Search found 55 matches
- Mon Dec 02, 2019 8:23 pm
- Forum: Conjugate Acids & Bases
- Topic: the conjugate seesaw
- Replies: 5
- Views: 427
Re: the conjugate seesaw
There is an inverse relationship between acid and conjugate base/ base and conjugate acid.
- Mon Dec 02, 2019 8:20 pm
- Forum: Amphoteric Compounds
- Topic: Amphoteric Compound
- Replies: 5
- Views: 613
Re: Amphoteric Compound
Amphoteric compounds are compounds that can act as both an acid and a base. In class he showed examples of acid and base reactions that involved water. Water can act as both an acid and a base as it can accept H+ to become H3O+ and donate H+ to become OH-. There are also a couple of nonmetal and me...
- Mon Dec 02, 2019 8:20 pm
- Forum: Identifying Acidic & Basic Salts
- Topic: Acid protonation
- Replies: 4
- Views: 351
Re: Acid protonation
What exactly is protonation? What is happening to an acid undergoing protonation?
- Mon Dec 02, 2019 8:17 pm
- Forum: Calculating the pH of Salt Solutions
- Topic: pH vs. pOH
- Replies: 17
- Views: 2478
Re: pH vs. pOH
Emma Popescu 3D wrote:If you have the pH of a solution, you can calculate its pOH or vice versa. For example, if the pH is 2 then the pOH will be 14-2=12
Just to clarify, they always add to 14?
- Mon Dec 02, 2019 8:15 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Class wed 11/27
- Replies: 7
- Views: 526
Re: Class wed 11/27
Can someone explain what pOH is in a little bit more detail?
- Tue Nov 26, 2019 4:09 pm
- Forum: General Science Questions
- Topic: Rusty on High School Chem [ENDORSED]
- Replies: 347
- Views: 432178
Re: Rusty on High School Chem [ENDORSED]
Does a lot of the material from 14a come up again in 14b or is it mainly new concepts?
- Tue Nov 26, 2019 4:06 pm
- Forum: Sigma & Pi Bonds
- Topic: Question on Test 2
- Replies: 11
- Views: 924
Question on Test 2
Can someone explain the sigma and pi bond question from Test 2? I believe there was also a hydrogen bonding component to the question as well.
- Tue Nov 26, 2019 4:05 pm
- Forum: Amphoteric Compounds
- Topic: Oxides
- Replies: 4
- Views: 397
Re: Oxides
rohun3H wrote:It is most useful to know the patterns of the periodic table.
Are there any exceptions?
- Tue Nov 26, 2019 4:04 pm
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: difficulties recognizing weak acids and bases
- Replies: 9
- Views: 1513
Re: difficulties recognizing weak acids and bases
daniella_knight1I wrote:If you see a Ka or Kb constant that's a giveaway. If not you'll have to draw out the lewis structure and look at the lone pairs to determine if it's a weak acid or base.
Just clarifying that if you see a constant it is most likely a weak acid, not a strong one.
- Tue Nov 26, 2019 4:03 pm
- Forum: Lewis Acids & Bases
- Topic: Lewis vs Bronsted Acids and Bases
- Replies: 3
- Views: 533
Re: Lewis vs Bronsted Acids and Bases
Will we use both in this class? Or will we use one more so than the other?
- Tue Nov 19, 2019 8:37 pm
- Forum: Ionic & Covalent Bonds
- Topic: strength of bonds
- Replies: 14
- Views: 1018
Re: strength of bonds
Does strength of bonds relate to its melting point?
- Tue Nov 19, 2019 8:35 pm
- Forum: Student Social/Study Group
- Topic: Final Jitters
- Replies: 457
- Views: 357651
Re: Final Jitters
I always get really bad anxiety during test week to the point where I get sick and throw up or am constantly nauseous. Anyone have ideas for how to help while also being able to study? You could try meditating and planning little study breaks to look forward to. Also, try to think about tests in th...
- Tue Nov 19, 2019 8:32 pm
- Forum: Ideal Gases
- Topic: Reading the textbook
- Replies: 262
- Views: 150027
Re: Reading the textbook
I have had a problem on a test that had an answer that was only covered in the textbook, not in lecture at all. So, I would say definitely at least skim the textbook chapters.
- Tue Nov 19, 2019 8:31 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: formal charge
- Replies: 6
- Views: 522
Re: formal charge
A very simplified/juvenile way to think of formal charge is FC = Valence E - (# sticks + # dots). Sticks being bonds and dots being single electrons in lone pairs.
- Tue Nov 19, 2019 8:29 pm
- Forum: *Liquid Structure (Viscosity, Surface Tension, Liquid Crystals, Ionic Liquids)
- Topic: Hydrogen Bonding/Pi bonds
- Replies: 11
- Views: 1620
Re: Hydrogen Bonding/Pi bonds
What are all of the kinds of intermolecular forces?
- Fri Nov 15, 2019 11:59 pm
- Forum: Trends in The Periodic Table
- Topic: Question
- Replies: 17
- Views: 1405
Re: Question
Can someone explain the exception for oxygen and nitrogen? It was on the midterm and I got it wrong.
- Fri Nov 15, 2019 11:58 pm
- Forum: Dipole Moments
- Topic: Polarity
- Replies: 12
- Views: 619
Re: Polarity
What is dipole-dipole? I know we talked about it a lot in class but I am still confused.
- Fri Nov 15, 2019 11:57 pm
- Forum: Octet Exceptions
- Topic: electron number in octet
- Replies: 8
- Views: 616
Re: electron number in octet
Can anyone sum up the exceptions into one post so I can memorize?
- Fri Nov 15, 2019 11:56 pm
- Forum: Student Social/Study Group
- Topic: Chemistry News
- Replies: 135
- Views: 167030
Re: Chemistry News
Jose Robles 1D wrote:https://www.sciencedaily.com/releases/2019/11/191104130450.htm
Interesting article that I thought you should look at.
For anyone who is feeling lazy, the article is titled: Light-based 'tractor beam' assembles materials at the nanoscale
- Fri Nov 15, 2019 11:56 pm
- Forum: Student Social/Study Group
- Topic: Chemistry News
- Replies: 135
- Views: 167030
Re: Chemistry News
Muj_Rahman_3B wrote:Researchers have been using two different types of brain scanners on babies to check, as the title says, their "brain chemistry." Here's it is.
https://www.sciencedaily.com/releases/2 ... 160940.htm
Thats interesting!
- Fri Nov 15, 2019 11:55 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: regions of electron density
- Replies: 7
- Views: 560
Re: regions of electron density
Basically an atom that has electron lone pairs or is sharing electrons I believe.
- Fri Nov 08, 2019 2:02 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Question regarding definition of molecules
- Replies: 5
- Views: 552
Re: Question regarding definition of molecules
A mole is the SI unit for a quantity of something
- Fri Nov 08, 2019 2:01 pm
- Forum: Electronegativity
- Topic: Website
- Replies: 3
- Views: 355
Re: Website
McKenna_4A wrote:I can't log into Dr. Lavelle's website. I enter my password and it just loads for a second and nothing happens. I don't get any "wrong password" notification or anything. Anybody else having this problem/have a solution?
Switch your browser to chrome or safari.
- Fri Nov 08, 2019 2:00 pm
- Forum: Lewis Structures
- Topic: Midterm grades
- Replies: 26
- Views: 1439
Re: Midterm grades
VioletKo3F wrote:Does anyone know if we'll get the actual midterm back? Or is it only our grades?
You should get the whole midterm back.
- Fri Nov 08, 2019 1:59 pm
- Forum: SI Units, Unit Conversions
- Topic: Converting SI units
- Replies: 3
- Views: 360
Re: Converting SI units
Do we need to write out and cross out every unit or is it safe to skip a few of these steps as long as our answer is in the correct unit?
- Fri Nov 08, 2019 1:44 pm
- Forum: Lewis Structures
- Topic: Formal Charges
- Replies: 15
- Views: 965
Re: Formal Charges
Is it okay to break the octet rule in order to minimize formal charge?
- Thu Oct 31, 2019 9:58 am
- Forum: Photoelectric Effect
- Topic: Work Function
- Replies: 9
- Views: 633
Re: Work Function
Is work function, Φ used in any other equations besides the KE/Energy of a photon one?
- Thu Oct 31, 2019 9:55 am
- Forum: Photoelectric Effect
- Topic: kinetic energy
- Replies: 6
- Views: 325
Re: kinetic energy
Kinetic energy + O[strike] = the energy of a photon? What is the O with a strike through it representing?
- Thu Oct 31, 2019 9:54 am
- Forum: Properties of Electrons
- Topic: Formal Charge of an Atom
- Replies: 5
- Views: 321
Re: Formal Charge of an Atom
Can you explain what the lone pairs part of the equation means and maybe give an example? Thank you!
- Thu Oct 31, 2019 9:53 am
- Forum: Einstein Equation
- Topic: Joules units
- Replies: 6
- Views: 941
Re: Joules units
So the units are essentially equivalent to each other?
- Thu Oct 31, 2019 9:51 am
- Forum: Properties of Light
- Topic: All the formulas
- Replies: 5
- Views: 215
Re: All the formulas
Vuong_4G wrote:Not sure if this is what you're looking for but...
c =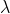
E(photon) = hm*
= E(photon) -
= h/p
Could you explain wavelength = h/p to me please?
- Thu Oct 31, 2019 9:47 am
- Forum: Properties of Light
- Topic: All the formulas
- Replies: 5
- Views: 215
Re: All the formulas
Will we ever use wavelength x frequency = velocity? or will it always be =c(speed of light)
- Thu Oct 24, 2019 1:42 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Energy of spdf orbitals
- Replies: 11
- Views: 399
Re: Energy of spdf orbitals
Can someone explain what a shell is vs a subshell?
- Thu Oct 24, 2019 1:41 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Test 1 [ENDORSED]
- Replies: 107
- Views: 20486
Re: Test 1 [ENDORSED]
Will any of the content from test 1 be on the midterm and/or test 2?
- Thu Oct 24, 2019 1:40 pm
- Forum: Electronegativity
- Topic: How to determine which bond is more covalent?
- Replies: 2
- Views: 2278
Re: How to determine which bond is more covalent?
Any other tips for what to study/how to prepare for test two?
- Thu Oct 24, 2019 1:40 pm
- Forum: Lewis Structures
- Topic: Ion lewis structure
- Replies: 9
- Views: 386
Re: Ion lewis structure
Emily_4B wrote:Is there any case where the atoms lewis structure doesn’t have a full octet?
I also was wondering about this question? Are there any exceptions to the rules that we should memorize?
- Thu Oct 24, 2019 1:39 pm
- Forum: Electronegativity
- Topic: Trend of Electronegativity
- Replies: 18
- Views: 4208
Re: Trend of Electronegativity
In discussion someone mentioned a noble gas rule/shortcut when we were discussing periodic table trends? Does this relate to electronegativity? If not, can someone explain what it is?
- Thu Oct 24, 2019 1:36 pm
- Forum: Ionic & Covalent Bonds
- Topic: strength of bonds
- Replies: 14
- Views: 1018
Re: strength of bonds
Is an ionic or a covalent bond typically stronger?
- Wed Oct 16, 2019 5:38 pm
- Forum: Einstein Equation
- Topic: kJ/mol
- Replies: 4
- Views: 300
Re: kJ/mol
I have a question regarding one of the post assessment questions (28b) from the atomic spectra module. In this problem, the units kJ/mol are used. In solving it, I just thought to use the kJ->J conversion but still ended up with the wrong answer. I'm not sure if I made just a calculation mistake or...
- Wed Oct 16, 2019 5:36 pm
- Forum: DeBroglie Equation
- Topic: De Broglie Wavelength
- Replies: 7
- Views: 317
Re: De Broglie Wavelength
I think it just means to use the De Broglie equation because the De Broglie equation is derived from the equations E=pc, c= wavelength*frequency, and E=hv which we have learned before. However, this equation only works for any particle with a rest mass, momentum, and wavelength so if it asks for th...
- Wed Oct 16, 2019 5:35 pm
- Forum: Trends in The Periodic Table
- Topic: electronegativity
- Replies: 10
- Views: 3328
Re: electronegativity
Electronegativity is related to the electron-pulling ability of an atom. Elements at the top right corner of the periodic table have the highest electronegativity, meaning that electronegativity increases as you move right across the table and decreases as you move down. I have been looking for an ...
- Wed Oct 16, 2019 5:33 pm
- Forum: *Shrodinger Equation
- Topic: What is Shrodinger's for?
- Replies: 6
- Views: 353
Re: What is Shrodinger's for?
Schrodinger's equation predicts future dynamic systems via calculating the partial differentiation of wave functions, or how the electron will behave around its nucleus. Most likely, you will not have to calculate anything with the equation itself, but you should at least know what each part of the...
- Wed Oct 16, 2019 5:32 pm
- Forum: Properties of Light
- Topic: Difference between Quanta and photons?
- Replies: 6
- Views: 628
Re: Difference between Quanta and photons?
Sam McNeill 3A wrote:A photon is the quanta, or smallest measurement, of light.
What unit is this measurement in?
- Thu Oct 10, 2019 11:47 pm
- Forum: Significant Figures
- Topic: Question About Significant Figures and Rounding
- Replies: 22
- Views: 3249
Re: Question About Significant Figures and Rounding
Is anyone familiar with Dr. Lavelle's rule for rounding to the nearest even number when there are 4 sig figs and you can only have 3? I don't think I've heard him talk about this. Is this a rule that isn't standard, but Dr. Lavelle wants us to apply? And what do you mean by the nearest even number ...
- Thu Oct 10, 2019 11:44 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Advice from a Medical Student [ENDORSED]
- Replies: 297
- Views: 408800
Re: Advice from a Medical Student [ENDORSED]
Do you recommend taking chem 17 before chem 14a?
- Thu Oct 10, 2019 11:43 pm
- Forum: DeBroglie Equation
- Topic: How to know which equation to use
- Replies: 9
- Views: 609
Re: How to know which equation to use
What is a real-world application of this equation?
- Thu Oct 10, 2019 11:41 pm
- Forum: Student Social/Study Group
- Topic: Final Jitters
- Replies: 457
- Views: 357651
Re: Final Jitters
Getting a good night's sleep is huge! If you've been studying for hours, just go to bed!! Give your brain some time to process everything in the meantime.
- Thu Oct 10, 2019 11:40 pm
- Forum: Significant Figures
- Topic: Question About Significant Figures and Rounding
- Replies: 22
- Views: 3249
Re: Question About Significant Figures and Rounding
Is anyone familiar with Dr. Lavelle's rule for rounding to the nearest even number when there are 4 sig figs and you can only have 3?
- Thu Oct 10, 2019 11:38 pm
- Forum: Administrative Questions and Class Announcements
- Topic: KAREN SUN 5-7PM WORKSHOP - DOWNLAOD WORKSHEETS HERE
- Replies: 53
- Views: 5926
Re: KAREN SUN 5-7PM WORKSHOP - DOWNLAOD WORKSHEETS HERE
What was the scam for workshops that Dr. Lavelle mentioned in lecture? Something to do with asking for our phone numbers?
- Thu Oct 10, 2019 11:36 pm
- Forum: Trends in The Periodic Table
- Topic: ionization energy
- Replies: 11
- Views: 1035
Re: ionization energy
When would I use this in a calculation, like what would the problem be asking?
- Thu Oct 03, 2019 10:19 am
- Forum: Administrative Questions and Class Announcements
- Topic: Tips for Tests [ENDORSED]
- Replies: 4
- Views: 310
Re: Tips for Tests [ENDORSED]
I spoke with one of the UA's and went to their Step-Up session on Tuesday and they really recommended taking advantage of the resources available. For instance, the Step-Up session really helped me solidify my confidence in certain skills and identify areas I am weaker in. They gave us a bunch of p...
- Thu Oct 03, 2019 10:17 am
- Forum: Administrative Questions and Class Announcements
- Topic: Will there be lecture on Nov. 27?
- Replies: 3
- Views: 245
Re: Will there be lecture on Nov. 27?
It is looking like I will have to fly home on the Tuesday before Thanksgiving too. Will I be penalized for missing lecture?
- Thu Oct 03, 2019 10:15 am
- Forum: Administrative Questions and Class Announcements
- Topic: First Test in Discussion
- Replies: 13
- Views: 762
Re: First Test in Discussion
Do we need to bring any materials/resources to the test? Calculator, Periodic Table, etc?
- Thu Oct 03, 2019 10:13 am
- Forum: SI Units, Unit Conversions
- Topic: Homework for Week 1
- Replies: 16
- Views: 875
Re: Homework for Week 1
Does anyone know if we will get our homework back with comments or corrections from our ta?
- Thu Oct 03, 2019 10:10 am
- Forum: Significant Figures
- Topic: Significant Figures
- Replies: 10
- Views: 798
Re: Significant Figures
Here are a couple rules I found online: Non-zero digits are always significant. Any zeros between two significant digits are significant. A final zero or trailing zeros in the decimal portion ONLY are significant. My question: When you say 'any zeros between two significant digits are significant',...

