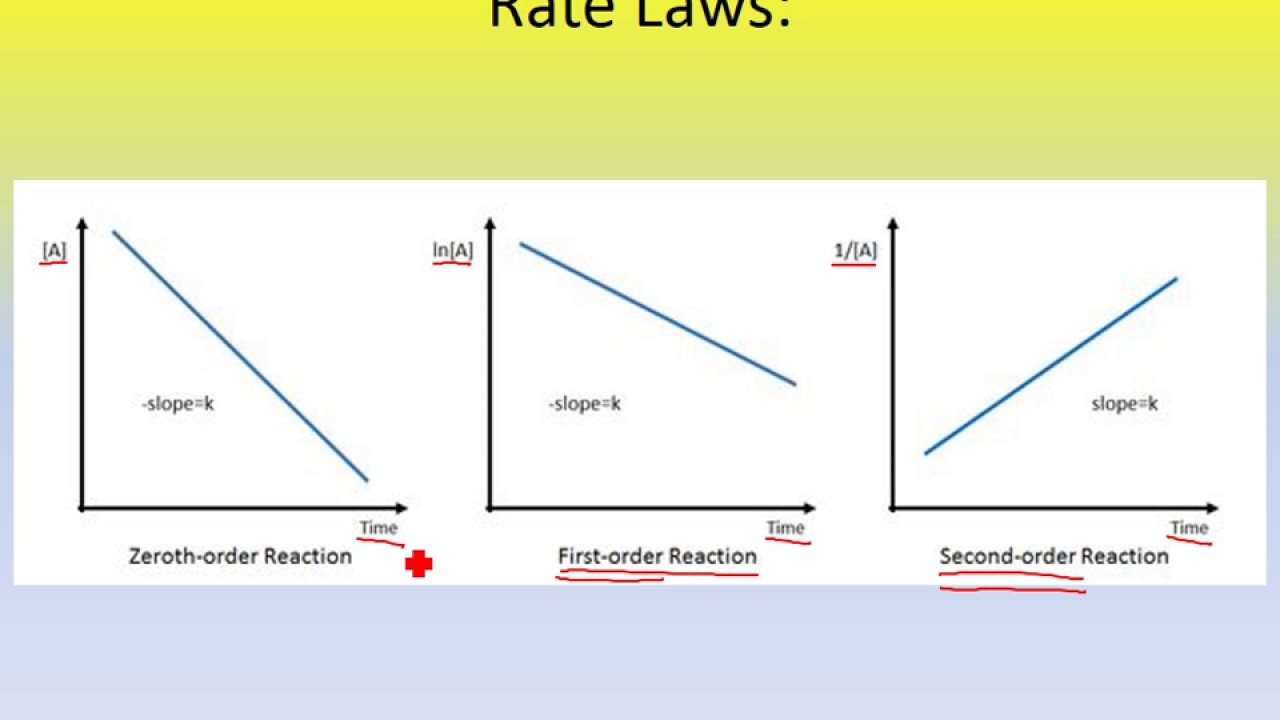
Veronica Lu 2H wrote:when using PV=nRT is the temperature supposed to be celsius or kelvin?
To add on, you use kelvin especially since the constant R is in kelvin, not celsius. However, if you use PV=nR∆T, the you may use either celsius or kelvin since the change in temperature would be the same.