Search found 90 matches
- Tue Mar 17, 2020 10:46 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: ignoring solids & liquids for K
- Replies: 7
- Views: 547
Re: ignoring solids & liquids for K
So an aqueous solution isn't considered a liquid? Yes, they are different. To sum it up, you would omit a species from the equilibrium expression if it's in a solid(s) or pure liquid (l) state. If they are in an aqueous (aq) or gas (g) state, then you would include the species in the equilibrium ex...
- Mon Mar 16, 2020 1:51 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Redox from cell diagram
- Replies: 6
- Views: 482
Re: Redox from cell diagram
the notation for cell diagrams is (anode) II (cathode), and I've heard some TAs say that on each side it should be written as reactant/product but in reality, there's kind of inconsistencies with the order in which they are written. so just assume that the species on the left are being oxidized and ...
- Mon Mar 16, 2020 1:48 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: adding platinum
- Replies: 8
- Views: 582
Re: adding platinum
when there is no conducting solid in reactants or products, add platinum as a common inert conductor
- Mon Mar 16, 2020 1:46 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: reverse half reaction
- Replies: 4
- Views: 456
Re: reverse half reaction
Why is that? Why is the anode reaction reversed? i believe it is because when you are given two equations and their potential values, it is the standard reduction potential values for both. therefore for the anode cell reaction where oxidation is taking place, you must reverse it to account for the...
- Mon Mar 16, 2020 1:41 am
- Forum: Zero Order Reactions
- Topic: zero order reactions
- Replies: 5
- Views: 402
Re: zero order reactions
You can identify a zero order reaction by looking at experimental data or looking at the graph, which should yield a linear line for the plot of [A] vs time
- Mon Mar 16, 2020 1:38 am
- Forum: Zero Order Reactions
- Topic: kind of reaction
- Replies: 25
- Views: 1210
Re: kind of reaction
a zero order reaction graph of [A] v. time would turn out to be a negative slope linear line
- Mon Mar 16, 2020 1:34 am
- Forum: Zero Order Reactions
- Topic: 0 order
- Replies: 7
- Views: 691
Re: 0 order
0 order indicates that the rate of reaction is independent of the concentration of the reactants
- Sun Mar 15, 2020 8:05 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: heterogeneous vs homogeneous catalysts
- Replies: 2
- Views: 229
heterogeneous vs homogeneous catalysts
is there any significance in knowing the difference between a homogeneous and a heterogeneous catalyst?
- Sun Mar 15, 2020 8:05 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: temp vs k
- Replies: 3
- Views: 320
temp vs k
how does changing temperature affect the reaction constant?
- Sun Mar 15, 2020 8:03 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: catalyst vs intermediate
- Replies: 3
- Views: 261
catalyst vs intermediate
what's the difference between a catalyst and an intermediate?
- Sun Mar 15, 2020 8:03 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: catalyst
- Replies: 4
- Views: 365
catalyst
how can determine if a species is a catalyst?
- Wed Mar 04, 2020 12:52 am
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: k
- Replies: 10
- Views: 615
Re: k
605395381 wrote:k is unit-less because it is a ration of concentrations of products and reactants
thanks for all the answers everyone but i forgot to clarify in my original question that i'm referring to the k that is in rate laws. i thought that k in the context of kinetics had units?
- Wed Mar 04, 2020 12:50 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: adding Pt
- Replies: 1
- Views: 162
adding Pt
for 6L.5b, why is there platinum added to the reaction I-(aq) --> I2(s) ? i thought inert conductors were only needed when there is no conducting solid or liquid in the reaction. what constitutes a 'conducting' solid?
- Mon Mar 02, 2020 1:44 am
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: factors that affect k
- Replies: 8
- Views: 713
factors that affect k
what factors can affect the value of k?
- Mon Mar 02, 2020 1:42 am
- Forum: First Order Reactions
- Topic: deriving the integrated rate law
- Replies: 4
- Views: 465
deriving the integrated rate law
how do you derive the integrated rate law?
- Mon Mar 02, 2020 1:40 am
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: k
- Replies: 10
- Views: 615
k
what are the units of k?
- Mon Mar 02, 2020 1:35 am
- Forum: Interesting Applications: Rechargeable Batteries (Cell Phones, Notebooks, Cars), Fuel Cells (Space Shuttle), Photovoltaic Cells (Solar Panels), Electrolysis, Rust
- Topic: biological examples
- Replies: 3
- Views: 369
biological examples
how is electrochemistry applicable in biology? please give examples!
- Mon Mar 02, 2020 1:34 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: deriving nernst equation
- Replies: 4
- Views: 288
deriving nernst equation
how would you derive the nernst equation?
- Mon Mar 02, 2020 1:33 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: predicting is metals will dissolve
- Replies: 3
- Views: 229
predicting is metals will dissolve
how would you predict if a metal will dissolve in a solution? (it's one of the bullet points in the outline)
- Mon Mar 02, 2020 1:31 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: work
- Replies: 7
- Views: 454
Re: work
Eugene Chung 3F wrote:the deltaG for a cell is the amount of useful work obtainable from a system at constant T and P.
thanks for your answer! how is work related to cell potential though?
- Mon Mar 02, 2020 1:29 am
- Forum: First Order Reactions
- Topic: preferred form of rates
- Replies: 2
- Views: 239
preferred form of rates
i have written in my notes from wednesday (feb 26), that for the following reaction 2NO2 --> 2NO + O2 , the differential rate equation -½ d[NO2]/dt = ½ d[NO]/dt = d[O2]/dt is preferred over d[NO2]/dt = d[NO]/dt = 2d[O2]/dt
can someone explain why the first one is preferred over the latter?
can someone explain why the first one is preferred over the latter?
- Mon Mar 02, 2020 1:24 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: salt bridge
- Replies: 4
- Views: 350
salt bridge
how exactly do ions move across the salt bridge? why is a salt bridge and the movement of ions necessary in the first place?
- Mon Mar 02, 2020 1:21 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: concentration related to current?
- Replies: 4
- Views: 383
Re: concentration related to current?
Eugene Chung 3F wrote:electrons will flow from area of high concentration to an area of low concentration. So, the concentration difference makes potential.
so once there is no concentration difference, there is no more potential?
- Mon Mar 02, 2020 1:17 am
- Forum: Balancing Redox Reactions
- Topic: E cell and Standard
- Replies: 2
- Views: 216
Re: E cell and Standard
according to the textbook, the standard E cell is defined through deltaG(standard) = -n*F*E(cell, standard) where deltaG(standard) is defined as the difference of the molar Gibbs free energies of the products and reactants all in their standard states (all gases at 1 bar, all participating solutes a...
- Mon Mar 02, 2020 1:07 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: pH
- Replies: 1
- Views: 171
pH
how would you calculate pH using the nernst equation?
- Mon Mar 02, 2020 12:40 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: concentration related to current?
- Replies: 4
- Views: 383
concentration related to current?
how do the concentrations change as current flows from anode to cathode?
- Mon Mar 02, 2020 12:39 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: work
- Replies: 7
- Views: 454
work
how is work related to free energy or cell potentials?
- Mon Mar 02, 2020 12:37 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: gibbs free energy vs cell standard potential
- Replies: 3
- Views: 220
gibbs free energy vs cell standard potential
how come when you multiply a chemical equation, the gibbs free energy changes but the standard potential remains unaffected?
- Mon Mar 02, 2020 12:35 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: what's happening in galvanic cells
- Replies: 2
- Views: 226
what's happening in galvanic cells
in which direction are electrons and ions flowing with respect to the anode/cathode? why are the ions moving in the direction that they are moving?
- Mon Mar 02, 2020 12:32 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: inert conductor
- Replies: 2
- Views: 203
inert conductor
when is platinum used as an inert conductor? during lecture, he mentioned that there were other inert conductors, do we need to know them? also how come sometimes it's only added on one side of the cell diagram and other times it's on both sides?
- Mon Mar 02, 2020 12:30 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: cell notation
- Replies: 1
- Views: 166
cell notation
why are commas used sometimes instead of the normal vertical lines?
- Mon Mar 02, 2020 12:29 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: standard potential calculations
- Replies: 1
- Views: 166
standard potential calculations
if the direct value of the standard potential for a specific equation isn't given, how would you calculate it indirectly?
- Mon Mar 02, 2020 12:28 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: cell diagrams
- Replies: 1
- Views: 91
cell diagrams
what's the step-by-step for making a cell diagram? what are the usually the trickiest questions they can ask us about this?
- Tue Feb 11, 2020 10:57 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Pizza Rolls REVIEW Session DOWNLOAD HERE
- Replies: 67
- Views: 5809
Re: Pizza Rolls REVIEW Session DOWNLOAD HERE
Can someone explain how to solve #11 on the practice questions? What steps do you need to take? 1) since you're dealing with an acid, you want to convert the pKb to Ka 2) given the concentration of the acid, you can set up an ice table to help you write the equilibrium expression 3) after setting u...
- Tue Feb 11, 2020 10:51 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Pizza Rolls 6
- Replies: 3
- Views: 261
Re: Pizza Rolls 6
I also can't get the answer for q and w; it seems like he subtracted 23.3 and 9.12 to get 14.2, but I don't understand why you wouldn't add the work values you get for each part of the problem together instead. Edit: it seems that work will be positive if the system is being compressed, like in the...
- Sun Feb 02, 2020 11:39 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: state functions
- Replies: 10
- Views: 380
state functions
what’s a state function?
- Sun Feb 02, 2020 11:38 pm
- Forum: Phase Changes & Related Calculations
- Topic: intensive/extensive
- Replies: 4
- Views: 173
intensive/extensive
what’s the difference between intensive and extensive properties? what are some examples of them?
- Sun Feb 02, 2020 11:37 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: closed vs isolated?
- Replies: 7
- Views: 1310
closed vs isolated?
what are some examples of closed versus isolated systems??
- Sun Feb 02, 2020 11:36 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: constant pressure
- Replies: 1
- Views: 68
constant pressure
how would you calculate enthalpy if the pressure isn’t constant?
- Sun Feb 02, 2020 11:35 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: enthalpy
- Replies: 7
- Views: 245
enthalpy
can someone explain what enthalpy is?
- Sun Jan 26, 2020 8:17 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Hess's Law
- Replies: 4
- Views: 228
Re: Hess's Law
Method 2 that Lavelle went over in class, aka the one that uses bond enthalpies to calculate the enthalpy.
- Sun Jan 26, 2020 8:16 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Hess's Law
- Replies: 9
- Views: 451
Re: Hess's Law
Hess's Law states that regardless of the multiple stages or steps of a reaction, the total enthalpy change for the reaction is the sum of all changes (demonstrating that enthalpy is a state function).
- Sun Jan 26, 2020 8:06 pm
- Forum: Phase Changes & Related Calculations
- Topic: Standard State
- Replies: 1
- Views: 119
Standard State
One of the bullet points on the outline says "Define the standard state of a substance and recognize when a substance is in its standard state." How exactly would you know whether a substance is in its standard state or not.
- Sun Jan 26, 2020 8:03 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Method 2
- Replies: 4
- Views: 175
Method 2
Hi can someone please summarize how to use Method 2? Also, will there ever really be a case where only Method 2 is applicable (would Lavelle test us on this method specifically)?
- Sun Jan 26, 2020 8:01 pm
- Forum: Phase Changes & Related Calculations
- Topic: Phase Changes
- Replies: 7
- Views: 245
Phase Changes
For reactions where substances are not in their most stable/standard phase, how would you account for that when calculating enthalpies?
- Sun Jan 12, 2020 5:55 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5I.23
- Replies: 2
- Views: 91
Re: 5I.23
Correct me if I'm wrong but I think it's because they are gases, not aqueous.
- Sun Jan 12, 2020 5:53 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Chatelier's Principle
- Replies: 7
- Views: 241
Re: Chatelier's Principle
You typically use this principle if there is a scenario where equilibrium conditions are disturbed (whether it be by temperature, pressure, etc). Applying the principle will help you determine the effect of the change.
- Sun Jan 12, 2020 5:47 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Different types of K
- Replies: 9
- Views: 293
Re: Different types of K
chari_maya 3B wrote:How do you convert from Kc to Kp?
Lavelle hasn't mentioned anything about this in lecture so I'm not sure if this is necessary information, but the relationship between the two is Kp=Kc(RT)^Δn
- Sun Jan 12, 2020 5:45 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: K
- Replies: 10
- Views: 407
Re: K
Kc is the equilibrium constant when it's found using concentrations, while Kp is the equilibrium constant when it's found using partial pressures.
- Sun Jan 12, 2020 5:43 pm
- Forum: Ideal Gases
- Topic: ICE tables
- Replies: 4
- Views: 323
Re: ICE tables
ICE tables show the concentrations of reactants and products at different points in a chemical reaction. We usually use ICE tables to determine the K of the reaction. I stands for initial concentration, C stands for change in concentration, and E stands for equilibrium concentration. Once you fill o...
- Sat Dec 07, 2019 11:58 pm
- Forum: Properties & Structures of Inorganic & Organic Bases
- Topic: Acidosis
- Replies: 3
- Views: 389
Acidosis
How does carbon dioxide lead to respiratory acidosis?
- Sat Dec 07, 2019 11:55 pm
- Forum: Air Pollution & Acid Rain
- Topic: Options to Reduce Acid Rain
- Replies: 2
- Views: 478
Re: Options to Reduce Acid Rain
sarahsalama1G wrote:Additionally, has anyone been able to find the acid rain problems in the textbook?
Someone on another post said H.13, Focus 3 3.69, Example 5G.2, Box 6E.1 all mention acid rain/have practice problems for acid rain.
- Sat Dec 07, 2019 10:44 pm
- Forum: Biological Examples
- Topic: Chemotherapy drugs
- Replies: 7
- Views: 506
Re: Chemotherapy drugs
Cisplatin is one biological example of how coordination compounds are relevant. But I believe we should also know the biological functions of Cr, Fe, Co, Mn, Ni, Cu, Zn. The outline for this unit mentions knowing that Co functions in vitamin B12 specifically. What is the biological function of vita...
- Sat Dec 07, 2019 10:42 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Chelates
- Replies: 5
- Views: 476
Re: Chelates
I think the best way to approach this if you are having difficulty is to memorize the common polydentates like oxalate and ethylenediamine. What are the common polydentates that we need to know? The ones listed in the textbook are ethylenediamine (en), diethylenetriamine (dien), oxalato (ox), and e...
- Sat Dec 07, 2019 10:39 pm
- Forum: Naming
- Topic: bis- tris- tetrakis-
- Replies: 8
- Views: 632
bis- tris- tetrakis-
Can someone give examples for how to apply these roots when naming complexes?
- Sun Dec 01, 2019 11:56 pm
- Forum: Properties & Structures of Inorganic & Organic Bases
- Topic: strong and weak acid and bases
- Replies: 2
- Views: 178
Re: strong and weak acid and bases
I think it would be fair to say that we should the common strong acids and the trend for the strong bases.
- Sun Dec 01, 2019 11:54 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: neutralization
- Replies: 4
- Views: 274
Re: neutralization
Neutralization always forms water and, in most cases, a salt.
Re: Naming
Yes, that's just the rule. The cation has the same name as its element and the anion will have the suffix -ide.
- Sun Dec 01, 2019 11:49 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: charge and coordination number
- Replies: 2
- Views: 99
Re: charge and coordination number
I don't think there is a relationship between the two, and if there were, I don't believe Lavelle has mentioned it so you don't need to worry about it.
- Sun Dec 01, 2019 11:46 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Coordination Numbers and Ligands
- Replies: 3
- Views: 241
Re: Coordination Numbers and Ligands
That's all there is to it, the coordination number is the number of ligands attached to the central atom.
- Mon Nov 18, 2019 1:50 am
- Forum: Sigma & Pi Bonds
- Topic: Sigma and Pi Bonds
- Replies: 8
- Views: 534
Re: Sigma and Pi Bonds
005162520 wrote:Why are sigma and pi bonds relevant?
They are used to predict the behavior of molecules in molecular orbital theory.
- Mon Nov 18, 2019 1:49 am
- Forum: Sigma & Pi Bonds
- Topic: Quantum Numbers
- Replies: 9
- Views: 626
Re: Quantum Numbers
Nathan Rothschild_3D wrote:How do you tell ML again and can someone explain what it is?
ML is the magnetic quantum number (it indicates which orbital the electron is in). It depends on the angular momentum quantum number, or L. Valid values for ML are -L, .. 0, .. L.
- Mon Nov 18, 2019 1:43 am
- Forum: Sigma & Pi Bonds
- Topic: Test 2 Sigma and Pi bonds
- Replies: 5
- Views: 208
Re: Test 2 Sigma and Pi bonds
905289082 wrote:What is the relevance of having a sigma vs. a pi bond? is there an effect on bond strength and energy?
They are used to predict the behavior of molecules in molecular orbital theory. In general, sigma bonds are stronger than pi bonds simply because there is more overlap.
- Mon Nov 18, 2019 1:23 am
- Forum: Bond Lengths & Energies
- Topic: Lone pairs
- Replies: 7
- Views: 647
Re: Lone pairs
Nathan Rothschild_3D wrote:If a lone pair is a radical would it have a weaker repulsion than a complete lone pair?
I'm assuming so because a radical would occupy less space than a complete lone pair. Please correct me if I'm wrong though!
- Mon Nov 18, 2019 1:17 am
- Forum: Bond Lengths & Energies
- Topic: Test 2 Topics
- Replies: 40
- Views: 2238
Re: Test 2 Topics
KTran 1I wrote:Will we need to have the specific bond angles for each molecular shape memorized for Test 2?
Yes you do.
- Sun Nov 10, 2019 10:11 pm
- Forum: Trends in The Periodic Table
- Topic: Atomic Radius
- Replies: 30
- Views: 3137
Re: Atomic Radius
I still don't fully understand. If you're going horizontally across a row does atomic radius increase or decrease? And how about going down a column? Going horizontally across a period, the radius decreases. Going vertically down a column, the radius increases. The vertical trend plays a dominant r...
- Sun Nov 10, 2019 9:58 pm
- Forum: Coordinate Covalent Bonds
- Topic: Coordinate covalent bonds
- Replies: 9
- Views: 798
Re: Coordinate covalent bonds
A regular covalent bond is when two atoms both contribute one of their electrons to the bond. A coordinate covalent bond is when only one of the atoms is contributing the electrons to the bond. One example is carbon monoxide (CO) where two of its bonds are regular covalent, but the third is a coordi...
- Sun Nov 10, 2019 9:52 pm
- Forum: Dipole Moments
- Topic: Dipoles Cancelling Out
- Replies: 4
- Views: 300
Re: Dipoles Cancelling Out
I believe cancelling out has to do with the symmetry of a molecule. For example C2Cl4 has no net dipole moment because the molecule is symmetric so the moment cancels out When you say symmetric what does that exactly refer too, perhaps the shape of the element? Yes, if you draw out the molecule, yo...
- Sun Nov 10, 2019 9:07 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Lattice Energy
- Replies: 2
- Views: 196
Re: Lattice Energy
Charge and size of the ions in a compound can affect its lattice energy: increases in charge and size both lead to an increase in lattice energy.
- Sun Nov 10, 2019 9:03 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Test 2
- Replies: 6
- Views: 381
Re: Test 2
Test 2 is cumulative only for material covered after the midterm.
- Sun Nov 10, 2019 9:01 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Relation of the size of atoms with the strength of attractions
- Replies: 2
- Views: 126
Re: Relation of the size of atoms with the strength of attractions
The larger the molecule, the larger the electron clouds will be, which allows for a stronger temporary dipole and greater London dispersion forces.
- Sun Nov 10, 2019 8:40 pm
- Forum: Bond Lengths & Energies
- Topic: polarizability
- Replies: 9
- Views: 329
Re: polarizability
Jasmine 3L wrote:I don't really understand this too. Like how does it relate to melting and boiling point?
Polarizability determines the strength of the bonds. The stronger the bonds, the more energy is required for a change in state (from solid to liquid or liquid to gas).
- Sun Nov 10, 2019 7:55 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: 2E.17
- Replies: 2
- Views: 228
Re: 2E.17
Try searching up bond angle charts! First determine the VSEPR structure then you can find out what angle the bonds are at.
- Mon Nov 04, 2019 9:34 pm
- Forum: Ionic & Covalent Bonds
- Topic: Question 2D.3
- Replies: 2
- Views: 214
Re: Question 2D.3
Be is the central atom and the two Br atoms are placed symmetrically around Be, therefore the molecule is nonpolar.
- Mon Nov 04, 2019 9:29 pm
- Forum: Resonance Structures
- Topic: Resonance
- Replies: 2
- Views: 139
Resonance
So if there is more than one Lewis structure with formal charge of 0, are they considered resonant?
- Sun Oct 27, 2019 6:04 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: HW Helpp
- Replies: 2
- Views: 195
Re: HW Helpp
The number is front of the letter is the principal quantum number, n. For your orbital angular momentum number (l), the letters correspond to a value. s=0, d=1, p=2, and f=3.
- Sun Oct 27, 2019 5:45 pm
- Forum: Trends in The Periodic Table
- Topic: 1F.19
- Replies: 2
- Views: 100
1F.19
Why are s-block metals typically more reactive than p-block metals?
- Sun Oct 27, 2019 5:42 pm
- Forum: Ionic & Covalent Bonds
- Topic: 2A.24
- Replies: 3
- Views: 128
Re: 2A.24
a) magnesium has a charge of +2 and arsenic has a charge of -3. Therefore, you will need 3 of Mg and 2 of As to balance out the charges, which gives you the formula Mg_{3}{As_{2}} b) The charge of indium is given as (III), or +3, and sulfur has a charge of -2. Therefore, you will have the formula In...
- Sun Oct 27, 2019 5:30 pm
- Forum: Sigma & Pi Bonds
- Topic: Pi bonds
- Replies: 3
- Views: 184
Re: Pi bonds
They don't necessarily contribute to Lewis structures but you can look at your Lewis structure to determine what bonds you have. The first bond is always a sigma bond, and any bonds added after that are generally pi bonds.
- Sun Oct 27, 2019 5:12 pm
- Forum: Properties of Light
- Topic: All the formulas
- Replies: 5
- Views: 217
Re: All the formulas
Not sure if this is what you're looking for but...
c =
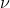
E(photon) = h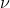
 m*
m*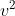 = E(photon) -
= E(photon) - 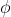
 = h/p
= h/p
c =
E(photon) = h
- Sun Oct 20, 2019 11:11 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Spin Quantum Number
- Replies: 3
- Views: 207
Re: Spin Quantum Number
Positive is represented by an up arrow, which means that it's spinning counterclockwise. Negative is represented by a down arrow, which means that it's spinning clockwise.
- Sun Oct 20, 2019 11:08 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Multi-electron atoms
- Replies: 7
- Views: 313
Re: Multi-electron atoms
The following are some that are isoelectronic to the H atom: He+, Li2+, Be3+ and B4+.
- Sun Oct 20, 2019 11:02 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Applying Pauli Exclusion Principle and Hund's Rule
- Replies: 5
- Views: 396
Re: Applying Pauli Exclusion Principle and Hund's Rule
Pauli exclusion states that no orbital can have more than 2 electrons. For example, if the 1s orbital was filled with 2 electrons then you cannot add another one to it, you would have to go to the next orbital, which is 2s. In addition to that, two electrons in the same orbital must have different s...
- Sun Oct 20, 2019 10:51 pm
- Forum: Trends in The Periodic Table
- Topic: Inert-Pair Effect
- Replies: 1
- Views: 121
Inert-Pair Effect
The textbook goes over this in F.6. Do we need to know this? If we do, can someone explain the concept more clearly?
- Sun Oct 20, 2019 10:49 pm
- Forum: Trends in The Periodic Table
- Topic: Ionization Energy
- Replies: 8
- Views: 328
Ionization Energy
Why is the second (and third and fourth, etc) ionization energy usually so much larger than the first one?
- Sat Oct 12, 2019 7:46 pm
- Forum: Properties of Electrons
- Topic: Wavelength and Electrons
- Replies: 4
- Views: 265
Re: Wavelength and Electrons
It depends on the speed the electron is traveling at. You can calculate the wavelength using De Broglie's equation (h/mv).
- Sat Oct 12, 2019 7:38 pm
- Forum: Properties of Electrons
- Topic: Diffraction
- Replies: 2
- Views: 114
Re: Diffraction
The waves are "in phase," so that the amplitude of the resulting wave is equal to the sum of the individual amplitudes of the original waves
- Sat Oct 12, 2019 7:34 pm
- Forum: Properties of Electrons
- Topic: Weight of Particles
- Replies: 4
- Views: 239
Re: Weight of Particles
According to the class constants and equations sheet:
Mass of electron = 9.109383 * 10^-31 kg
Mass of neutron = 1.674927 * 10^-27 kg
Mass of proton = 1.672622 * 10^-27 kg
Mass of electron = 9.109383 * 10^-31 kg
Mass of neutron = 1.674927 * 10^-27 kg
Mass of proton = 1.672622 * 10^-27 kg
- Sat Oct 12, 2019 7:07 pm
- Forum: Properties of Light
- Topic: 1A #11
- Replies: 3
- Views: 174
1A #11
The question is: In the spectrum of atomic hydrogen, several ines are generally classified together as belonging to a series (for example, Balmer series or Lyman series). What is common to the lines within a series that makes grouping them together logical? I know that the Balmer series is visible l...
- Sat Oct 12, 2019 6:55 pm
- Forum: DeBroglie Equation
- Topic: Measurable V. Non-Measurable
- Replies: 5
- Views: 191
Re: Measurable V. Non-Measurable
^ Because 10^-38 is a smaller value than 10^-15, therefore it is not measurable.

