Search found 107 matches
- Sat Mar 14, 2020 2:16 pm
- Forum: Administrative Questions and Class Announcements
- Topic: ENDGAME Review Session
- Replies: 71
- Views: 5703
Re: ENDGAME Review Session
Usually, aren't E* values by default given for reduction?
- Sat Mar 14, 2020 11:36 am
- Forum: Administrative Questions and Class Announcements
- Topic: Format?
- Replies: 11
- Views: 873
Re: Format?
Will the final still account for the 180 points on the syllabus or will it be worth less?
- Thu Mar 12, 2020 9:48 am
- Forum: Administrative Questions and Class Announcements
- Topic: FinalReview Sessions
- Replies: 3
- Views: 411
Re: FinalReview Sessions
The review is canceled but I imagine they will post solutions in the near future.
- Thu Mar 12, 2020 9:47 am
- Forum: Administrative Questions and Class Announcements
- Topic: Saying Thank You to Dr. Lavelle
- Replies: 490
- Views: 569997
Re: Saying Thank You to Dr. Lavelle
Dear Dr. Lavelle,
Thank you for being a great chemistry professor and an even better person. All the resources you put into this class have really helped all of us students and helping us through this stressful time will never be underappreciated!
Thank you for being a great chemistry professor and an even better person. All the resources you put into this class have really helped all of us students and helping us through this stressful time will never be underappreciated!
- Wed Mar 11, 2020 6:26 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Steady state
- Replies: 3
- Views: 250
Steady state
Are we expected to know how to use the steady-state approximation?
- Tue Mar 10, 2020 5:13 pm
- Forum: Administrative Questions and Class Announcements
- Topic: test 2 return
- Replies: 6
- Views: 641
test 2 return
Does anyone know how we will get our tests 2s back as classes are cancelled.
- Mon Mar 09, 2020 5:32 pm
- Forum: General Rate Laws
- Topic: negatives?
- Replies: 2
- Views: 228
Re: negatives?
Use negatives when looking at reactants as their concentrations decrease over time.
- Sat Mar 07, 2020 7:38 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: K=kforward/kreverse
- Replies: 1
- Views: 223
Re: K=kforward/kreverse
For hypothetical equation, A + B <-> C + D, K = [C][D]/[A][B]. We know that the rate of the forward reaction is k [A][B], and the rate of the reverse is k'[C][D]. At equilibrium (so we can use K), the forward rate is equal to the reverse rate. So, k [A][B] = k'[C][D]. If you divide [A][B] from both ...
- Wed Mar 04, 2020 7:54 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 6N.1.b)
- Replies: 4
- Views: 311
Re: 6N.1.b)
Yeah you would get 1*10^4 if 2 electrons were transferred but that is not the case here. The answer should be 107
- Wed Mar 04, 2020 5:03 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: When to use Platinum as an electrode
- Replies: 4
- Views: 343
Re: When to use Platinum as an electrode
Does anyone know which elements are considered conductive enough that platinum is not used to transfer electrons? I remember seeing liquid mercury as something that can transfer electrons but I forgot whether that was true or not.
- Wed Mar 04, 2020 3:13 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6.L.5 D
- Replies: 2
- Views: 212
Re: 6.L.5 D
I'm don't really understand your question. The oxidation would be Au+ going to Au3+ with 2 electrons being on the products side, signifying oxidation.
- Tue Mar 03, 2020 5:08 pm
- Forum: Van't Hoff Equation
- Topic: Equation Sheet
- Replies: 18
- Views: 1132
Equation Sheet
Is the Van't Hoff Equation on the equation sheet?
- Sat Feb 29, 2020 3:59 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3649539
Re: Post All Chemistry Jokes Here
Albert was a chemist. Albert is no more.
What he thought was H2O. Was H2SO4
What he thought was H2O. Was H2SO4
- Sat Feb 29, 2020 3:56 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: No Salt Bridge
- Replies: 7
- Views: 559
Re: No Salt Bridge
In the absence of a salt bridge, the anode will keep losing electrons and hence get more positive. A salt bridge would pump anions to the anode making it less positive.
- Sat Feb 29, 2020 3:54 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Salt bridges
- Replies: 11
- Views: 778
Re: Salt bridges
Salt bridges ensure neutrality on both the cathode and the anode. As the cathode gets more negative over time and the anode gets more positive over time, salt bridges pump anions to the anode and cations to the cathode.
- Sat Feb 29, 2020 3:51 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Study Advice
- Replies: 73
- Views: 7178
Re: Study Advice
Honestly just doing all the homework problems will probably give you the best grasp of what will be expected from you on the test.
- Sat Feb 29, 2020 3:46 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: diamond
- Replies: 4
- Views: 315
Re: diamond
A diamond is thermodynamically unstable as it has a negative delta G value indicating that the reaction from diamond to graphite is favorable. however, it is kinetically stable as the reaction barrier is very high, so the reaction takes place at a very slow rate.
A diamond is not forever.
A diamond is not forever.
- Sun Feb 23, 2020 2:14 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3649539
Re: Post All Chemistry Jokes Here
What was oxidation again? My science is a bit rusty.
- Sun Feb 23, 2020 2:08 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Recommended Pathway for Chem Series
- Replies: 13
- Views: 1608
Re: Recommended Pathway for Chem Series
I would highly recommend doing all A-D first as some upper-division science classes require C or D as prerequisites. For example, LS 107 requires C as a prerequisite and it is a part of many life science majors, so getting BL and CL done is not that important right now.
- Sun Feb 23, 2020 2:06 pm
- Forum: Administrative Questions and Class Announcements
- Topic: 14BL and 14C
- Replies: 8
- Views: 476
Re: 14BL and 14C
I've heard BL is a LOT of work so keep that in mind as there will probably be a good amount of studying needed for 14C.
- Sun Feb 23, 2020 2:05 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Test 2 Material
- Replies: 16
- Views: 1064
Re: Test 2 Material
I believe we are covering kinetics next week which will NOT be on test 2.
- Sun Feb 23, 2020 2:03 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Curve?
- Replies: 5
- Views: 358
Re: Curve?
Tests aren't curved but I am also interested to see if he will adjust grades at the end in order to meet a specific distribution.
- Sun Feb 16, 2020 6:31 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3649539
Re: Post All Chemistry Jokes Here
Water molecule: Hey oil wanna hang out?
Oil molecule: I can't mix with you guys
Water molecule 2: You're such a hydrophobe!
Oil molecule: I can't mix with you guys
Water molecule 2: You're such a hydrophobe!
- Wed Feb 12, 2020 3:48 pm
- Forum: Ideal Gases
- Topic: STP
- Replies: 12
- Views: 1619
STP
Why is STP at 273.15 K (0 Celcius) and not 298 K like many constants are given at. Is there a separate constants list for values at STP?
- Wed Feb 12, 2020 3:45 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Pizza Rolls REVIEW Session DOWNLOAD HERE
- Replies: 67
- Views: 5854
Re: Pizza Rolls REVIEW Session DOWNLOAD HERE
Clara Cho 2K wrote:I feel like I'm doing #6 correctly , but I'm not getting the correct answer. Can someone explain how I calculate the work of expansion for the step with the reversible, isothermal expansion?
Wby = nRT (ln V2/V1), and -Wby = Won which is the component of dU = q + w.
- Wed Feb 12, 2020 3:43 pm
- Forum: Administrative Questions and Class Announcements
- Topic: A helpful equation sheet
- Replies: 4
- Views: 689
Re: A helpful equation sheet
Hiba Alnajjar_2C wrote:Could someone explain when to use Cp or Cv? Is Cp used when the pressure is constant, while Cv is used when the volume is constant? Thank you!
You got it, also remember how to calculate it when presented with an ideal gas, although it is on the formula sheet.
- Wed Feb 12, 2020 12:15 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Units for enthalpies
- Replies: 2
- Views: 197
Units for enthalpies
Can anyone give me examples of when the answer requested should be left as kJ or as kJ/mol. Some of the homework questions asked for standard reaction enthalpies and proceeded to answer in kJ.
- Sun Feb 09, 2020 5:04 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: U=3/2 nRT
- Replies: 4
- Views: 218
Re: U=3/2 nRT
U is the same as Eth I believe, which is the thermal energy an object has. Therefore, U is also determined by temperature and is equal to the average kinetic energy per mole multiplied by the number of moles (n).
- Sun Feb 09, 2020 5:01 pm
- Forum: Administrative Questions and Class Announcements
- Topic: memorizing things?
- Replies: 13
- Views: 644
Re: memorizing things?
Everything is given but you would have to know what each symbol/letter means.
- Sun Feb 09, 2020 4:56 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Calculating Average Kinetic Energy
- Replies: 1
- Views: 142
Re: Calculating Average Kinetic Energy
When converting celcius to kelvin, you added 273. For a more accurate conversion, add 273.15 as mentioned on Dr. Lavelle's formula sheet. Also, the textbook probably used R = 8.3145 as the gas constant. This should get the right answer.
- Sun Feb 09, 2020 4:50 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Strong acids and bases as gases
- Replies: 4
- Views: 232
Re: Strong acids and bases as gases
I think at least for the scope of this class acids and bases will be present in aqueous form.
- Sun Feb 09, 2020 4:48 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Isothermal expansion
- Replies: 2
- Views: 130
Re: Isothermal expansion
Delta U is equivalent to 3/2 NKB(Delta T), so if T is constant then Delta U is constant as well.
- Sat Feb 01, 2020 1:26 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Value of q
- Replies: 11
- Views: 593
Re: Value of q
In a perfect system with no energy lost or gained, q lost/gained by system = q gained/lost by surroundings.
- Sat Feb 01, 2020 1:24 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Calorimeter
- Replies: 3
- Views: 191
Re: Calorimeter
I believe that if it there is negative heat then the reaction is exothermic, so the surroundings increase in temperature and the system in question is releasing heat.
- Sat Feb 01, 2020 1:22 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Is U equal to delta Eth
- Replies: 2
- Views: 256
Is U equal to delta Eth
In physics classes the first law of thermodynamics was 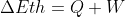 . However, in this class the equation is
. However, in this class the equation is 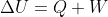 . I just want to make sure that U and Eth are the same so it doesn't get confusing.
. I just want to make sure that U and Eth are the same so it doesn't get confusing.
- Fri Jan 31, 2020 10:32 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3649539
Re: Post All Chemistry Jokes Here
What atoms make up aspirin?
As P Ir In
As P Ir In
- Fri Jan 31, 2020 10:23 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: How to derive ΔH = ΔU + nRΔT
- Replies: 1
- Views: 231
Re: How to derive ΔH = ΔU + nRΔT
The standard formula for the first law of thermodynamics is 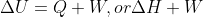 . Here, W is equivalent to P
. Here, W is equivalent to P 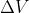 , which by the ideal gas law is the same as nR
, which by the ideal gas law is the same as nR 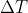 , which is where this equation came from.
, which is where this equation came from.
- Sat Jan 25, 2020 3:08 pm
- Forum: Phase Changes & Related Calculations
- Topic: Steam Burn
- Replies: 6
- Views: 240
Re: Steam Burn
As the latent heat of vaporization is very high, the amount of energy released when steam is condensed is much higher than the amount of energy released when boiling water is cooled, therefore the burn will be much more significant.
- Sat Jan 25, 2020 3:06 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: When to use Standard enthalpies of formation
- Replies: 3
- Views: 121
Re: When to use Standard enthalpies of formation
The standard enthalpies of formation are usually given at STP therefore it would be different under nonstandard conditions.
- Sat Jan 25, 2020 3:02 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Pressure and Enthalpy
- Replies: 5
- Views: 150
Re: Pressure and Enthalpy
Pressure has a direct relationship with enthalpy which is why it is usually easier to calculate enthalpies at STP, using 1 atm as standard.
- Sat Jan 25, 2020 3:01 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Melting and freezing
- Replies: 7
- Views: 283
Re: Melting and freezing
Freezing would be considered exothermic because it is converting particles from a less ordered state to a more ordered state, which would make the value of mass*latent heat of fusion, negative, hence exothermic.
- Sat Jan 25, 2020 2:57 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: state functions?
- Replies: 6
- Views: 763
Re: state functions?
State functions are functions where only the final state and the initial state matter when doing calculations and all intermediate states are effectively immaterial. For example, when calculating energy for a gas at two different temperatures, the fact that it is at any other value besides the start...
- Sun Jan 19, 2020 1:24 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: 5% vs. K < 10^-3
- Replies: 3
- Views: 137
5% vs. K < 10^-3
What is the difference between the 5% rule and the rule saying that you can disregard x in the denominator if K < 10-3, or are they the same thing. Also, is this mentioned in the textbook and if so, where?
- Sun Jan 19, 2020 1:21 pm
- Forum: Ideal Gases
- Topic: R Constant
- Replies: 7
- Views: 282
Re: R Constant
Use the R constant that uses the appropriate unit of pressure.
- Sun Jan 19, 2020 1:13 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3649539
Re: Post All Chemistry Jokes Here
Which element is the coldest?
Brrryllium
Brrryllium
- Sun Jan 19, 2020 1:11 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Quadratic Equation
- Replies: 8
- Views: 385
Re: Quadratic Equation
A lot of times when using ice tables, the equilibrium concentration equation will end up looking like x 2 /number-x = constant. Therefore, when you cross multiply by (number-x) and move it over to have one side of the equation equal to zero, you will end up with a quadratic equation, which can be so...
- Sun Jan 19, 2020 12:22 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: acids and bases
- Replies: 4
- Views: 137
Re: acids and bases
Yes, acids and bases are at equilibrium which can allow calculation of KA and KB
- Sun Jan 12, 2020 11:55 am
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3649539
Re: Post All Chemistry Jokes Here
Did you hear about the man who got cooled to absolute zero?
He's 0 K now.
He's 0 K now.
- Sun Jan 12, 2020 11:49 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: K vs Kc
- Replies: 3
- Views: 150
Re: K vs Kc
The textbook seems to not specify the K value, by this I mean Kc or Kp. The K values should all be the same though, it is just convention to use partial pressure when dealing with only gasses and concentration in other circumstances.
- Sun Jan 12, 2020 11:47 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: ICE box
- Replies: 9
- Views: 311
Re: ICE box
There is no such thing as a negative concentration, therefore this value should be disregarded and only the positive value should be used.
- Sun Jan 12, 2020 11:44 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: when to use Kc vs Kp
- Replies: 11
- Views: 459
Re: when to use Kc vs Kp
By using brackets you are denoting a value that is concentration, therefore you should plug in concentrations with the units molL-1, which will get you Kc.
- Sun Jan 12, 2020 11:43 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: solids and liquids
- Replies: 3
- Views: 123
Re: solids and liquids
I think it is because their concentrations do not change throughout the reaction and do not affect the concentrations at equilibrium. As the density of a pure solid or liquid remains the same no matter what volume, the concentration is also the same.
- Sat Dec 07, 2019 12:53 pm
- Forum: Administrative Questions and Class Announcements
- Topic: MARSHMALLOW- FINAL REVIEW SESSION [ENDORSED]
- Replies: 115
- Views: 13260
Re: MARSHMALLOW- FINAL REVIEW SESSION [ENDORSED]
Lauren Haight 1E wrote:for mini marshmallow 2b, why is the coordination number for Dihydroxoyoxolatocobalt (III) 4?
Oxalate is BIdentate, so it will form 2 coordinate covalent bonds. Add this to the two bonds made to hydroxide and the coordination number is 4.
- Sat Dec 07, 2019 12:52 pm
- Forum: Administrative Questions and Class Announcements
- Topic: MARSHMALLOW- FINAL REVIEW SESSION [ENDORSED]
- Replies: 115
- Views: 13260
Re: MARSHMALLOW- FINAL REVIEW SESSION [ENDORSED]
Joanne Kang 3I wrote:min marshmallows 1c... why isn't it neutral?
ammonium is a conjugate acid of a weak base, so it will lower pH by giving a proton (H+) to water to form hydronium.
- Sat Dec 07, 2019 12:51 pm
- Forum: Administrative Questions and Class Announcements
- Topic: MARSHMALLOW- FINAL REVIEW SESSION [ENDORSED]
- Replies: 115
- Views: 13260
Re: MARSHMALLOW- FINAL REVIEW SESSION [ENDORSED]
For mini marshmallows 2c, why is it cupperate instead of copper? Isn't it a +1 oxidation state? The coordination complex has a 1- charge, so it is cuprate. This is shown because it forms an ionic bond with potassium which has a +1 charge, so you know the coordination complex must have a -1 charge. ...
- Thu Dec 05, 2019 2:44 pm
- Forum: Identifying Acidic & Basic Salts
- Topic: HW 6D.11 e and f
- Replies: 1
- Views: 93
Re: HW 6D.11 e and f
For e, Al has a strong charge (+3) so it can take away protons. Remember that small highly charged metals act as acids. As Cl - is a conjugate base of a strong acid, it does not affect pH. For f, Cu again is a metal that has a strong charge (+2, which is kind of borderline). I think that Copper can ...
- Thu Dec 05, 2019 2:35 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Midterm question with Rydberg
- Replies: 1
- Views: 190
Re: Midterm question with Rydberg
I'm kind of lost so I'll just walk through the problem. Ok to start we have 1.94 *10 -18 J for the change in energy by converting 102.557 nm to Energy which you have done. Then this value is equivalent to E final - E initial . However, as you are subtracting a negative (the E initial ), it becomes E...
- Thu Dec 05, 2019 2:23 pm
- Forum: Einstein Equation
- Topic: units for energy
- Replies: 2
- Views: 366
Re: units for energy
The unit for energy is Joules, which is equivalent to a Newton*meter. Planck's constant h is in the units of m 2 kgs -1 and the unit for v frequency v is s -1 . Multiplying these together you would get m 2 kgs -2 . As a newton is in the units of kgms -2 , you can see that this equation indeed does f...
Re: 9C 1C
It is a negative ion, so you must add -ate to the end of the transition metal name. For reference Dr. Lavelle has the naming guide and it should be there.
- Thu Dec 05, 2019 2:12 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Hydrogen bonds in Uric Acid
- Replies: 1
- Views: 159
Hydrogen bonds in Uric Acid
What would be the total number of hydrogen bonding sites in Uric Acid?


- Fri Nov 29, 2019 9:54 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Test 2 Grades
- Replies: 10
- Views: 678
Re: Test 2 Grades
I think if you haven't gotten them already you will get them next week in discussion.
- Fri Nov 29, 2019 9:53 pm
- Forum: General Science Questions
- Topic: hydrogen vs hydronium
- Replies: 5
- Views: 278
Re: hydrogen vs hydronium
Technically it is the concentration of Hydronium as the H+ will attach to a water molecule. However, it is easier to write H+ concentration which is why it is common.
- Fri Nov 29, 2019 9:51 pm
- Forum: SI Units, Unit Conversions
- Topic: midterm/final
- Replies: 18
- Views: 1498
Re: midterm/final
I think there will be a good balance between the two as it should probably follow the format of the midterms.
- Fri Nov 29, 2019 9:50 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: pH scale
- Replies: 4
- Views: 330
Re: pH scale
Most of the substances that we will work with, if not all, will have pH values between 0 and 14 corresponding to Hydronium ion concentrations that range from 1 * 100 M*L-1 to 1* 10-14 M*L-1
- Fri Nov 29, 2019 9:47 pm
- Forum: Biological Examples
- Topic: cisplatin
- Replies: 6
- Views: 429
Re: cisplatin
Does anyone remember what specific nucleotide Cisplatin attaches to?
- Wed Nov 20, 2019 9:00 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3649539
Re: Post All Chemistry Jokes Here
Why is working with ammonia easy?
Because its quite basic.
Because its quite basic.
- Wed Nov 20, 2019 8:56 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Test 2
- Replies: 5
- Views: 315
Re: Test 2
I believe it goes up to 2F.1.
- Wed Nov 20, 2019 8:55 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3649539
Re: Post All Chemistry Jokes Here
Thank you Dr. Lavelle for putting the Cation in education.
- Wed Nov 20, 2019 8:54 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3649539
Re: Post All Chemistry Jokes Here
How often do I tell chemistry jokes?
Periodically.
Periodically.
- Wed Nov 20, 2019 8:52 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Test 2 related HW problems
- Replies: 2
- Views: 249
Re: Test 2 related HW problems
Aman Sankineni 3E wrote:The questions on the syllabus from 3F, 2E, and anything that covers sigma and pi bonds, so 2F.1 will cover topics on the test.
Thank you.
- Wed Nov 20, 2019 8:50 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Test #2
- Replies: 22
- Views: 2946
Re: Test #2
Probably up to "Explain how hybridization arises from atomic orbitals"
- Wed Nov 13, 2019 6:04 pm
- Forum: Dipole Moments
- Topic: Hydrogen bonds
- Replies: 17
- Views: 822
Re: Hydrogen bonds
Just with N, O, or F.
- Wed Nov 13, 2019 6:02 pm
- Forum: Dipole Moments
- Topic: Textbook question 3F.1
- Replies: 5
- Views: 320
Re: Textbook question 3F.1
All molecules have London Dispersion forces.
Molecules with uneven sharing of electrons (think polar/non-polar) will have dipole-dipole.
Molecules with Hydrogen bonded to a N, O, or F will have Hydrogen bonding.
Molecules with uneven sharing of electrons (think polar/non-polar) will have dipole-dipole.
Molecules with Hydrogen bonded to a N, O, or F will have Hydrogen bonding.
- Wed Nov 13, 2019 6:01 pm
- Forum: Dipole Moments
- Topic: HW Question 3F1
- Replies: 5
- Views: 403
Re: HW Question 3F1
SO2 is polar, hence the dipole-dipole interactions.
- Wed Nov 13, 2019 5:59 pm
- Forum: Dipole Moments
- Topic: 3F.3
- Replies: 5
- Views: 329
Re: 3F.3
As CH4 and CCl4 are symmetrical (and tetrahedral) they are nonpolar and dipole dipole interactions will not be that important. However, they still have London dispersion forces despite being nonpolar.
- Wed Nov 13, 2019 5:57 pm
- Forum: Dipole Moments
- Topic: How does O3 have dipole-dipole interactions?
- Replies: 2
- Views: 785
Re: How does O3 have dipole-dipole interactions?
Ozone is polar as it is bent, therefore it has a net dipole.
- Wed Nov 13, 2019 5:55 pm
- Forum: Dipole Moments
- Topic: 3F.5
- Replies: 6
- Views: 252
Re: 3F.5
To finish off this problem,
C. CHI3 will have a higher melting point because iodine is bigger than chlorine with more electrons and therefore higher IMFs.
D. Methanol will have a higher melting point because of the presence of Hydrogen bonds.
C. CHI3 will have a higher melting point because iodine is bigger than chlorine with more electrons and therefore higher IMFs.
D. Methanol will have a higher melting point because of the presence of Hydrogen bonds.
- Wed Nov 13, 2019 5:53 pm
- Forum: Dipole Moments
- Topic: 3F.5
- Replies: 6
- Views: 252
Re: 3F.5
Amy Luu 3I wrote:How do you determine that buthanol has h-bonds? How come diethyl ether doesn't have h bonds?
H bonds form when H is bonded to N, O or F.
- Wed Nov 13, 2019 5:52 pm
- Forum: Dipole Moments
- Topic: 3F.5
- Replies: 6
- Views: 252
Re: 3F.5
Yeah, doesn't ether also have H bonds? They're bonded to the carbon just as in Butanol, so what's the difference? Is it because unlike in Ether, theres a carbon bonded to an Oxygen? If so, why is this important?? I don't think so because the Hydrogen is not bonded to N, O, or F, therefore there are...
- Wed Nov 06, 2019 3:21 pm
- Forum: *Shrodinger Equation
- Topic: Explanation of Shrodinger Equation and Hamiltonian
- Replies: 3
- Views: 349
Re: Explanation of Shrodinger Equation and Hamiltonian
Victor James 4I wrote:how does this relate to orbitals?
I believe that valid solutions of Schrodinger's equation, psi, correspond to orbitals.
- Wed Nov 06, 2019 3:18 pm
- Forum: Administrative Questions and Class Announcements
- Topic: DINO NUGGETS Review Session! Download Problems HERE [ENDORSED]
- Replies: 52
- Views: 6809
Re: DINO NUGGETS Review Session! Download Problems HERE [ENDORSED]
ayushibanerjee06 wrote:For 6b., I am confused about why GarBreadium has the longer deBroglie wavelength. Shouldn't it be He because it weighs more?
As mass is on the denominator for deBroglie wavelength calculations, a lower mass atom will have a longer wavelength as it is inversely proportional.
- Thu Oct 31, 2019 5:56 pm
- Forum: Student Social/Study Group
- Topic: Midterm
- Replies: 7
- Views: 498
Re: Midterm
I believe he said in class it will include up to Focus 2D.
- Thu Oct 31, 2019 5:55 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3649539
Re: Post All Chemistry Jokes Here
OMG did you hear Oxygen and Magnesium were a couple?
- Thu Oct 31, 2019 5:53 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3649539
Re: Post All Chemistry Jokes Here
Don't throw sodium chloride around. It's a salt.
- Thu Oct 31, 2019 5:49 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3649539
Re: Post All Chemistry Jokes Here
Me: Hey Siri, can you give me the formula for Nitric Acid?
Siri: NO
Me: ...
Siri: NO
Me: ...
- Wed Oct 30, 2019 9:37 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Midterm
- Replies: 16
- Views: 740
Re: Midterm
Fundamentals, Quantum World, and Chemical Bonds up to Section 2D
- Wed Oct 23, 2019 11:32 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: how to do 1.D.23?
- Replies: 2
- Views: 152
Re: how to do 1.D.23?
I think you may have looked at the answer key incorrectly, mine says the right answer. Anyways, the thing that really matters is the l value, as it determines the orientation of the different orbitals. An l of 1 corresponds to a p orbital, which can be oriented 3 ways. When the m l is given, then th...
- Wed Oct 23, 2019 11:29 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: What's the difference between subshell vs orbitals?
- Replies: 8
- Views: 663
Re: What's the difference between subshell vs orbitals?
Hopefully this helps. Also there is a chemistry stackexchange post on this topic with helpful images. If electrons share the value n, it is part of a shell. If electrons share n and l, it is part of the same sub-shell. If electrons share n, l and ml then it is in the same orbital. https://chemistry....
- Wed Oct 23, 2019 11:27 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: l values
- Replies: 4
- Views: 285
Re: l values
l can go up to n-1, but in this class, we are only required to know up to the f orbital. Most likely we won't even see f orbitals as it doesn't really apply to living systems.
- Wed Oct 23, 2019 11:26 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: order
- Replies: 3
- Views: 177
Re: order
With some exceptions where the s orbital is lower energy, since the d orbital is in a different shell you order that first. Eg. 4d then 5s.
- Wed Oct 23, 2019 11:24 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: subshell or orbital?
- Replies: 3
- Views: 174
Re: subshell or orbital?
If electrons share the value n, it is part of a shell.
If electrons share n and l, it is part of the same sub-shell.
If electrons share n, l and ml then it is in the same orbital.
If electrons share n and l, it is part of the same sub-shell.
If electrons share n, l and ml then it is in the same orbital.
- Sat Oct 19, 2019 9:25 pm
- Forum: Properties of Light
- Topic: light
- Replies: 5
- Views: 266
Re: light
Yes theoretically speaking there is nothing that can exceed the speed of light so if you calculate the velocity of an election exceeding the speed of light that is not possible.
- Wed Oct 16, 2019 5:26 pm
- Forum: Properties of Light
- Topic: Constant for Speed of Light
- Replies: 14
- Views: 615
Re: Constant for Speed of Light
Given he provides 2.99792 × 10^8 m/s on the formula sheet pdf, I would use that, although I'm sure he would specify/be fine with either on homework.
- Mon Oct 14, 2019 9:42 pm
- Forum: Einstein Equation
- Topic: Lecture Question!
- Replies: 5
- Views: 220
Re: Lecture Question!
I don't believe so, which is why there are discrete absorption/emission line spectra for each element. If an element were to absorb all frequencies and therefore all energies of light, then there would be no black spots in the emission spectrum. If you google hydrogen emission spectrum you'll see on...
- Mon Oct 14, 2019 9:39 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Test 1 [ENDORSED]
- Replies: 107
- Views: 22682
Re: Test 1 [ENDORSED]
DesireBrown3K wrote:If someone was able to complete the Angstrom question, can you please explain to me (step by step) how to solve the problem?
An angstrom is 10-10m, so there are .1 angstrom in a nanometer and 100 angstroms in a picometer.
- Mon Oct 14, 2019 9:37 pm
- Forum: Einstein Equation
- Topic: 1B.7 Part c
- Replies: 3
- Views: 222
Re: 1B.7 Part c
As you are given 1 mol of photons, and the energy per photon is calculated in part A, you take the energy (part A) in J/photon multiplied by 6.022 photons/mol which would yield the units J/mol, which is what the answer is looking for. You multiply this my 1 mol, and get the answer.
- Mon Oct 14, 2019 8:10 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Heisenberg indeterminacy equation
- Replies: 2
- Views: 142
Heisenberg indeterminacy equation
In class, we learned it as Delta P* Delta X >= h/4pi, but in the solutions manual it states that it is greater than or equal to (1/2)h. Can someone please clarify which one is the correct formula? Thanks.
- Thu Oct 10, 2019 4:23 pm
- Forum: Properties of Electrons
- Topic: Planck's Constant
- Replies: 3
- Views: 183
Re: Planck's Constant
Planck's constant relates the energy in one photon of electromagnetic radiation to the frequency of that radiation. m2*kg/s is the same as Joule*second, as a joule is a kg *m2*s-2, so this multiplied by frequency in Hz (or s-1) would yield energy.
- Thu Oct 10, 2019 4:18 pm
- Forum: Properties of Light
- Topic: HW 1B9
- Replies: 2
- Views: 134
Re: HW 1B9
You would want to take 32W * 2 seconds and divide it by your result from the energy released by each photon. You then should take the photons you have and divide by 6.022 *10^23 photons/mol, leaving you with moles of photons.
- Thu Oct 10, 2019 4:15 pm
- Forum: Properties of Electrons
- Topic: Understanding Balmer & Lyman Series
- Replies: 3
- Views: 194
Re: Understanding Balmer & Lyman Series
As Romina said, the Lyman series refers to UV light, which has more energy than the visible light spectrum which is why it corresponds to going from n=1 to n=3, for example. The further away an electron is from the nucleus, less energy is required to jump to the next level, so lower energy light wil...

