Feb 09, 2020 5:12 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3591732
Re: Post All Chemistry Jokes Here
Only in the world of chemistry can a Pb & J sandwich kill you. Unless you're allergic. Then you're also dead.
- Sun Feb 09, 2020 5:00 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Intensive vs Extensive
- Replies: 7
- Views: 367
Re: Intensive vs Extensive
Extensive properties depend on the sample size, such as mass and volume. Intensive properties, on the other hand, do not depend on the quantity of the sample we are looking at, such as the melting point of a sample. Samples with extensive properties can be calculated by subtracting the final amount ...
- Fri Jan 31, 2020 9:30 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Equilibrium shift by pressure
- Replies: 7
- Views: 269
Re: Equilibrium shift by pressure
Equilibrium constant does not change (it only changes if temperature changes), but the equilibrium will shift in such a way that the equilibrium constant remains the same after calculation.
- Fri Jan 31, 2020 9:16 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Equilibrium shift by pressure
- Replies: 7
- Views: 269
Re: Equilibrium shift by pressure
The inverse is also true. If the pressure is decreased (more volume available in the system), the equilibrium will shift towards the side that has more moles.
- Fri Jan 31, 2020 9:12 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Strong/weak acids & bases
- Replies: 14
- Views: 949
Re: Strong/weak acids & bases
You should be able to tell if something is a weak acid or base, based on if it can accept a proton or not. Also, you should have the strong acids memorized.
- Fri Jan 31, 2020 9:08 pm
- Forum: Ideal Gases
- Topic: final exam pickup
- Replies: 10
- Views: 497
Re: final exam pickup
If you still haven't picked up your final exam, don't fret! They still have them until the end of the quarter ... but its best to pick them up ASAP.
- Fri Jan 31, 2020 9:05 pm
- Forum: Calculating Work of Expansion
- Topic: Difference between irreversible/reversible reaction?
- Replies: 2
- Views: 177
Re: Difference between irreversible/reversible reaction?
Aren't reversible reactions where the reactants can go towards the products and the products can go back toward the reactants, like weak acid and base dissociation? That would mean that irreversible reactions would only go in one direction (from reactants to products), such as combustion reactions. ...
- Sun Jan 26, 2020 6:46 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: bar conversion
- Replies: 5
- Views: 225
Re: bar conversion
Also, if you are given the concentration and need to convert into bar, torr, etc., you would use the PV = nRT equation with the corresponding R.
- Sun Jan 26, 2020 6:41 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Pressure and equilibrium
- Replies: 9
- Views: 274
Re: Pressure and equilibrium
Also, solids and liquids are NOT affected by pressure.
- Sun Jan 26, 2020 6:37 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Order of homework for Thermochem unit
- Replies: 6
- Views: 348
Re: Order of homework for Thermochem unit
Honestly, I would just read the chapter and do the problems before the lecture, so that we know what he is talking about in the lecture - making it easier to understand.
- Sun Jan 26, 2020 6:36 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: state functions
- Replies: 4
- Views: 603
Re: state functions
State functions are any functions that can be derived by subtracting the final result by the initial result.
- Sun Jan 26, 2020 6:35 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: System vs Surroundings
- Replies: 14
- Views: 2137
Re: System vs Surroundings
The system is the area where the reaction is occurring. The surroundings are the outside of where the reaction is occurring. Think of the reaction happening in a beaker. If the reaction is endothermic, it requires energy to come to fruition. Therefore, it will take energy from the surroundings, or o...
- Sun Jan 19, 2020 5:02 pm
- Forum: Ideal Gases
- Topic: 5I.15
- Replies: 5
- Views: 457
Re: 5I.15
The solid would not matter when setting up the ICE table. The products do matter. What I do is cross out the section for the reactant, and add x to both the products. Then, you can solve using k.
- Sun Jan 19, 2020 4:59 pm
- Forum: Ideal Gases
- Topic: ph
- Replies: 10
- Views: 513
Re: ph
The pH scale is not restricted per se, but it is at room temp. I don't think that they will ever ask you a question where the answer is pH 15.45 though. Oh, and pH can never be negative.
- Sun Jan 19, 2020 4:56 pm
- Forum: Ideal Gases
- Topic: Topics on Test 1
- Replies: 37
- Views: 1385
Re: Topics on Test 1
Yeah, it is everything in outlines 1 and 2. Make sure you know the ICE table, Le'chat, and how to manipulate the important equations, Also, pH is very important.
- Sun Jan 19, 2020 4:55 pm
- Forum: Ideal Gases
- Topic: Solids and liquids
- Replies: 4
- Views: 310
Re: Solids and liquids
Think about it. We are focusing in on an equilibrium reaction between concentrations or gases. If we were to take in account for solids and liquids, we would have to take into account another form of k - muddling up the equation.
- Sun Jan 19, 2020 4:45 pm
- Forum: Ideal Gases
- Topic: PV=nRT
- Replies: 13
- Views: 697
Re: PV=nRT
We can use the equation to relate the values before and after the reaction, like comparing pressure, volume, and concentration.
- Sun Jan 12, 2020 9:54 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Values of K and Meaning
- Replies: 3
- Views: 195
Re: Values of K and Meaning
I feel like K>10^3 and K<10^-3 is the absolute bounds for the favoritism of the reaction.
- Sun Jan 12, 2020 9:52 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: "quick" way?
- Replies: 4
- Views: 182
Re: "quick" way?
The long way is comparing the K with the newly made Q that occurs after Le Chat occurs. We do this by calculating the equilibrium constant K and Q, see which one is greater, and decide whether the reaction shifta to the left or right. The quick way is by simply looking at the reaction and counting t...
- Sun Jan 12, 2020 9:46 pm
- Forum: Ideal Gases
- Topic: Le Chatelier's Principle
- Replies: 7
- Views: 468
Re: Le Chatelier's Principle
Le Chatelier's Principle is a way to make sure a reaction stays at equilibrium when a different force, such as pressure or volume, is altered.
- Thu Jan 09, 2020 3:01 pm
- Forum: Ideal Gases
- Topic: non gases
- Replies: 3
- Views: 546
Re: non gases
In ICE tables (which we will learn about later), we ignore solids and liquids and only worry about aqueous solutions and gases. Would the ideal gas law be relevant to why we ignore those two states?
- Thu Jan 09, 2020 2:59 pm
- Forum: Ideal Gases
- Topic: K
- Replies: 10
- Views: 523
Re: K
K is the equilibrium constant, which can be measured by either comparing the concentrations of the reactants and the products in the reaction, or the partial pressures of the reactants and products in the said reaction. Since K is a constant, whenever it is used (as Kc or Kp), it should equate. With...
- Sat Dec 07, 2019 8:57 pm
- Forum: Lewis Acids & Bases
- Topic: Lewis structure of acids and bases
- Replies: 2
- Views: 233
Re: Lewis structure of acids and bases
The filling of the octets is the most important aspect - double and triple bonds come in later. Double bonds should be used when both the central and attached atom has an extra lone pair each, and if the formal charge conditions are most favorable. I am not sure what you are talking about regarding ...
- Sat Dec 07, 2019 8:24 pm
- Forum: Hybridization
- Topic: what are terminal atoms?
- Replies: 3
- Views: 8488
Re: what are terminal atoms?
Actually, the Cl would have the hybridization of sp3.
- Sat Dec 07, 2019 7:55 pm
- Forum: Industrial Examples
- Topic: what examples should we know?
- Replies: 6
- Views: 1282
Re: what examples should we know?
Cisplatin is for cancer prevention. On Marshmallow, this explanation was posted: Chemotherapy drug, Cl on same side are involved in the binding of cisplatin to Guanine base pairs in DNA that are adjacent or near each other. The binding at 2 base pairs allows cisplatin to block DNA replication machin...
- Fri Dec 06, 2019 1:42 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: naming
- Replies: 1
- Views: 148
Re: naming
I am not sure what you mean by "going both ways", but you would name a coordination compound by starting off by the ligand names in alphabetical order. Then you would write down the cation names in alphabetical order. Finally, if there are any anions, you would write them at the end.
- Fri Dec 06, 2019 1:38 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: structure
- Replies: 1
- Views: 150
Re: structure
We would look at the naming chart Lavelle provided us and write out the formula. Then using that formula, we would draw the structure.
Here is the naming chart:
https://lavelle.chem.ucla.edu/wp-conten ... pounds.pdf
Here is the naming chart:
https://lavelle.chem.ucla.edu/wp-conten ... pounds.pdf
- Sun Dec 01, 2019 2:29 pm
- Forum: Sigma & Pi Bonds
- Topic: Drawing Sigma and Pi bonds
- Replies: 7
- Views: 768
Re: Drawing Sigma and Pi bonds
For my exam, I simply labeled each bond as a sigma bond and each double bond with an extra pi bond. That was the correct way to do it, which means you probably got full points unless you accidentally missed one.
- Sun Dec 01, 2019 2:26 pm
- Forum: Electronegativity
- Topic: H-bonding Question
- Replies: 2
- Views: 245
Re: H-bonding Question
Certain questions may ask for all hydrogen bonding sites, which would be all the lone pairs and hydrogen bondings to N O and F in a molecule.
- Sun Dec 01, 2019 2:22 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Ions
- Replies: 3
- Views: 376
Re: Ions
Not all atoms can create ionic bonds, but I believe that they all can be ionic to a point.
- Sun Dec 01, 2019 2:21 pm
- Forum: Sigma & Pi Bonds
- Topic: Ionic bond --> sigma and pi bonds
- Replies: 8
- Views: 1544
Re: Ionic bond --> sigma and pi bonds
Sigma bonds are formed when two molecules share electrons. Ionic bonds do not share electrons - but exchange them. Therefore, bonds such as NaCl do not form sigma bonds.
- Sat Nov 30, 2019 6:41 pm
- Forum: Significant Figures
- Topic: Sig Figs on Tests
- Replies: 24
- Views: 2243
Re: Sig Figs on Tests
Now, when multiplying two or more numbers, it is important to have the final answer in the form of the smallest sig figs. Values found on the periodic table do not count in this sig fig process. Values found through calculation or in the problem count as sig figs. If the sig figs are not clear, it i...
- Sun Nov 24, 2019 6:01 pm
- Forum: Naming
- Topic: Latin names
- Replies: 1
- Views: 154
Re: Latin names
The IUPAC naming system is used in organic chemistry. I believe that we have the freedom to choose which naming scheme we use in this class. BUT, we will need to have memorized the naming scheme. We will not be provided anything on the test. I believe that is how it works.
Re: Testing
Probably naming compounds that he gives to us on the test. It can also be part of a problem where you have a specific compound that you have just used VSEPR for in finding the shape, lewis structure, etc.
- Sun Nov 24, 2019 5:55 pm
- Forum: Naming
- Topic: Oxidation Numbers
- Replies: 4
- Views: 432
Re: Oxidation Numbers
Oxidation numbers are rules that you need to memorize. The following link is from Khan Academy regarding identifying Oxidation numbers. It is pretty helpful. https://www.khanacademy.org/science/chemistry/chemical-reactions-stoichiome/types-of-chemical-reactions/a/oxidation-number I really do hope th...
- Sun Nov 24, 2019 5:52 pm
- Forum: Biological Examples
- Topic: Examples
- Replies: 1
- Views: 189
Re: Examples
There are many examples of biological coordination compounds, like chlorophyll or hemoglobin. I think that you find the following website helpful:
https://chemistrynotesinfo.com/coordina ... -class-12/
I hope this helps!
https://chemistrynotesinfo.com/coordina ... -class-12/
I hope this helps!
- Sun Nov 24, 2019 3:14 pm
- Forum: Naming
- Topic: Chemistry Community
- Replies: 4
- Views: 294
Re: Chemistry Community
I suggest reading the comments. Honestly, after reading what others have to ask, I realize that there are certain topics that I had forgotten to study. It is indeed a helpful tool.
- Tue Nov 12, 2019 9:32 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Molecular Shape
- Replies: 3
- Views: 210
Re: Molecular Shape
The surface area of the shape is important when it comes to dipole moments. If the two molecules expose more surface area to one another, then they have more London attractions. The molecules will be highly polarizable and have a greater intermolecular force than two molecules that expose less surfa...
- Tue Nov 12, 2019 9:26 am
- Forum: Formal Charge and Oxidation Numbers
- Topic: Formal Charge Placement
- Replies: 4
- Views: 505
Re: Formal Charge Placement
Negative charges tend to be put on the more electronegative atom, while the positive charge is put on the atom affected by the electronegative atom. Look at SO2. There is a negative charge on the Oxygen (the more electronegative atom), and a positive charge on Sulfur (the element affected by the ele...
- Tue Nov 12, 2019 9:20 am
- Forum: Formal Charge and Oxidation Numbers
- Topic: Formal Charge Cancellation
- Replies: 3
- Views: 578
Re: Formal Charge Cancellation
Exactly. Look at CO for example. The lewis structure would indicate a triple bond between the two atoms. If we are to fill the octet correctly, the final formal charge for Oxygen is +1 and the final formal charge for Carbon is -1. These two cancel out to 0, which is the overall charge of the molecule.
- Tue Nov 12, 2019 9:17 am
- Forum: Formal Charge and Oxidation Numbers
- Topic: Best Formal Charge
- Replies: 7
- Views: 484
Re: Best Formal Charge
For the question right above this comment:
Exactly. Atoms such as fluorine and oxygen are highly electronegative and are able to pull electrons closer to themselves. Putting a negative on them only makes sense because they have a natural tendency to be negative in a molecule.
Exactly. Atoms such as fluorine and oxygen are highly electronegative and are able to pull electrons closer to themselves. Putting a negative on them only makes sense because they have a natural tendency to be negative in a molecule.
- Tue Nov 12, 2019 9:11 am
- Forum: Sigma & Pi Bonds
- Topic: Sigma & Pi Bonds
- Replies: 5
- Views: 260
Re: Sigma & Pi Bonds
Pi bonds are also more commonly found in double and triple bonds, while they are not included in single bonds. Pi bonds add bond strength to the bonds - hence why double and triple bonds are stronger than single bonds. All types of bonds have sigma bonds.
- Fri Nov 08, 2019 1:40 pm
- Forum: Lewis Structures
- Topic: Exceptions to the Octet Rule Question
- Replies: 5
- Views: 615
Re: Exceptions to the Octet Rule Question
Only N, C, F and O follow the octet rule. The exceptions are when radicals are involved.
- Fri Nov 08, 2019 1:37 pm
- Forum: Lewis Structures
- Topic: Favorable Bonds
- Replies: 5
- Views: 244
Re: Favorable Bonds
Although N and O have favorable bonds, certain structures require them to have more or fewer bonds - causing them to have a charge. For example, NO+ shows a triple bond between the N and the O. The N is pleased with a formal charge of 0, but the O has a formal charge of +1, which causes the positive...
- Fri Nov 08, 2019 1:32 pm
- Forum: Lewis Structures
- Topic: Radicals
- Replies: 5
- Views: 270
Re: Radicals
This is more of an organic chemistry topic when you have to draw resonance structures regarding radical molecules. In orgo, the radical is most commonly found on the C, but in general, any atom can exhibit a radical. Radicals are notable when they appear on structures that should have their octet fi...
- Fri Nov 08, 2019 1:29 pm
- Forum: Lewis Structures
- Topic: Formal Charges
- Replies: 15
- Views: 965
Re: Formal Charges
It is best to have the negative charge on the most electronegative atom in the molecule, and the positive charge on the least electronegative atom in the molecule.
- Fri Nov 08, 2019 1:28 pm
- Forum: Lewis Structures
- Topic: Midterm grades
- Replies: 26
- Views: 1439
Re: Midterm grades
Think about it. There were some long questions, and because we had to write in pen - much of it would be messy. I would be surprised if we get our grades back before next week. Also, I think that we will get the papers back, as it had a scoring sheet for the students to see, and we were allowed to t...
- Fri Nov 01, 2019 1:14 pm
- Forum: Coordinate Covalent Bonds
- Topic: Distinguishing a coordinate covalent bond
- Replies: 5
- Views: 265
Re: Distinguishing a coordinate covalent bond
You have to remember the scale for covalent vs ionic character. If the difference between the electronegativities of the two atoms are less than 1.5, then it is a covalent bond.
- Fri Nov 01, 2019 1:10 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Formal Charges on Atoms Summed in Ions?
- Replies: 6
- Views: 205
Re: Formal Charges on Atoms Summed in Ions?
Also, the more formal charges there are that equal to zero, the more stable the molecule actually is.
- Fri Nov 01, 2019 1:08 pm
- Forum: Resonance Structures
- Topic: hybrid structure
- Replies: 4
- Views: 172
Re: hybrid structure
Hybrid structures are just resonance structures interpreted through one diagram. The double bonds are represented as one solid and one dotted, and there are enough electrons on each atom considering a double bond is present. Any bond can have resonance.
- Tue Oct 29, 2019 8:41 am
- Forum: Lewis Structures
- Topic: How to know where each atom should go for bonding?
- Replies: 3
- Views: 240
Re: How to know where each atom should go for bonding?
If you are looking at examples such as ONF from question 2B.1, you would see that to get a zero formal charge, O needs two bonds, N needs three, and F needs 1. Since N has the most bonding possibilities, N would be the central atom, and the O and F would be around it.
- Tue Oct 29, 2019 8:37 am
- Forum: Lewis Structures
- Topic: 2B. 1C
- Replies: 5
- Views: 178
Re: 2B. 1C
You can also try checking the formal charge. If we were to put a double bond between the N and the F, and a single bond between the N and the O, the formal charge for F would be +1, and the formal charge for O would be -1. This is worse than the zero formal charges across the board when the double b...
- Wed Oct 23, 2019 1:35 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Electron configuration for Cerium
- Replies: 2
- Views: 144
Re: Electron configuration for Cerium
Cerium is actually the first element in the Lanthanoid f-block, giving it the configuration: [Xe] 4f1 5d1 6s2. Lanthanum is part of the d-block with a configuration of [Xe] 5d1 6s2.
- Wed Oct 23, 2019 1:30 pm
- Forum: Trends in The Periodic Table
- Topic: Shielding
- Replies: 8
- Views: 573
Re: Shielding
Think of it like people around a campfire. The people close to the campfire will receive the most warmth, will people farther away won't receive as much. Therefore, the people farther away are more likely to go to a different fire where they get more warmth. The people close to the campfire are the ...
- Wed Oct 23, 2019 1:23 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Yet Another Electron Spin Question
- Replies: 5
- Views: 267
Re: Yet Another Electron Spin Question
I don't think so, but it is an interesting concept to think about.
- Wed Oct 23, 2019 1:22 pm
- Forum: Trends in The Periodic Table
- Topic: Radioactive elements
- Replies: 1
- Views: 108
Radioactive elements
Why don't we compare the periodic trends for radioactive elements? For example, according to the trends, Francium should have a larger atomic radius than Caesium, but we don't mention Francium or any other group 7 elements for that matter. Also, how do we compare the trends for the Lanthanoids and a...
- Tue Oct 22, 2019 8:41 am
- Forum: Quantum Numbers and The H-Atom
- Topic: Yet Another Electron Spin Question
- Replies: 5
- Views: 267
Re: Yet Another Electron Spin Question
The electrons would not collide with one another, but repel each other when they get close, shifting into a different spin. I think that is what happens, but I'm not a 100% sure.
- Tue Oct 22, 2019 8:38 am
- Forum: Quantum Numbers and The H-Atom
- Topic: Test Question
- Replies: 3
- Views: 183
Re: Test Question
I believe that the test might be more calculation heavy, but have parts where we might need to explain a certain circumstance such as explaining the trends. I took AP chem, and that is what we had to do. I acknowledge this class may be more difficult, so it might adopt a different formula for tests,...
- Thu Oct 17, 2019 4:11 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Advice for studying
- Replies: 92
- Views: 7142
Re: Advice for studying
My main way of studying is reading the entire chapter and then doing all the homework problems. If I do not understand any of the homework problems, I either go here, or go to my TA. If there is something I do not understand, I seek out help.
- Thu Oct 17, 2019 4:08 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Orbitals, 1D.11
- Replies: 2
- Views: 136
Re: Orbitals, 1D.11
Nice explanation!
Anyway, these are what I got:
a. 1 orbital
b. 5 orbitals
c. 3 orbitals
d. 7 orbitals
Anyway, these are what I got:
a. 1 orbital
b. 5 orbitals
c. 3 orbitals
d. 7 orbitals
- Thu Oct 17, 2019 4:06 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: HW Help
- Replies: 3
- Views: 140
Re: HW Help
I got this:
a. 3
b. 1
c. 4
d. 1
I just wrote the number of orbitals.
a. 3
b. 1
c. 4
d. 1
I just wrote the number of orbitals.
- Thu Oct 17, 2019 4:03 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: HW D13
- Replies: 3
- Views: 172
Re: HW D13
This is what I got after doing the question:
b. There are five values: 2, 1, 0, -1 and -2
c. There are three values: 1, 0 and -1
d. There are four subshells: 4s, 4p, 4d, and 4f
I hope this helps.
b. There are five values: 2, 1, 0, -1 and -2
c. There are three values: 1, 0 and -1
d. There are four subshells: 4s, 4p, 4d, and 4f
I hope this helps.
- Thu Oct 17, 2019 3:57 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Spin State
- Replies: 17
- Views: 422
Re: Spin State
A +1/2 spin is spinning up, while a -1/2 spin is spinning down. Remember, this is not like the electron is spinning north or south if it is spinning up or down. They are spinning in different directions. That's about it.
- Thu Oct 10, 2019 8:36 pm
- Forum: Properties of Light
- Topic: For future tests
- Replies: 3
- Views: 170
Re: For future tests
We should know where to apply the equation and how to use it, but not the specific experiments of history behind it. Tests - based on the first one - are more calculation based rather than history-based.
- Thu Oct 10, 2019 8:33 pm
- Forum: Einstein Equation
- Topic: Final Grade
- Replies: 5
- Views: 5934
Re: Final Grade
From Lavelle's website: "Each test and exam has a total score but is not assigned a grade. Only at the end of the class when the class average score (out of 500 points) is known are final grades assigned. This class does not use a curve. Group learning (Chemistry Community, Study Groups, Peer L...
- Thu Oct 10, 2019 8:32 pm
- Forum: Einstein Equation
- Topic: Planck's constant
- Replies: 9
- Views: 670
Re: Planck's constant
The equation helps us calculate the energy of light. Planks constant has the units: J*s, which cancels out with the frequency units: 1/s. It helps us find the energy in J (Joules).
- Thu Oct 10, 2019 8:27 pm
- Forum: Properties of Electrons
- Topic: Polar vs Non polar
- Replies: 15
- Views: 4764
Re: Polar vs Non polar
I love polar vs nonpolar stuff! The polarity of a compound depends on the charges and electronegativity of its structure. The electronegativity of atoms can be found on a periodic table such as this: http://www.thecatalyst.org/electabl.html Using the values from the periodic table above, you can mea...
- Thu Oct 10, 2019 8:13 pm
- Forum: Properties of Electrons
- Topic: Planck's Constant
- Replies: 3
- Views: 180
Re: Planck's Constant
Planck's constant is not really explored in depth in high school/college chem. If you would like to learn about the history of Planck's Constant, I recommend this Wikipedia article:
https://en.wikipedia.org/wiki/Planck_constant
https://en.wikipedia.org/wiki/Planck_constant
- Thu Oct 10, 2019 8:08 pm
- Forum: Properties of Light
- Topic: Frequency
- Replies: 2
- Views: 137
Re: Frequency
If you're interested in frequency, I recommend reading the Wikipedia page behind it:
https://en.wikipedia.org/wiki/Frequency
v (or nu) can also be expressed in Hz (Hertz), which is pretty interesting.
https://en.wikipedia.org/wiki/Frequency
v (or nu) can also be expressed in Hz (Hertz), which is pretty interesting.
- Fri Oct 04, 2019 1:19 pm
- Forum: SI Units, Unit Conversions
- Topic: Fundamental E.1
- Replies: 5
- Views: 193
- Thu Oct 03, 2019 2:13 pm
- Forum: Significant Figures
- Topic: Does molar mass count for sig figs?
- Replies: 6
- Views: 3031
Re: Does molar mass count for sig figs?
The molar mass from the periodic table does not affect our calculations, but the molar mass we get from a question or from our calculations do affect our calculations.
- Thu Oct 03, 2019 2:11 pm
- Forum: Significant Figures
- Topic: significant figures
- Replies: 5
- Views: 447
Re: significant figures
I posted this on another thread...so here is a copy:
What tricks me up the most is the zeroes involved in sig-figs. I'll show you why:
250000 has 2 sig-figs
250000. has 6-sig-figs
250.000 has 6 sig-figs
250.001 has 6 sig-figs
1.00025 has 6 sig-figs
0.00025 has 2 sig-figs
What tricks me up the most is the zeroes involved in sig-figs. I'll show you why:
250000 has 2 sig-figs
250000. has 6-sig-figs
250.000 has 6 sig-figs
250.001 has 6 sig-figs
1.00025 has 6 sig-figs
0.00025 has 2 sig-figs

