Search found 110 matches
- Sat Mar 14, 2020 4:58 am
- Forum: Administrative Questions and Class Announcements
- Topic: Is there a time limit on the final?
- Replies: 1
- Views: 222
Re: Is there a time limit on the final?
Oh nevermind... "The final is 6 questions in 3-4 hrs (still to be decided). Time will not be an issue."
- Sat Mar 14, 2020 4:52 am
- Forum: Administrative Questions and Class Announcements
- Topic: Is there a time limit on the final?
- Replies: 1
- Views: 222
Is there a time limit on the final?
Time limit or due date for the final? I didn't see anything about that in the emails.
- Sat Mar 14, 2020 4:19 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 6N.5
- Replies: 1
- Views: 188
Re: 6N.5
If we are just looking at the concentration of [H+], then it would be ^{-64})
But in the solutions manual, they combined^{-64}) with the other concentrations.
with the other concentrations.
This would be(10^{-64})} = 10^{66})
But in the solutions manual, they combined
This would be
- Tue Mar 10, 2020 8:09 pm
- Forum: Biological Examples
- Topic: Chem Final Review Worksheet
- Replies: 2
- Views: 785
Re: Chem Final Review Worksheet
Look up "endgame" to find the worksheets for Lyndon's electrochem/kinetics review.
- Tue Mar 10, 2020 4:33 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Worksheet for Review Session?
- Replies: 3
- Views: 402
Worksheet for Review Session?
I know for Lyndon's review session, I can look up endgame to find it, but is there a worksheet for the review session on Thursday, March 12, 6-9pm, Moore 100, Riya Shah, Matthew Tran, Kate Santoso, Chemical Equilibrium, Acids & Bases, Thermochem, Thermodynamics?
- Sun Mar 08, 2020 7:22 pm
- Forum: Zero Order Reactions
- Topic: examples of zero order reactions?
- Replies: 6
- Views: 537
Re: examples of zero order reactions?
I know in class, Lavelle used an example of a reaction that needs an enzyme or catalyst to describe a zero order reaction. Pretty much the catalyst is at max capacity, and the reaction can't go any faster. By adding more reactant, the reaction rate doesn't change, so it is a zero order reaction. I r...
- Sun Mar 08, 2020 5:13 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5.57
- Replies: 1
- Views: 188
Re: 5.57
Here's a picture of how to do it: I set up an ICE table. Our initial amounts are 0.245M SO3 and a certain amount of NO that we don't know yet. Since we know that 0.24M SO2 will be formed at equilibrium we can say the change is 0.24. Then by setting up the mass action, we can isolate the initial conc...
- Thu Mar 05, 2020 8:26 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6L.5
- Replies: 5
- Views: 417
Re: 6L.5
Usually for anodes, the solid is the reactant of the half reaction. In this case, the iodine solid is the product. I don't have an explanation why this needs the platinum because I don't know as well, but I think it does have something to do with the solid being a product. Hello, How did you determ...
- Thu Mar 05, 2020 8:11 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 6.61
- Replies: 2
- Views: 304
Re: 6.61
E⁰ means reduction potential under standard conditions, i.e. concentrations of 1 M in each cell and pressures of 1 atmosphere at 25 C degrees. In a concentration cell, the only thing driving the reaction is the difference in concentration. Under standard conditions, since both half cells have 1M, th...
- Thu Mar 05, 2020 7:53 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6L.5
- Replies: 5
- Views: 417
Re: 6L.5
Usually for anodes, the solid is the reactant of the half reaction. In this case, the iodine solid is the product. I don't have an explanation why this needs the platinum because I don't know as well, but I think it does have something to do with the solid being a product. Hello, How did you determ...
- Sun Mar 01, 2020 11:27 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6L.5
- Replies: 5
- Views: 417
Re: 6L.5
Usually for anodes, the solid is the reactant of the half reaction. In this case, the iodine solid is the product. I don't have an explanation why this needs the platinum because I don't know as well, but I think it does have something to do with the solid being a product.
- Sun Mar 01, 2020 11:13 pm
- Forum: Balancing Redox Reactions
- Topic: 6L.5d
- Replies: 2
- Views: 255
Re: 6L.5d
Write the half-reactions, the balanced equation for the cell reaction, and the cell diagram for each of the following skeletal equations: (d) Au^+ (aq) \rightarrow Au(s) + Au^{ 3+} (aq) Here are the half reactions I used: 2[Au^+(aq)+e^-\rightarrow Au(s)] and ...
- Sun Mar 01, 2020 10:48 pm
- Forum: Balancing Redox Reactions
- Topic: 6L.5d
- Replies: 2
- Views: 255
6L.5d
Write the half-reactions, the balanced equation for the cell reaction, and the cell diagram for each of the following skeletal equations: (d) Au^+ (aq) \rightarrow Au(s) + Au^{ 3+} (aq) Here are the half reactions I used: 2[Au^+(aq)+e^-\rightarrow Au(s)] and A...
- Sun Mar 01, 2020 10:15 pm
- Forum: Balancing Redox Reactions
- Topic: 6K 3 part a)
- Replies: 4
- Views: 391
Re: 6K 3 part a)
Here is how to balance the oxidation reaction in acidic solution:
- Wed Feb 26, 2020 4:03 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Diamond Reaction
- Replies: 4
- Views: 396
Diamond Reaction
Lavelle said that the reaction from diamond to graphite was spontaneous but had a very high activation energy and would occur very slowly. Does this mean that over time, say a few trillions years, the diamond is slowly collecting enough energy to overcome the energy barrier? Or would the reaction ev...
- Sun Feb 23, 2020 11:59 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: surroundings
- Replies: 4
- Views: 455
Re: surroundings
My explanation is sort of modeled off of 4I.9. ΔS of the surr depends on the circumstances. For a reversible process, the system is doing work to push the piston, but the surroundings is giving heat/energy in order to keep internal energy of the system consistent. This means ΔS(surr) = -ΔS(sys) and ...
- Sun Feb 23, 2020 11:58 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: 4F.13
- Replies: 2
- Views: 271
Re: 4F.13
The units for change in entropy is J/K. That's why we multiply by moles to cancel it. I also think you're confusing state function with intensive property. A state function doesn't necessarily mean it is intensive. Change in entropy definitely depends on how many moles of substance we have.
- Sun Feb 23, 2020 11:45 pm
- Forum: Interesting Applications: Rechargeable Batteries (Cell Phones, Notebooks, Cars), Fuel Cells (Space Shuttle), Photovoltaic Cells (Solar Panels), Electrolysis, Rust
- Topic: Respiration and Fermentation
- Replies: 1
- Views: 235
Re: Respiration and Fermentation
My mind was blown when I learned about cellular respiration in LS7A. I remember in high school chem, I merely knew about the overall chemical reaction, glucose and oxygen yielding energy, CO2 and H2O. And now in LS7A, I'm starting to see just how crazy complicated the process really is. The human bo...
- Sun Feb 23, 2020 9:13 pm
- Forum: Balancing Redox Reactions
- Topic: Hydroxide and H+
- Replies: 6
- Views: 433
Re: Hydroxide and H+
Well if it's a neutral solution, that should mean the redox reaction wouldn't be using or creating any H+ or OH-. If we had to balance a redox reaction in neutral solution, we would probably be given something like this where H+ or OH- ions wouldn't be needed: Cu+(aq)+Fe(s)→Fe3+(aq)+Cu(s)
- Sun Feb 23, 2020 8:59 pm
- Forum: Balancing Redox Reactions
- Topic: 6K.3 part d
- Replies: 3
- Views: 267
Re: 6K.3 part d
Some people have said that it's a typo, so it should say 2Cl-(aq) as the product rather than Cl2(g)
- Wed Feb 12, 2020 4:51 pm
- Forum: Calculating Work of Expansion
- Topic: Pizza Rolls #6
- Replies: 1
- Views: 167
Pizza Rolls #6
"You have a system consisting of 0.40 moles of an ideal gas contained in a 100.0L container at 1.0 atm. You just love chemistry to a fault, so you perform a series of steps to the system. First, you perform an isobaric compression of the container to 10.0L. Then, you pressurize the system to 10...
- Tue Feb 11, 2020 4:01 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: 4I.9
- Replies: 3
- Views: 342
4I.9
"Initially an ideal gas at 323 K occupies 1.67 L at 4.95 atm. The gas is allowed to expand to 7.33 L by two pathways: (a) isothermal, reversible expansion; (b) isothermal, irreversible free expansion. Calculate ΔStot, ΔS, and ΔSsurr for each pathway." For part b, why is the ΔS of the irrev...
- Tue Feb 11, 2020 3:56 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: 4I.9
- Replies: 2
- Views: 271
Re: 4I.9
When you use w=P_{ex}\Delta V , external pressure is 0 because free expansion means the system isn't pushing against a pressure. It is freely expanding without any use of energy. This means w=0. For isothermal processes, ∆U = 0 = q + w, so q and w = 0 in this case. I'm not sure why the ∆S is the sam...
- Sun Feb 09, 2020 8:45 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: 4H.9
- Replies: 2
- Views: 186
Re: 4H.9
"Container B has 1.0 mol of atoms bound together as diatomic molecules that are not vibrationally active. Container C has 1.0 mol of atoms bound together as diatomic molecules that are vibrationally active." I think the wording is just tricky. "1.0 mol of atoms bound together as diato...
- Sun Feb 09, 2020 8:12 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Finding concentration of H30 and OH from Kw
- Replies: 2
- Views: 318
Re: Finding concentration of H30 and OH from Kw
A solution of water is neutral, so Ka = Kb. Taking the square root of Kw will give Ka and Kb.
- Sun Feb 09, 2020 7:56 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Hw 5I.23
- Replies: 1
- Views: 170
Re: Hw 5I.23
This is how I did the question.
- Sun Feb 09, 2020 7:25 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 4D.21
- Replies: 3
- Views: 220
Re: 4D.21
Also getting -138.18, so probably inputting into the calculator wrong.
- Sun Feb 09, 2020 6:51 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Favorability of Endothermic Reactions
- Replies: 5
- Views: 227
Re: Favorability of Endothermic Reactions
Yes, endothermic reactions mean heat is a reactant, so in high temperature (lots of heat), the reaction will proceed to the right in order to decrease the temperature/heat.
- Wed Jan 29, 2020 9:28 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: HW 4A.5
- Replies: 1
- Views: 109
Re: HW 4A.5
When work is a positive value, it means the surroundings is doing work on the system while a negative value means the system is doing work on the surroundings. The more negative the value, the more work the system is doing.
- Tue Jan 28, 2020 10:40 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 4E.5, 4E.7
- Replies: 7
- Views: 619
Re: 4E.5, 4E.7
For 4E.5) you used the C-C bond enthalpy (-348kJ/mol) when it should be the C-H bond enthalpy which is 518kJ/mol For 4E.7) a) Your work looks right. You should be getting -202 kJ/mol. Maybe you plugged it into your calculator wrong? b) You forgot to include the bond enthalpy of C-C in the products. ...
- Tue Jan 28, 2020 10:15 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: 4A.9
- Replies: 4
- Views: 164
Re: 4A.9
Heat released = - heat absorbed
Your work here looks right. I'm assuming you used the negative to change (T-100) into (100-T) right?
Your work here looks right. I'm assuming you used the negative to change (T-100) into (100-T) right?
- Tue Jan 28, 2020 9:20 pm
- Forum: Calculating Work of Expansion
- Topic: 4A.3
- Replies: 2
- Views: 123
Re: 4A.3
My answer book shows 28J. Your statement regarding internal energy and work is correct.
- Tue Jan 28, 2020 9:08 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 4D.9
- Replies: 2
- Views: 180
Re: 4D.9
I think enthalpy density is a value like regular density or volume, in which the value can't be negative.
- Tue Jan 21, 2020 12:18 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: 5.35 Textbook
- Replies: 2
- Views: 101
Re: 5.35 Textbook
Well let's look at the changes for A B and C. A: -10 B: +5 C: +10 The ratio of A/B/C is -10/+5/+10 or simplified -2/1/2. Negative change indicates the reactant while positive indicates products. The ratio above represents the coefficients for the equation. For every 2 A molecules used, 1 B and 2 C m...
- Mon Jan 20, 2020 11:36 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6A.23
- Replies: 3
- Views: 266
Re: 6A.23
Ba(OH)2 is a strong acid, so you can assume all of the reactant turns into products. No need for an ICE table or K value. Just be careful and know that there will be double [OH] compared to [Ba2+].
- Mon Jan 20, 2020 11:32 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: 6C.7
- Replies: 1
- Views: 287
Re: 6C.7
NH3OH+ is the conjugate acid of hydroxylamine (NH2OH) which is given in Table 6C.2. (CH3)2NH2+ is the conjugate acid of dimethylamine, (CH3)2NH which is also given in Table 6C.2. Just do 14 - pKb to find pkA.
- Mon Jan 20, 2020 11:19 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kc to Kp conversion
- Replies: 2
- Views: 126
Re: Kc to Kp conversion
That equation given by your TA comes from this equation (PV=CRT) given by Lavelle. Here is an example I made illustrating where the difference in moles comes from.
Edit: accidentally put exponent inside B brackets twice
Edit: accidentally put exponent inside B brackets twice
- Mon Jan 20, 2020 10:56 pm
- Forum: *Making Buffers & Calculating Buffer pH (Henderson-Hasselbalch Equation)
- Topic: 6G.5
- Replies: 1
- Views: 658
Re: 6G.5
NaCl doesn't affect the pH of the solution because the Na+ and Cl- ions don't react with anything. This means we completely ignore NaCl. We are given 0.20 M of NH2NH2, which is a weak base. Create an ICE table and look up the Kb for NH2NH2 in one of the tables in the textbook (6C.1 , 6C.2 and 6E.1)....
- Wed Jan 15, 2020 3:18 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Homework Question 5I.13
- Replies: 2
- Views: 165
Re: Homework Question 5I.13
When doing the approximation, you have to double check the ionization percentage. Lavelle told us in lecture today that if this ionization percentage is above 5%, then the approximation isn't accurate enough, and we should use other methods like quadratic formula. To find this percentage, do the amo...
- Wed Jan 15, 2020 2:58 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6B.9a
- Replies: 1
- Views: 86
Re: 6B.9a
Yeah it looks like the answer key is wrong. The pH should be -0.176. With this pH, the pOH would now be 14.176 and  =
= 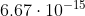
- Wed Jan 15, 2020 2:48 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6B.5
- Replies: 1
- Views: 85
Re: 6B.5
For each mole of Ba(OH)2 that dissociates, 2 moles of OH are created, so 0.0092 M Ba(OH)2 turns into 0.0184 M OH.
- Tue Jan 14, 2020 11:42 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5H.2
- Replies: 2
- Views: 112
Re: 5H.2
My solutions manual only has odds and not even numbered problems. Could you post a picture of it so I could see?
- Tue Jan 14, 2020 11:24 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Thermodynamically Stable?
- Replies: 3
- Views: 141
Re: Thermodynamically Stable?
Being thermodynamically stable means being in a low energy state. The less energy or enthalpy a molecule has, the more stable it is. Now to the problem, we are asked which reactant (Cl2 or F2) is more stable. The more stable a reactant is, the less it wants to dissociate into product. Comparing 1.1x...
- Mon Jan 13, 2020 1:46 pm
- Forum: Properties of Electrons
- Topic: Experiments of electrons
- Replies: 5
- Views: 405
Re: Experiments of electrons
The photoelectric experiment (photons shined on a metal surface which ejected electrons) showcases the particle property of electrons. For the wave property of electrons, I don't know the name, but I think the experiment involved particles passing through a hole in a wall, then a detector at the end...
- Tue Jan 07, 2020 5:54 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5.35 Part b
- Replies: 2
- Views: 103
5.35 Part b
This is the answer to part b: (in attachment). I understand the arrangement of the equilibrium constant and where the values (5, 10, and 18) come from, but I don't understand why these values are over 100. Is it because of units? The graph uses P/pKa, but what does that mean? Pressure per kiloPascal?
- Mon Jan 06, 2020 10:46 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Textbook question 5G.11
- Replies: 2
- Views: 123
Re: Textbook question 5G.11
5G.4 is all about Gibbs free energy which doesn't have anything to do with question 5G.11. Read through 5G.2 and you should be able to solve this problem. It's all partial pressures and mentions "Pure liquids and solids do not appear in K" (5G.2)
- Mon Jan 06, 2020 10:32 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Le Chatelier's and Endo/Exothermic
- Replies: 5
- Views: 245
Re: Le Chatelier's and Endo/Exothermic
Exothermic means energy is released while endothermic means energy is taken in, so exothermic:energy is a product, and endothermic: energy is a reactant. What I use to help me determine which is which is to think that endo sounds like "in", so it takes in energy. In exothermic reactions, e...
- Mon Jan 06, 2020 10:22 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Module: Equilibrium Part 3 Question 17
- Replies: 3
- Views: 152
Re: Module: Equilibrium Part 3 Question 17
If you can solve the problem, without the x^2 approximation, then do it that way. In this case, making x^2 equal to 0 is too much of an approximation. This is how I solved for x: (in file attachment)
Edit: Click on the image to see it right side up
Edit: Click on the image to see it right side up
- Mon Jan 06, 2020 9:44 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Very Large K
- Replies: 12
- Views: 2703
Re: Very Large K
A very large K value means the reaction at equilibrium will heavily lean towards the products. The equilibrium constant is basically concentration of products over concentration of reactants, so for K to be a large value, the numerator (product) should be big while denominator (reactant) should be s...
- Mon Jan 06, 2020 9:33 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: HW Problem 5G.7
- Replies: 4
- Views: 151
Re: HW Problem 5G.7
My solutions manual uses partial pressures. Is your's seventh edition?
- Mon Jan 06, 2020 9:23 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5l.7
- Replies: 1
- Views: 91
Re: 5l.7
Use this equation: 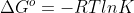
- Thu Dec 05, 2019 3:52 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: 6B 1
- Replies: 2
- Views: 170
Re: 6B 1
Doing log[.88] means the concentration was reduced by 12%, but the problem is asking for when it decreases to 12%. The equation for the initial pH is: pH = -log[HCl]_i Final pH: pH = -log(0.12[HCl]_i) To find the change, just do final pH - initial pH. Through some algebra, you should get -lo...
Re: naming
Wait there was a worksheet he emailed us? When did he email it? I don't see it.
- Thu Dec 05, 2019 2:45 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: water
- Replies: 3
- Views: 168
Re: water
When figuring out whether a ligand is monodenate or polydentate, we have to look at the lone pairs. Both of the lone pairs of water are on the oxygen right next to each other. When lone pairs are on the same atom, only one can bond because the other lone pair will point away from the bonding metal a...
- Thu Dec 05, 2019 2:12 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: CH4 versus CCl4 (Boiling Point)
- Replies: 3
- Views: 6046
Re: CH4 versus CCl4 (Boiling Point)
When it comes to boiling point, we have to look at the intermolecular forces (e.g. dipole-dipole and london dispersion forces) not the intramolecular strength (like bond strength within a molecule). Since both CH4 and CCl4 are nonpolar, they both only have london dispersion forces for intermolecular...
- Thu Dec 05, 2019 2:03 pm
- Forum: *Titrations & Titration Calculations
- Topic: Wednesday Lecture
- Replies: 3
- Views: 545
Wednesday Lecture
In this lecture, what information do we have to know since Lavelle said some of the information was 14B material? Like for example, would we have to know stoichiometric point and the polyprotic acids and bases for the final?
- Thu Nov 28, 2019 2:36 pm
- Forum: Hybridization
- Topic: sp^2 Hybridization of Carbon
- Replies: 2
- Views: 197
Re: sp^2 Hybridization of Carbon
The answer is electron-electron repulsion. There is more repulsion when two electrons share an orbital making it less favorable and unstable. Therefore, to avoid this, one of the electrons in the s-orbital jumps to fill an empty p-orbital. Hope this helps! I'm still confused. In the first photo, th...
- Thu Nov 28, 2019 12:58 pm
- Forum: Bronsted Acids & Bases
- Topic: Acid vs Base
- Replies: 4
- Views: 336
Re: Acid vs Base
Yeah generally, molecules with OH will indicate a bronsted base and H for bronsted acids. For part a though, NH3 (ammonia) is a base. It will gain a proton to become NH4 (ammonium). Memorizing some of the common acids and bases will help in figuring out these molecules. B and D are the acids, while ...
- Thu Nov 28, 2019 12:47 pm
- Forum: Hybridization
- Topic: sp^2 Hybridization of Carbon
- Replies: 2
- Views: 197
sp^2 Hybridization of Carbon
Why is it that when we use sp^2 orbitals for Carbon, it involves three 2sp^2 and one 2p^1 orbital? Why not three 2sp^2 orbitals and one 2s^1 orbital?
- Thu Nov 28, 2019 12:28 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Concentration
- Replies: 2
- Views: 187
Re: Concentration
It depends on what we are given. If a weak acid is involved, then we need to use the equilibrium constant (Ka) along with the given concentrations to make an ice table and calculate [H+] or [OH-], but I don't think we'll be facing these types of problems in this course. I haven't seen the practice p...
- Thu Nov 28, 2019 12:06 pm
- Forum: Bronsted Acids & Bases
- Topic: J17 Help
- Replies: 2
- Views: 292
Re: J17 Help
The problem is asking for equations involving a cation that is a weak acid reacting with water or an anion that is a weak base reacting with water, so they will look something like this: \textup{A}^++\textup{H}_2\textup{O}\rightleftharpoons \textup{A}+\textup{H}_3\textup{O}^+ or \textup{B}^-+\textup...
- Tue Nov 26, 2019 1:30 am
- Forum: Hybridization
- Topic: Unpaired electrons
- Replies: 2
- Views: 178
Re: Unpaired electrons
Yes each hybridized orbital represents bonds in the molecule. Let's take methane (CH4) for example. Because there are four regions of electron density around the Carbon atom, the s and p orbitals combine to create sp^3 orbitals: https://ibb.co/DGWMx8v Each of these sp^3 orbitals has an unpaired elec...
- Tue Nov 19, 2019 6:10 pm
- Forum: Hybridization
- Topic: Hybridization
- Replies: 4
- Views: 165
Re: Hybridization
Do all molecules use hybridized orbitals? No. Hybridized orbitals are only considered when necessary. For example, H2 molecules use the 1s orbital and don't need hybridized orbitals. You will have to look at the electron configuration of the atom to figure out if there are enough unpaired electrons...
- Mon Nov 18, 2019 11:07 pm
- Forum: Hybridization
- Topic: 2.57
- Replies: 4
- Views: 409
Re: 2.57
Image of Lewis Structure: https://ibb.co/FbB3TwM There are 2 electron densities around the right carbon, so sp hybridized orbitals are used for the triple bond. Here is the electron configuration shown by my epic MS paint skills: https://ibb.co/kQTh67k For the left carbon atom, sp^3 orbitals are use...
- Mon Nov 18, 2019 10:07 pm
- Forum: Hybridization
- Topic: Hybridization
- Replies: 4
- Views: 165
Re: Hybridization
Hybridization is just a model. It's used whenever necessary. If the current orbitals of a molecule don't have enough unpaired electrons to make all of the necessary bonds, then we use hybridized orbitals to explain for the lack of unpaired electrons. For example, a molecule like H2 won't have hybrid...
- Mon Nov 18, 2019 9:46 pm
- Forum: Hybridization
- Topic: Energy in Hybridization
- Replies: 3
- Views: 224
Re: Energy in Hybridization
Hybridization is just another addition to the bonding model to fix the issue that there weren't enough unpaired electrons to make all of the bonds, so I don't think energy of the molecule changes. But when it comes to the hybridized orbitals, I thought Lavelle said the energy of these orbitals were ...
- Mon Nov 18, 2019 9:23 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: 2E13 part d
- Replies: 1
- Views: 147
Re: 2E13 part d
https://imgur.com/a/AUAeA2Z Out of these three structures, the top right one is the best structure. Negative formal charge should be placed on the most electronegative atom if possible. In this case, O is more electronegative than N, so O should get the negative formal charge over N. Edit: Is the i...
- Mon Nov 18, 2019 9:05 pm
- Forum: Dipole Moments
- Topic: non polar dipole moments
- Replies: 2
- Views: 198
Re: non polar dipole moments
Nonpolar molecules can't have dipole-dipole interactions. Within each molecule, all of the dipoles cancel out, so there should be no dipole interaction between molecules. The only intermolecular force of nonpolar molecules is london dispersion forces.
- Thu Nov 14, 2019 4:39 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: why are double bonds equally weighted as single ones when drawing models?
- Replies: 10
- Views: 1980
Re: why are double bonds equally weighted as single ones when drawing models?
I think double/triple bonds can distort the molecule geometry slightly, but the VSEPR model ignores that for simplicity I guess. It's just a model, so there are probably limitations and exceptions.
- Thu Nov 14, 2019 4:19 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Vapor Pressure + IMF
- Replies: 3
- Views: 337
Re: Vapor Pressure + IMF
Imagine a closed container half filled with liquid. The temperature is then increased to the point where some of the liquid evaporated into gas. The gas particles colliding with the liquid creates what's called vapor pressure, pressure exerted by gas against it's solid or liquid phase. Vapor pressur...
- Thu Nov 14, 2019 3:59 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: 2F 9
- Replies: 1
- Views: 78
Re: 2F 9
Lavelle hasn't taught us hybridized orbitals yet, but it's pretty much combining orbitals into one type. For example, the two 2s electrons and two 2p electrons could combine to create 2sp^3 orbitals. Here is a table I made back from AP chem to help determine what hybridized orbitals are used. I woul...
- Thu Nov 14, 2019 2:10 am
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Rotation of Polar Molecules in Dipole-Dipole Forces
- Replies: 1
- Views: 182
Re: Rotation of Polar Molecules in Dipole-Dipole Forces
Alright, I'll pretty much paraphrase the paragraph above the figure. In the gas phase, molecules are rotating rapidly. An assumption you can make is that there is no net interaction between the molecules because the attraction between opposite charges are canceled by the repulsion of like charges. T...
- Thu Nov 14, 2019 1:48 am
- Forum: Ionic & Covalent Bonds
- Topic: HW 3F #19
- Replies: 1
- Views: 187
Re: HW 3F #19
Vapor pressure is relative to boiling point. If the boiling point of one molecule is lower than a different molecule, then the first molecule will have more gas molecules, leading to more vapor pressure. To tie all of this in terms of IMFs, you can say a weaker IMF makes the molecules easier to brea...
- Wed Nov 06, 2019 4:39 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Mo and Ag exceptions
- Replies: 4
- Views: 2094
Re: Mo and Ag exceptions
Yes, Mo and Ag want to have the half filled d-orbital over the full s-orbital, just like Cr and Cu above them.
- Wed Nov 06, 2019 4:35 pm
- Forum: Photoelectric Effect
- Topic: Photoelectric Effect vs. De Broglie
- Replies: 8
- Views: 765
Re: Photoelectric Effect vs. De Broglie
Do we use the De Broglie equation for the midterm review question 13a? Yes we do. We know this because we are using the wavelength of a potassium ion, which has mass. The speed of light equation (\textup{c}=\lambda \upsilon ) on the other hand would be used for situations involving electrom...
- Wed Nov 06, 2019 1:43 am
- Forum: Properties of Light
- Topic: Remembering order of EM radiation
- Replies: 1
- Views: 219
Re: Remembering order of EM radiation
Well for visible light, I use (ROY G BIV) to remember colors from weakest to strongest energy. Then to the left of visible light is infraRED light and to the right of visible light is ultraVIOLET light. Before infrared light and the start of the EM spectrum is radio waves followed by microwaves. I r...
- Wed Nov 06, 2019 12:28 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: 2A #21
- Replies: 1
- Views: 203
Re: 2A #21
The electron configuration of Ag is: [\textup{Kr}] 4\textup{d}^{10} 4\textup{s}^1 . Silver is one of the exceptions of the Aufbau principle because it is more stable to have a full d-subshell and one s-electron rather than a full s-subshell and 9 d-electrons. Since the question is asking for \textup...
- Tue Nov 05, 2019 11:46 pm
- Forum: Photoelectric Effect
- Topic: Photoelectric Effect vs. De Broglie
- Replies: 8
- Views: 765
Re: Photoelectric Effect vs. De Broglie
Do we use the De Broglie equation for the midterm review question 13a? Yes we do. We know this because we are using the wavelength of a potassium ion, which has mass. The speed of light equation (\textup{c}=\lambda \upsilon ) on the other hand would be used for situations involving electrom...
- Tue Nov 05, 2019 11:30 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Polarizing Strength of Cations
- Replies: 8
- Views: 348
Re: Polarizing Strength of Cations
I think if the cation's radius is small, the electrons of the anion would be closer to the cation nucleus, creating a stronger pull and polarizing the electrons more easily.
- Tue Nov 05, 2019 11:16 pm
- Forum: Trends in The Periodic Table
- Topic: ionic radii
- Replies: 4
- Views: 260
Re: ionic radii
Actually, when we add electrons, the attractive pull between the nucleus and electrons are weaker. With more electrons to pull in, the force is weaker. There is also added repulsion between the electrons that cause them to be further apart, creating a larger radius.
- Tue Nov 05, 2019 11:03 pm
- Forum: Trends in The Periodic Table
- Topic: Half Filled Orbitals
- Replies: 2
- Views: 325
Half Filled Orbitals
So for number 9 in the Dino Nuggets Midterm Review, the UA's explained that oxygen actually has less ionization energy than nitrogen despite being on the right of nitrogen. I know it's because of half-filled orbitals being pretty stable, but does this concept apply to the periods below nitrogen and ...
- Fri Nov 01, 2019 10:28 pm
- Forum: Lewis Structures
- Topic: Expanded Shells
- Replies: 3
- Views: 174
Re: Expanded Shells
Well d-subshells can hold 10 electrons, so I guess 18 could be the max? I don't think we need to worry about a max because we won't ever get to that point.
- Wed Oct 30, 2019 6:07 pm
- Forum: Ionic & Covalent Bonds
- Topic: Homework 2A.11
- Replies: 2
- Views: 96
Re: Homework 2A.11
If we were looking at \textup{M}^{2+} , then your answer of Iron and Manganese would be correct, but we are looking at metal cations with a 3+ charge or in other words elements that lose 3 electrons. Let's take a look at Iron. The electron configuration of Iron is [\textup{Ar}]3\textup{d}^64\textup{...
- Wed Oct 30, 2019 5:33 pm
- Forum: Electronegativity
- Topic: 3F. 5C
- Replies: 1
- Views: 201
Re: 3F. 5C
The size of the atoms largely contribute to the melting point of molecules. Fluorine may have stronger dipole-dipole attraction than Iodine, but that difference in electronegativity is trumped by the difference in size. Iodine is bigger than Fluorine, meaning more electrons and stronger London Dispe...
- Wed Oct 30, 2019 5:20 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: 1E.25 Part C
- Replies: 2
- Views: 147
Re: 1E.25 Part C
In my solution manual, the answer to part c is ns^2 (n-1)d^3. That's the answer you said in your post right? ns^2 is the 2 valence electrons, and the (n-1)d^3 is the outermost d-orbital electrons.
- Wed Oct 30, 2019 5:10 pm
- Forum: Ionic & Covalent Bonds
- Topic: ground state electron configuration
- Replies: 1
- Views: 157
Re: ground state electron configuration
For part a and b, keep in mind that the 4s orbital is higher in energy than the 3d orbital. The question is asking for metal cations with a 2+ charge, meaning the metal element will lose 2 electrons. Part a and b involve electron configurations with electrons in the 3d and 4s orbitals. Since 4s is h...
- Wed Oct 30, 2019 4:51 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: Practice Question for Midterm
- Replies: 2
- Views: 349
Re: Practice Question for Midterm
To solve this problem, we can use the \textup{M}_1{}\textup{V}_1 = \textup{M}_2{}\textup{V}_2 equation. To find the original molarity, we have to first convert the 5.00 g of KMnO4 into moles using KMnO4 molar mass ( 5.00\textup{g} \textup{KMnO}_4\div158.04\textup{g/mol}= 0.0316 \textup{mol KMnO}_4 )...
- Mon Oct 21, 2019 10:29 pm
- Forum: Lewis Structures
- Topic: Homework 2B.3 d
- Replies: 2
- Views: 118
Re: Homework 2B.3 d
BrF3 has 28 electrons. This molecule is one of the exceptions to the octet rule. Br can have an expanded octet. Br will be the central atom with single bonds to each F and 2 lone pairs to itself.
- Mon Oct 21, 2019 10:20 pm
- Forum: Trends in The Periodic Table
- Topic: Ionization Energies Trend
- Replies: 5
- Views: 219
Re: Ionization Energies Trend
I guess the increase in nuclear charge trumps the electron shielding. Maybe because when increasing across a period, the electrons added are valence electrons, so I think the electron shielding really isn't increasing much.
- Mon Oct 21, 2019 10:06 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Ground state electron configuration of ions
- Replies: 4
- Views: 265
Re: Ground state electron configuration of ions
No, the ground state of an ion is the ion itself. For example, the ground state of Ca+ is Ca+.
- Mon Oct 21, 2019 5:58 pm
- Forum: Properties of Light
- Topic: 1B.15 --> c=λv vs. λ= h/mv
- Replies: 2
- Views: 154
Re: 1B.15 --> c=λv vs. λ= h/mv
The equation is \textup{c}=\lambda\cdot v . The v actually represents frequency, not velocity. The units of the equation are (\textup{m/s}) = (\textup{m}))(s^{-1}) . As you mentioned, the units don't work if you plug in velocity into that equation. Checking units of each ...
- Mon Oct 21, 2019 5:45 pm
- Forum: Einstein Equation
- Topic: units
- Replies: 1
- Views: 133
Re: units
It stands for kilo-electron volt or 1000 electron volts. The SI unit of this is joules. The conversion is 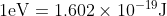
- Sun Oct 20, 2019 8:42 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: 1D.21
- Replies: 5
- Views: 497
Re: 1D.21
The question is asking for a specific subshell. When we are given an n and l value, we will have a subshell. For part a) since n=5 and l=2, we know the subshell is 5d. Subshell notation is simply just the n value followed by s, p, d, or f subshells. l=0 is the s-subshell, l=1 is the p-subshell, l=2 ...
- Sun Oct 20, 2019 8:26 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: 1E-11
- Replies: 4
- Views: 198
Re: 1E-11
Electron configuration is just an easy way to see where all of the electrons are in the orbitals. When we are stating all of the orbitals, we start from lowest energy to highest energy. The periodic table is a great way to figure out what orbitals are occupied. Here is a link to see the periodic tab...
- Sun Oct 20, 2019 8:00 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: 1E. 1
- Replies: 4
- Views: 313
Re: 1E. 1
Actually, all four of the values would still increase if the electron was in a hydrogen atom. All of the reasoning for the Lithium electron is the same for a hydrogen atom. In both cases, the the electron moves from an s-orbital to a p-orbital, which means the energy and n value is increased. When n...
- Sun Oct 20, 2019 7:30 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: 4s orbitals and 3d orbitals
- Replies: 2
- Views: 118
Re: 4s orbitals and 3d orbitals
When elements have z\leq 20 , then the 4s orbital is less in energy than 3d meaning 4s is written first. In other words, elements up to Calcium will have the 4s state come before 3d. But for elements after calcium in the periodic table, the 4s state is actually higher in energy than 3d. For example ...
- Sun Oct 20, 2019 7:15 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Problem 1a.11
- Replies: 3
- Views: 162
Re: Problem 1a.11
Each line represents energy released by the movement of a hydrogen electron from one energy level to a lower energy level. Each energy level is represented by a principal quantum number (n). What's common among each series is that their lines all involve the electron being in the same final energy l...
- Thu Oct 10, 2019 4:39 pm
- Forum: Student Social/Study Group
- Topic: Pen or Pencil for Homework Problems?
- Replies: 8
- Views: 490
Pen or Pencil for Homework Problems?
Does it matter if these practice problems are in pen or pencil?
- Tue Oct 08, 2019 11:20 pm
- Forum: Trends in The Periodic Table
- Topic: Chemistry Community
- Replies: 8
- Views: 495
Re: Chemistry Community
I think they are due every Sunday.
- Tue Oct 08, 2019 7:28 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Finding Textbook Questions on the Quantum World
- Replies: 4
- Views: 269
Re: Finding Textbook Questions on the Quantum World
In the seventh edition of the textbook, the 1A problems are right after Topic 1A on pg 9. Regarding turning in homework questions, I believe you can turn in questions just from Review of Chemical Principles if you want, or only from Quantum world, or a mix of the two.

