Search found 103 matches
- Thu Mar 12, 2020 12:17 pm
- Forum: General Rate Laws
- Topic: Factors Affecting k
- Replies: 83
- Views: 5707
Factors Affecting k
Can the rate constant (k) of a reaction change? If so, how?
- Wed Mar 11, 2020 3:43 pm
- Forum: General Rate Laws
- Topic: Rate limiting step
- Replies: 13
- Views: 798
Re: Rate limiting step
Dina 2k wrote:how would u determine which elementary step is the limiting?
I think that most likely, they would tell us which one is the slow step. At least that's what the problems in the textbook do.
- Wed Mar 11, 2020 3:40 pm
- Forum: *Enzyme Kinetics
- Topic: Do we need to know enzyme kinetics for the final? [ENDORSED]
- Replies: 4
- Views: 910
Re: Do we need to know enzyme kinetics for the final? [ENDORSED]
Enzymes are biological catalysts, and catalysts are mentioned in the outline. Maybe to be safe, you should know "how a catalyst lowers the activation energy of the transition state, how a catalyst increases the rate of a reaction, and also identify a catalyst in a reaction and give an example o...
- Wed Mar 11, 2020 3:35 pm
- Forum: Second Order Reactions
- Topic: Zeroth, First, Second Orders
- Replies: 4
- Views: 369
Re: Zeroth, First, Second Orders
Conceptually, orders of reactions generally relate to how changing the concentration of a reactant will affect the rate of a reaction.
- Wed Mar 11, 2020 3:27 pm
- Forum: General Rate Laws
- Topic: 7A.9
- Replies: 4
- Views: 418
Re: 7A.9
Yes. As the unit for k1 is s^-1, and the unit of k for first-order reactions is s^-1, the reaction is first order.
- Wed Mar 11, 2020 3:24 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Negative overall order
- Replies: 4
- Views: 630
Re: Negative overall order
I think that an order can be negative if as you increase the concentration of a particular reactant, the overall rate of reaction decreases.
- Sat Mar 07, 2020 9:12 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: A in the Arrhenius Equation
- Replies: 8
- Views: 554
A in the Arrhenius Equation
What exactly is A in the Arrhenius equation? From the lecture, I know that it is the "frequency or pre-exponential factor" and it has something to do with particles colliding in the correct orientation, but what does this actually mean?
- Sat Mar 07, 2020 9:09 pm
- Forum: First Order Reactions
- Topic: Negative sign in ln [A]t = -k t + ln [A]o
- Replies: 2
- Views: 304
Re: Negative sign in ln [A]t = -k t + ln [A]o
When you rearrange the equation ln [A]t = -k t + ln [A]o, you get ln [A]t-ln [A]o= -k t. Following the log rules, ln[A]t-ln[A]o is equal to ln([A]t/[A]o). Flipping the fraction to get ln([A]o/[A]t) is simply the negative, which explains the change in sign when you flip the fraction.
- Tue Mar 03, 2020 2:24 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6.N3 (a)
- Replies: 4
- Views: 375
Re: 6.N3 (a)
i think you're supposed to calculate n by the number of electrons that need to be transferred in the rxn. so you would have to write out the half-rxns of the cathode and anode, and see that only 1 electron is transferred and therefore n = 1 What would the half-reactions be if n=1? Would we still us...
- Tue Mar 03, 2020 11:34 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: pH and Oxidizing Strength
- Replies: 3
- Views: 303
Re: pH and Oxidizing Strength
I would assume that pH would only affect oxidizing strength if H+ or OH- ions are involved in the half-reactions. Therefore, for Br, I don't think that pH would affect its oxidizing strength as no H+ or OH- ions are involved in the reaction.
- Tue Mar 03, 2020 11:27 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Textbook question 6M.7
- Replies: 3
- Views: 298
Re: Textbook question 6M.7
For this question, you would want to find the least reduction potential as that implies that it is better as an oxidizing agent and thus will be better at reducing other compounds. What was your method for trying to solve this problem? For clarification, don't oxidizing agents oxidize other compoun...
- Sun Mar 01, 2020 12:33 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Outline 4 Learning Objective
- Replies: 2
- Views: 166
Re: Outline 4 Learning Objective
Q or K can refer to the equation:  , and
, and  Gº= -RTln(K), therefore pressure can affect
Gº= -RTln(K), therefore pressure can affect  .
.
- Sun Mar 01, 2020 12:26 am
- Forum: Interesting Applications: Rechargeable Batteries (Cell Phones, Notebooks, Cars), Fuel Cells (Space Shuttle), Photovoltaic Cells (Solar Panels), Electrolysis, Rust
- Topic: Electrolysis
- Replies: 1
- Views: 182
Re: Electrolysis
You would look at the potential required to reduce that particular species. For example, H+ and Na+ are present in solution and both are reducible. It takes +0.41V to reduce H+ to H2, and it takes +2.71V to reduce Na+ to Na, therefore H+ would be the species that is reduced, as it takes less potenti...
- Sun Mar 01, 2020 12:18 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Values of Standard Electrode Potentials
- Replies: 4
- Views: 378
Values of Standard Electrode Potentials
Why are some standard electrode potentials negative and some positive? What does this mean in relation to the hydrogen electrode?
- Sun Mar 01, 2020 12:04 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: ∆G and ∆G°
- Replies: 7
- Views: 542
Re: ∆G and ∆G°
I would assume that these principles still apply for ∆G.
- Sun Mar 01, 2020 12:03 am
- Forum: Balancing Redox Reactions
- Topic: 6K.5 Part B
- Replies: 1
- Views: 172
Re: 6K.5 Part B
Firstly, since it is in basic solution, you would add OH- in the reactants side. To balance the O and H atoms, you would then add H2O on the products side, giving the equation OH- + Br2 ----> BrO3- + Br- + H2O. For me, since there are 4 oxygens on the right side, I initially put 4 on OH-. As the num...
- Sun Feb 23, 2020 8:51 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Bike Example for Closed System?
- Replies: 2
- Views: 612
Re: Bike Example for Closed System?
I think Dr Lavelle was talking about a bike pump, which is a closed system. For closed systems, you can either heat or cool the system, or expand or compress the system to change the energy.
- Sun Feb 23, 2020 8:47 pm
- Forum: Balancing Redox Reactions
- Topic: Balancing in a Basic Solution
- Replies: 3
- Views: 223
Re: Balancing in a Basic Solution
Since it's a basic solution, you would need to add the OH- as basic solutions have OH- ions.
- Sun Feb 23, 2020 11:18 am
- Forum: Balancing Redox Reactions
- Topic: What is Being Reduced?
- Replies: 10
- Views: 585
What is Being Reduced?
For example, in the equation 2Fe3+(aq) + Cu(s) -> Cu2+(aq) + 2Fe2+ , would we have to say that Fe3+ is being reduced to Fe2+, or is it enough to say that iron is being reduced?
- Sun Feb 23, 2020 11:13 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Using Pt
- Replies: 7
- Views: 465
Re: Using Pt
When is it necessary to use Pt(s) in the skeletal equation of a redox reaction? In the example that Dr. Lavelle gave in class: 2Fe3+(aq) + Cu(s) -> Cu2+(aq) + 2Fe2+ although platinum is part of the redox reaction, platinum is an inert conductor that is transferring e-, as there is no conducting sol...
- Sun Feb 23, 2020 11:10 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: n
- Replies: 13
- Views: 785
Re: n
If you are referring to n in the equation 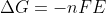 , then n is the number of moles of electrons in the balanced redox reaction.
, then n is the number of moles of electrons in the balanced redox reaction.
- Mon Feb 17, 2020 10:38 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Boltzmann Formula
- Replies: 11
- Views: 892
Re: Boltzmann Formula
Not necessarily, as not all molecules have resonance. It's the number of orientations a molecule can have, and a molecule can have multiple orientations even without resonance.
- Mon Feb 17, 2020 10:36 am
- Forum: Balancing Redox Reactions
- Topic: Possibly helpful: LEO the lion goes GER
- Replies: 2
- Views: 2247
Re: Possibly helpful: LEO the lion goes GER
Another helpful one is OIL RIG - Oxidation Is Loss, Reduction is Gain. Although this may be slightly confusing because this does not mention electrons!
- Mon Feb 17, 2020 10:29 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: importance of -RTlnk
- Replies: 7
- Views: 479
Re: importance of -RTlnk
I think that it's important if you only have the equilibrium constant and temperature of a reaction, therefore you wouldn't need to find enthalpy or entropy to measure delta G.
- Mon Feb 17, 2020 10:26 am
- Forum: Ideal Gases
- Topic: When to use equation
- Replies: 9
- Views: 768
Re: When to use equation
May I ask if the equation you're referring to is pV=nRT? If so, then you can use the equation to find concentration if you have the pressure and temperature. I don't think that you can use this equation to find the concentration/volume of both the reactants and the products, as the pressure or a cer...
- Tue Feb 11, 2020 9:29 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Standard Reaction Entropy/Enthalpy/Gibbs Free Energy
- Replies: 1
- Views: 88
Standard Reaction Entropy/Enthalpy/Gibbs Free Energy
In question 4J.7 a) in the homework problems, we are asked to calculate the standard reaction entropy, enthalpy and Gibbs Free Energy for the equation for the decomposition of hydrogen peroxide: 2H2O2 ---> 2H2O + O2 I thought that since we are calculating the standard entropy/enthalpy, we would be r...
- Sat Feb 08, 2020 10:52 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Addition of a solid product/reactant
- Replies: 3
- Views: 246
Re: Addition of a solid product/reactant
I don't think that the equilibrium would shift, because if you add more solid products/reactants, you would not be changing its concentration, thus the value of K would not change.
- Sat Feb 08, 2020 10:44 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Standard enthalpies of formation
- Replies: 5
- Views: 135
Re: Standard enthalpies of formation
Yes, I think that you would have to multiply them by their stoichiometric coefficients.
- Sat Feb 08, 2020 10:43 am
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Using heat capacity to determine molar entropy
- Replies: 3
- Views: 268
Re: Using heat capacity to determine molar entropy
I think that the heat capacity is used in the equation 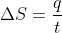 , where the heat capacity is used to calculate the value of q using the equation
, where the heat capacity is used to calculate the value of q using the equation 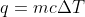
- Sat Feb 08, 2020 10:39 am
- Forum: Calculating Work of Expansion
- Topic: Units for -PV
- Replies: 5
- Views: 192
Units for -PV
When calculating work done using the equation: 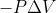 , what units should we use for pressure to obtain an answer in Joules?
, what units should we use for pressure to obtain an answer in Joules?
- Tue Feb 04, 2020 12:12 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: 4A.11
- Replies: 5
- Views: 284
Re: 4A.11
I think that because you are not required to calculate the specific heat capacity, and instead are only asked for heat capacity, mass or number of moles are not needed.
- Sat Feb 01, 2020 7:09 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Entropy
- Replies: 3
- Views: 125
Re: Entropy
I think that Dr Lavelle mentioned that later we will talk about how we can relate both entropy and enthalpy to determine the spontaneity of a reaction. However, I believe that generally, reactions which cause an increase in entropy tend to be more spontaneous, but I'm not too sure!
- Sat Feb 01, 2020 7:02 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: degeneracy usage and relevance
- Replies: 1
- Views: 76
Re: degeneracy usage and relevance
We can use degeneracy to calculate entropy, which was given by the equation S=kb*ln(W)
- Sat Feb 01, 2020 6:58 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Microstates
- Replies: 2
- Views: 147
Microstates
Dr Lavelle was talking about how atoms can have different "microstates" which have the same energy - but what exactly is a "microstate"? Are there any examples?
- Sat Feb 01, 2020 11:12 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Favored or Spontaneous
- Replies: 2
- Views: 131
Re: Favored or Spontaneous
I think that exothermic reactions tend to be more spontaneous because as the reaction commences, the products end up having a lower energy than the reactants because energy is released. Therefore, the products end up being more stable, therefore this tends to be favored.
- Sat Feb 01, 2020 10:52 am
- Forum: Calculating Work of Expansion
- Topic: ∆H = ∆U
- Replies: 1
- Views: 51
∆H = ∆U
Are there any instances where ∆H is not equal to ∆U for a system?
- Sat Jan 25, 2020 1:52 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Using different methods
- Replies: 4
- Views: 138
Re: Using different methods
I think it would depend on what information is given to you. For example, if the bond enthalpies are given, use method 2.
- Sat Jan 25, 2020 1:48 am
- Forum: Phase Changes & Related Calculations
- Topic: Bond enthalpy
- Replies: 4
- Views: 102
Re: Bond enthalpy
I think that there is a correlation, as shorter bond lengths would take more energy to be broken. I found that a C-C bond has a bond enthalpy of 347kJ/mol, whereas a shorter C=C bond has a bond enthalpy of 611kJ/mol.
- Sat Jan 25, 2020 1:43 am
- Forum: Phase Changes & Related Calculations
- Topic: Bond Enthalpies
- Replies: 5
- Views: 291
Re: Bond Enthalpies
To add on, bond breaking is an endothermic process and has a positive value, and bond forming is an exothermic process and has a negative value.
- Tue Jan 21, 2020 1:25 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: H2O in K Expressions
- Replies: 6
- Views: 326
H2O in K Expressions
Water as a liquid is not included in K expressions, but would we still include H2O gas in K expressions?
- Tue Jan 21, 2020 1:13 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Gas Constant (R)
- Replies: 3
- Views: 117
Gas Constant (R)
On the constants sheet, there are multiple values for the gas constant (R). When would we use each of these values? Specifically, how do we know when to use 8.314 and not 8.314x10^-2?
- Thu Jan 16, 2020 6:35 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Product Yield
- Replies: 6
- Views: 195
Re: Product Yield
Depending on the reaction, changing the temperatures and pressures can also affect the yield product.
- Thu Jan 16, 2020 6:31 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5.35
- Replies: 1
- Views: 145
Re: 5.35
You can see from the graph that the partial pressure of A decreases by 10, and similarly the partial pressure of C increases by 10. However, the partial pressure of B only increases by 5, half of A and C. This explains why the molar ratio ends up being 2A ---> B + 2C
- Thu Jan 16, 2020 6:25 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Negative pH
- Replies: 6
- Views: 260
Re: Negative pH
You can definitely have an acid with a negative pH, although this would be very rare. Additionally, very strong bases can also have a pH of more than 14.
- Wed Jan 15, 2020 11:19 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Units in ICE Table
- Replies: 8
- Views: 261
Units in ICE Table
If we were to use an ICE table, would the values need to be concentrations, or can we use moles when using the ICE table?
- Wed Jan 15, 2020 11:17 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Ice Box
- Replies: 9
- Views: 242
Re: Ice Box
If you are looking at a reaction where the reactants form products, the concentrations of the products would increase (a positive value in the C row) whereas the concentrations of the reactants would decrease (a negative value in the C row)
- Fri Jan 10, 2020 2:20 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Le Chatelier's Principle
- Replies: 7
- Views: 313
Re: Le Chatelier's Principle
Le Chatelier's principle is when a change is applied to a reaction at equilibrium, the equilibrium adjusts to minimize the effect of the change. When N2 is added, there are more reactants than products, or Q<K. Because of this, the equilibrium will shift towards the right to make more products (in t...
- Fri Jan 10, 2020 2:01 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Pressure and Volume
- Replies: 8
- Views: 242
Re: Pressure and Volume
Changing the pressure of a container by decreasing or increasing its volume does not affect K. This is because the partial pressures of all the gases involved in calculating K change by a common factor when a gas is compressed or otherwise, therefore the overall ratio of products to reactants remain...
- Fri Jan 10, 2020 1:52 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Equilibrium Constant for Multiples of the Chemical Equation
- Replies: 2
- Views: 177
Equilibrium Constant for Multiples of the Chemical Equation
The textbook states that "if a chemical equation is multiplied through by a factor N, K is raised to the Nth power". Why is this the case? When would it be necessary to multiply a chemical equation by a factor?
- Fri Jan 10, 2020 10:46 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: K for Heterogeneous Equilibria
- Replies: 4
- Views: 241
K for Heterogeneous Equilibria
For heterogeneous equilibrium equations where there are both gases and aqueous products or reactants, would you write the equilibrium constant in terms of Kp or Kc?
- Fri Jan 10, 2020 10:39 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kp vs Kc
- Replies: 6
- Views: 165
Re: Kp vs Kc
Kp is used when you use partial pressures, which is for gases. Kc is normally used if you use molar concentrations in the equation, which is mostly for substances other than gases, however using the ideal gas equation (PV=nRT), you can convert pressure to concentration.
- Sat Dec 07, 2019 1:37 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Chelate vs Polydentate
- Replies: 6
- Views: 233
Re: Chelate vs Polydentate
Chelates form a ring around the central atom, whereas polydentate ligands allow more than one of its binding sites to be occupied. I think that a polydentate ligand can be a chelate, but they are not necessarily a chelate. In the review session i'm pretty sure Matthew said all polydentate ligands c...
- Sat Dec 07, 2019 1:37 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: [Co(OH)2C2O4]-
- Replies: 1
- Views: 263
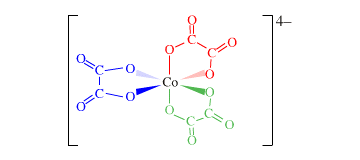
Re: [Co(OH)2C2O4]-
From this Lewis structure, I found that 2 of the oxygens in C2O4 bind to the central metal atom. It is bidentate and is a chelate.
- Sat Dec 07, 2019 1:30 am
- Forum: Lewis Acids & Bases
- Topic: Small, Highly Charged Metal Cations in Water
- Replies: 2
- Views: 229
Small, Highly Charged Metal Cations in Water
Why do small, highly charged metal cations such as Fe3+ act as Lewis acids in water? Is there an equation to demonstrate this?
- Sat Dec 07, 2019 1:01 am
- Forum: Naming
- Topic: Isocyanido
- Replies: 2
- Views: 171
Re: Isocyanido
It depends on which atom is bonded to the metal atom. Cyanido is used when the carbon is bonded to the metal atom, whereas isocyanido is used when the nitrogen is bonded to the metal atom.
- Sat Dec 07, 2019 12:57 am
- Forum: Amphoteric Compounds
- Topic: As2O3
- Replies: 2
- Views: 271
Re: As2O3
The element As is a metalloid, which can form amphoteric oxides. They can react with both acids and bases.
- Fri Dec 06, 2019 11:46 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Chelate vs Polydentate
- Replies: 6
- Views: 233
Re: Chelate vs Polydentate
Chelates form a ring around the central atom, whereas polydentate ligands allow more than one of its binding sites to be occupied. I think that a polydentate ligand can be a chelate, but they are not necessarily a chelate.
- Sat Nov 30, 2019 10:48 am
- Forum: Hybridization
- Topic: Homework 2F.11
- Replies: 1
- Views: 385
Re: Homework 2F.11
All of the phosphorus atoms have 3 other phosphorus atoms attached to them. Therefore, the shape of the molecule would be as follows: https://qph.fs.quoracdn.net/main-qimg-f5db806cc5f659130ab2eb07e69972fe.webp There is one lone pair around each phosphorus atom. Therefore, there are 4 regions of elec...
- Sat Nov 30, 2019 10:31 am
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: Ka constant
- Replies: 5
- Views: 430
Re: Ka constant
Ka is the equilibrium constant for acids. It's used to determine the level of dissociation an acid in an aqueous solution at equilibrium by finding the ratio between the products and reactants. Because of this, a higher Ka will indicate a stronger acid, as it dissociates more. Inversely, a lower pKa...
- Tue Nov 26, 2019 10:31 am
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Structure of H2SO4
- Replies: 2
- Views: 548
Structure of H2SO4
Why is this the Lewis structure of sulfuric acid? https://www.fishersci.com/us/en/products/chemicals/acids-bases/acids/inorganic-acids/sulfuric-acid/jcr%3Acontent/browse-results/browse_content/imageandrte_66e2.img.jpg/1535483076248.jpg I initially thought that sulfuric acid consisted of hydrogen cat...
- Tue Nov 26, 2019 10:20 am
- Forum: Amphoteric Compounds
- Topic: How can compounds be amphoteric?
- Replies: 5
- Views: 270
How can compounds be amphoteric?
How do some compounds have both basic and acidic character?
- Tue Nov 26, 2019 10:17 am
- Forum: Lewis Acids & Bases
- Topic: Electron acceptor / donor
- Replies: 2
- Views: 146
Re: Electron acceptor / donor
Normally, electron acceptors (Lewis acids) are positively charged, and electron donors (Lewis bases) are negatively charged (this may not be the case all the time). For me, drawing the Lewis structure helps determine which species has lone pairs of electrons, which helps to determine the Lewis base....
- Sun Nov 24, 2019 10:40 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Understanding Chelate
- Replies: 2
- Views: 191
Re: Understanding Chelate
Would we always need to draw out Lewis structures to determine if a molecule can form chelate complexes?
- Sun Nov 24, 2019 10:28 am
- Forum: Biological Examples
- Topic: Transplatin?
- Replies: 3
- Views: 247
Re: Transplatin?
Cisplatin can form coordination compounds with DNA by binding to the lone pair of electrons on nitrogen in the DNA base guanine, whereas transplatin is unable to do so.
- Sun Nov 24, 2019 10:23 am
- Forum: Hybridization
- Topic: Hybridization Confusion
- Replies: 3
- Views: 181
Re: Hybridization Confusion
I think you would probably need to know how to determine what kind of hybridized orbitals are present in a molecule, as well as the concept behind hybridization, and how it is caused by the merging of orbitals to form a hybridized orbitals with the same energy.
- Mon Nov 18, 2019 8:55 pm
- Forum: Dipole Moments
- Topic: Dipole moments
- Replies: 3
- Views: 230
Re: Dipole moments
Yup, when you draw dipoles the arrow is pointing towards the more electronegative atom. The dipoles cancel out because there is no net dipole, because the two dipoles are going in opposite directions.
- Mon Nov 18, 2019 8:37 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Intermolecular Forces in CO2
- Replies: 4
- Views: 1603
Intermolecular Forces in CO2
What are the intermolecular forces present in CO2? Does a non-polar molecule with polar bonds have dipole-dipole interactions?
- Sat Nov 16, 2019 10:57 am
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Polarizability vs. Electronegativity
- Replies: 3
- Views: 147
Re: Polarizability vs. Electronegativity
Electronegativity is the ability of an atom to attract electrons. Polarizability refers to how much an atom's electron cloud will be distorted when it is bonded to another atom.
- Sat Nov 16, 2019 10:51 am
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: London Forces
- Replies: 2
- Views: 75
London Forces
Why and how are London forces present in all molecules?
- Sat Nov 16, 2019 10:40 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: How to study for VSEPR?
- Replies: 9
- Views: 723
Re: How to study for VSEPR?
I think that visualizing the molecules in 3-D really helps, especially when you can't remember the names of the shapes.
- Sat Nov 16, 2019 10:13 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bond Angle with Lone Pairs
- Replies: 3
- Views: 137
Bond Angle with Lone Pairs
Why are bond angles smaller when there are lone pairs of electrons present? For example, why are the bond angles in a tetrahedral structure larger than the bond angles in a trigonal pyramidal structure?
- Fri Nov 15, 2019 6:39 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSEPR Formula Exceptions
- Replies: 6
- Views: 455
Re: VSEPR Formula Exceptions
If you know the formula, you would be able to draw the Lewis structures, and you would know the number of bonding and non-bonding pairs of electrons around the central atom, so, I think it would be easier to apply VSEPR theory in that case.
- Sat Nov 09, 2019 6:24 pm
- Forum: Electronegativity
- Topic: Polar Molecules
- Replies: 4
- Views: 322
Polar Molecules
What exactly determines the polarity of a particular molecule? Is there a specific difference in electronegativity between two elements that is needed in order for a molecule to be polar?
- Sat Nov 09, 2019 5:00 pm
- Forum: Bond Lengths & Energies
- Topic: Bonds
- Replies: 6
- Views: 431
Re: Bonds
To add on, sigma bonds are a result of direct overlaps between orbitals, whereas pi bonds are a result of an overlap between two adjacent parallel p-orbitals.
- Sat Nov 09, 2019 4:51 pm
- Forum: Ionic & Covalent Bonds
- Topic: Hydrogen Bond Strength
- Replies: 8
- Views: 481
Re: Hydrogen Bond Strength
Hydrogen bonds are intermolecular forces, whereas ionic and covalent bonds are bonds between atoms. Therefore, hydrogen bonds are weaker than ionic and covalent bonds.
- Sat Nov 09, 2019 4:50 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Dipole-dipole v. Induced Dipole-Induced Dipole
- Replies: 2
- Views: 158
Dipole-dipole v. Induced Dipole-Induced Dipole
What is the difference between a dipole-dipole force and an induced dipole-induced dipole intermolecular force? Are molecules that experience dipole-dipole forces polar?
- Sat Nov 09, 2019 4:04 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: 3F.5 (b)
- Replies: 4
- Views: 633
Re: 3F.5 (b)
Butanol has hydrogen bonds, where the electronegative oxygen pulls in electrons, causing a slight negative charge on the oxygen, and a slight positive charge on the hydrogen. The slight positive charge on the hydrogen in one molecule is attracted to the lone pair of electrons on oxygen in another mo...
- Sat Nov 02, 2019 11:19 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Cu and Cr
- Replies: 11
- Views: 582
Re: Cu and Cr
Yes, this trend continues for elements below copper and chromium, because a half-filled d orbital is more energetically stable.
- Sat Nov 02, 2019 11:10 am
- Forum: Octet Exceptions
- Topic: Expanded Octet
- Replies: 3
- Views: 184
Re: Expanded Octet
Elements from the third period onwards generally can have an expanded octet because they have an empty 3d orbital which can accommodate for additional electrons.
- Wed Oct 30, 2019 11:27 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Why does 3d come before 4s?
- Replies: 2
- Views: 221
Re: Why does 3d come before 4s?
When the 3d orbitals contain electrons, the 3d orbitals have a lower energy than the 4s orbitals. This is caused by electron-electron repulsions, which lower the energy.
- Wed Oct 30, 2019 11:22 pm
- Forum: Octet Exceptions
- Topic: Radicals
- Replies: 5
- Views: 270
Re: Radicals
Additionally, radicals are also highly reactive. You can identify if a species is a free radical by looking at its Lewis structure - instead of a pair of valence electrons there should be an unpaired electron on the atom or molecule or ion. For example, this is what a methyl radical would look like:...
- Wed Oct 30, 2019 11:10 pm
- Forum: Octet Exceptions
- Topic: Octet Rule Exception (More than 8 electrons)
- Replies: 2
- Views: 139
Re: Octet Rule Exception (More than 8 electrons)
Elements in the p-block in the third period have valence electrons in the n=3 energy level. Yes, there are no electrons in the 3d orbital, and this is why these elements can have an expanded octet, because additional electrons can accommodate the empty 3d orbitals.
- Fri Oct 25, 2019 11:29 am
- Forum: Lewis Structures
- Topic: Exceptions to the Octet Rule
- Replies: 2
- Views: 158
Re: Exceptions to the Octet Rule
Atoms in period 3 or higher have d-orbitals in their valence shell which can accommodate more than 8 electrons, which is why these atoms are exceptions to the octet rule.
- Fri Oct 25, 2019 11:27 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Number of Valence Electrons
- Replies: 5
- Views: 358
Number of Valence Electrons
If a question asks you to "give the number of valence electrons (including d electrons)" for manganese, how many valence electrons are there? I thought that the electron configuration of manganese is [Ar]3d5 4s2, therefore should the number of valence electrons be 2 or 7?
- Thu Oct 24, 2019 11:59 am
- Forum: Ionic & Covalent Bonds
- Topic: valence
- Replies: 3
- Views: 242
Re: valence
Valence electrons are the number of electrons in the outer shell of an atom. The valence of an atom, however, refers to the "number of bonds the atom can form by sharing electron pairs". This determines how many bonds an atom can make when covalent bonds are formed.
- Thu Oct 24, 2019 11:52 am
- Forum: Trends in The Periodic Table
- Topic: Effective Nuclear Charge
- Replies: 5
- Views: 226
Re: Effective Nuclear Charge
Electron affinity is defined as the energy released when an electron is added to an atom in the gas phase. Generally, the elements on the right side of the periodic table have higher electron affinities. This is because the electrons are attracted to the higher effective nuclear charge, therefore en...
- Thu Oct 24, 2019 10:26 am
- Forum: Ionic & Covalent Bonds
- Topic: Double Bond vs Single Bond Length
- Replies: 6
- Views: 560
Re: Double Bond vs Single Bond Length
Additionally, in resonance structures, the bond lengths are equal due to the delocalization of electrons between the atoms. The molecule, as a result, has a lower energy, and this helps to stabilize the molecule.
- Thu Oct 17, 2019 11:10 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Orbitals in an H-Atom
- Replies: 3
- Views: 139
Orbitals in an H-Atom
In the textbook, it states that in a hydrogen atom, "all the orbitals of a given shell are degenerate (have the same energy)". Why do all the orbitals in a hydrogen atom have the same energy?
- Thu Oct 17, 2019 11:00 pm
- Forum: Properties of Electrons
- Topic: Wave Function
- Replies: 5
- Views: 241
Re: Wave Function
A wave function is a mathematical function that can tell us the shape of an orbital. If you square the wavefunction, you can find the probability density of an electron being found within that particular region.
- Thu Oct 17, 2019 6:41 pm
- Forum: DeBroglie Equation
- Topic: SI Units
- Replies: 4
- Views: 198
Re: SI Units
If you are using the equation wavelength=h/p, you need to convert the wavelength to metres, but you do not have to convert the mass of the neutron to grams, and you have to leave this mass in kilograms because the units for momentum(p) is kgm/s
- Thu Oct 17, 2019 6:04 pm
- Forum: Properties of Electrons
- Topic: HW Question Topic 1B #1B.5
- Replies: 2
- Views: 209
Re: HW Question Topic 1B #1B.5
If you look at the "Constants and Equations" sheet (which can be found on the class website), you will find the conversion for 1eV, which is equal to 1.602x10^-19J.
- Thu Oct 17, 2019 6:00 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Stern and Gerlach Experiment
- Replies: 2
- Views: 113
Re: Stern and Gerlach Experiment
Silver atoms were chosen because they have one unpaired electron. Therefore, the atom acts as a single unpaired electron. As a stream of silver atoms are passed through a magnetic field, they split into two streams, one where the unpaired electron is spin-up, and the other where the electron is spin...
- Fri Oct 11, 2019 12:13 pm
- Forum: Photoelectric Effect
- Topic: Photoelectric Effect Clarification
- Replies: 3
- Views: 163
Re: Photoelectric Effect Clarification
Light sources with short wavelengths have high frequencies, and therefore high energy. Electrons are only ejected from a metal surface if the frequency or energy of the incoming light is equal to or is higher than the threshold energy of that particular metal. The reason why increasing the intensity...
- Fri Oct 11, 2019 12:08 pm
- Forum: Properties of Electrons
- Topic: Energy Level Relationship
- Replies: 4
- Views: 148
Re: Energy Level Relationship
If an electron moves to a lower energy level, light is emitted. Therefore, the energy change of the electron has decreased or is negative, however, the energy of the photon emitted is positive.
- Thu Oct 10, 2019 3:44 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Rydberg vs Balmer Series
- Replies: 2
- Views: 114
Re: Rydberg vs Balmer Series
Do you mean the Balmer and Lyman series? In the emission spectrum, the Balmer series refers to the visible light spectrum, which occurs when electrons move to n=2. The Lyman series occurs when electrons move to the first energy level, and this is in the UV spectrum.
- Thu Oct 10, 2019 1:45 pm
- Forum: Properties of Light
- Topic: Waves/Particles
- Replies: 14
- Views: 560
Re: Waves/Particles
Photons act as particles of electromagnetic radiation, therefore they have particle characteristics
- Thu Oct 10, 2019 12:15 pm
- Forum: Properties of Electrons
- Topic: Photoelectric Effect Experiment
- Replies: 5
- Views: 187
Photoelectric Effect Experiment
I am not entirely sure of how the experiment demonstrating the photoelectric effect works... How and why are electrons ejected when light of a certain frequency is directed towards a metal surface?
- Thu Oct 03, 2019 10:07 pm
- Forum: Properties of Light
- Topic: Spectroscopy
- Replies: 3
- Views: 108
Re: Spectroscopy
When an electron gets excited and moves up higher energy levels, light is absorbed. Electrons can get excited by heat.
- Thu Oct 03, 2019 11:24 am
- Forum: Balancing Chemical Reactions
- Topic: Chemical Principles Section L Question 35
- Replies: 2
- Views: 132
Re: Chemical Principles Section L Question 35
If you look under the "Solution Manual Errors 7th Edition" on the class page, you will find that there is an error in question L35, and that the compound should be Fe3Br8.
- Thu Oct 03, 2019 9:26 am
- Forum: Significant Figures
- Topic: Significant Figures
- Replies: 10
- Views: 809
Re: Significant Figures
Generally, if a question is asking for you to add or subtract, you round your answer based on the value in your working with the least number of decimal places. For example, 33.567+2.1 = 35.7. For multiplication and division questions, you round off your answer based on the value in your working wit...

