Search found 50 matches
- Sat Mar 14, 2020 8:34 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Saying Thank You to Dr. Lavelle
- Replies: 490
- Views: 577066
Re: Saying Thank You to Dr. Lavelle
Dr. Lavelle, Although I know that my comment will be lost alongside many, I would like to say thank you. I switched majors over the summer from political science to a stem major and my first quarter was extremely rough; I didn't know how I was going to survive 14 B. Although I still struggled (and d...
- Sat Mar 14, 2020 8:22 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: elementary rate law
- Replies: 4
- Views: 364
Re: elementary rate law
What was most helpful in understanding the mechanism functionality of an overall rate law?
- Sat Mar 14, 2020 8:16 pm
- Forum: Administrative Questions and Class Announcements
- Topic: ENDGAME Review Session
- Replies: 71
- Views: 5724
Re: ENDGAME Review Session
You're great! thank you for posting a comprehensive answer key despite not being able to have the review session!!
Your sessions were very helpful!
Your sessions were very helpful!
- Sat Mar 14, 2020 8:13 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: order of reaction
- Replies: 6
- Views: 592
Re: order of reaction
This is a good video! I watched another of his videos on other general kinetics problems, and it was good. https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=15&cad=rja&uact=8&ved=2ahUKEwiOp82665voAhXMi54KHRqgAZsQtwIwDnoECAkQAQ&url=https%3A%2F%2Fwww.you...
- Sat Mar 14, 2020 8:10 pm
- Forum: Second Order Reactions
- Topic: Graphs
- Replies: 9
- Views: 803
Re: Graphs
This is a good chart for reference.
- Sat Mar 14, 2020 8:07 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Endgame 15b
- Replies: 5
- Views: 417
Re: Endgame 15b
TanveerDhaliwal3G wrote:I think the k' is supposed to signify the combination of the first rate constant and the concentration of A
How did you get that from the problem? Is there a simple way of figuring that out?
- Sat Mar 14, 2020 12:37 pm
- Forum: General Rate Laws
- Topic: Study Resources for Kinetics
- Replies: 1
- Views: 248
Study Resources for Kinetics
Does anyone have a good video/ website that helped them understand kinetics better?
I have been studying kinetics a lot, but seem to need some additional review!
I have been studying kinetics a lot, but seem to need some additional review!
- Wed Mar 11, 2020 12:09 pm
- Forum: First Order Reactions
- Topic: determining Kr
- Replies: 5
- Views: 379
Re: determining Kr
JasonLiu_2J wrote:Also to add on, remember that for a first order reaction, the slope of the graph ln[A] vs time is -k, so you would need to multiply it by negative 1 to find the value of the rate constant.
Why are we able to just multiply the slope by -1 to find the rate constant?
- Mon Mar 09, 2020 6:39 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3680606
Re: Post All Chemistry Jokes Here
Chem jokes
- Sun Mar 01, 2020 10:22 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Nernst Equation
- Replies: 2
- Views: 219
Re: Nernst Equation
the Nernst Equation relates cell potential with concentrations: E = E^naught - (RT/nF)*lnQ E^naught is the cell potential at standard conditions. R = Gas constant T = Temp in Kelvin N = mols of e- F = Faraday's constant Q = Reaction Quotient Another way of explaining n, is by saying it is the numbe...
- Sun Mar 01, 2020 10:16 pm
- Forum: Interesting Applications: Rechargeable Batteries (Cell Phones, Notebooks, Cars), Fuel Cells (Space Shuttle), Photovoltaic Cells (Solar Panels), Electrolysis, Rust
- Topic: Metal dissolution
- Replies: 10
- Views: 1034
Re: Metal dissolution
Is there a way of knowing if the metal will be oxidized? Do certain metals oxidize easier than others? Which metals are least likely to oxidize and dissolve into solution? Refer to the ordered electrochemical series. Metals with half-reactions that appear later in the table (more negative E^0) are ...
- Sun Mar 01, 2020 10:10 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagrams
- Replies: 6
- Views: 489
Re: Cell Diagrams
205007651 wrote:The only order that matters is anode on the left and cathode on the right, and having aqueous next to salt bridge.
Why does it matter to have aqueous next to the salt bridge?
- Sun Mar 01, 2020 10:08 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: How to determine anode and cathode in 6.57?
- Replies: 6
- Views: 458
Re: How to determine anode and cathode in 6.57?
Natalie Benitez 1E wrote:The cathode should be the one with the larger E value.
Why again is the cathode the one with the larger E value?
- Sun Mar 01, 2020 10:03 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Writing cell diagrams
- Replies: 7
- Views: 571
Re: Writing cell diagrams
When writing cell diagrams from a given reaction when do we separate species with a comma? is this due to the species being in the same phase? If this is true would you also separate products and reactants in the same phase with a comma as well? In addition to this, you separate the sides of the re...
- Sun Mar 01, 2020 10:01 pm
- Forum: Balancing Redox Reactions
- Topic: OH and H
- Replies: 8
- Views: 499
Re: OH and H
In a test style problem, would the question say something alongs the lines of "In the acidic rxn..." or must we be able to identify that? Otherwise, if we were to accidentally use OH- when we were supposed to use H+, would a significant number of points be taken off?
- Sun Feb 23, 2020 10:44 pm
- Forum: Student Social/Study Group
- Topic: Sapling Learning
- Replies: 4
- Views: 353
Re: Sapling Learning
When I took chem 14A with Caram, for the most part the Sapling was not that helpful. I do believe Lavelle has some problems he's pulled from there for test/exam questions. However, doing all the textbook problems is your better bet, along with going to step up sessions/ workshops.
- Sun Feb 23, 2020 10:35 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: test 2 material clarification
- Replies: 10
- Views: 681
Re: test 2 material clarification
Test 2 will cover the information on the 2nd page of the Thermodynamics and the electrochemistry outline.
- Sun Feb 23, 2020 10:31 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Spontaneity
- Replies: 8
- Views: 542
Re: Spontaneity
Yes, due to their inverse relationship in the equation 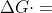 -nFEcell, when the cell potential of the battery is positive, the
-nFEcell, when the cell potential of the battery is positive, the 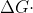
becomes negative
becomes negative
- Sun Feb 23, 2020 10:26 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: cell diagrams
- Replies: 5
- Views: 363
Re: cell diagrams
Is it important to have a detailed cell diagram for your answer/work in your problems, or is supposed to function as more of a basis for your equation?
- Tue Feb 18, 2020 10:14 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3680606
Re: Post All Chemistry Jokes Here
Found on Pinterest
- Tue Feb 18, 2020 10:12 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3680606
Re: Post All Chemistry Jokes Here
Ahh you Noble Gasses
- Tue Feb 18, 2020 10:01 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Activation Energy
- Replies: 16
- Views: 1512
Re: Activation Energy
The activation energy of a reaction is the peak of an energy diagram. Beginning where the products are located to the height of the peak is how much energy is needed to cause the reaction to happen. Some reactions have very low activation energies, and others have high activation energies. It is imp...
- Tue Feb 18, 2020 9:56 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: intermediate
- Replies: 26
- Views: 2084
Re: intermediate
If an intermediate is formed during a reaction, and then used, do non-state functions include those changes and quantities?
- Tue Feb 18, 2020 9:53 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Study Advice
- Replies: 73
- Views: 7205
Re: Study Advice
I end up doing the readings from the book and I make sure to do the example problems as I go along to make sure I have grasp the subject. The practice problems are helpful and the evening step up sessions are helpful if you need extra guidance on certain problems.
- Tue Feb 18, 2020 9:49 pm
- Forum: Van't Hoff Equation
- Topic: Van't Hoff Temperature Dependence
- Replies: 4
- Views: 409
Re: Van't Hoff Temperature Dependence
What equation do you use if you are not sure if the equation has a  equal to 0?
equal to 0?
Is there a way to check that is 0 if it is not explicitly stated in the problem?
is 0 if it is not explicitly stated in the problem?
What happens if you use the equation for a reaction that is not at equilibrium?
Is there a way to check that
What happens if you use the equation for a reaction that is not at equilibrium?
- Tue Feb 18, 2020 9:38 pm
- Forum: Environment, Fossil Fuels, Alternative Fuels
- Topic: Why ethanol?
- Replies: 7
- Views: 1781
Re: Why ethanol?
Ethanol is a byproduct of the fermentation of plants, which makes it readily available. We will always have a fuel source if we are producing plants. Although they still contribute to global warming, our society cannot immediately switch to fuel sources powered by solar, nuclear or wind/water becaus...
- Mon Feb 17, 2020 3:44 pm
- Forum: Balancing Redox Reactions
- Topic: Reduction vs. oxidation
- Replies: 29
- Views: 1212
Re: Reduction vs. oxidation
Reduction and oxidation is how an electron is transferred. The notation taught in LS is OIL RIG. Oxidation is Loss of electrons Reduction is Gain of electrons When something becomes oxidized, the oxidation number becomes more positive. Being reduced means your oxidation number becomes more negative.
- Mon Feb 17, 2020 3:34 pm
- Forum: Interesting Applications: Rechargeable Batteries (Cell Phones, Notebooks, Cars), Fuel Cells (Space Shuttle), Photovoltaic Cells (Solar Panels), Electrolysis, Rust
- Topic: Metal dissolution
- Replies: 10
- Views: 1034
Re: Metal dissolution
Is there a way of knowing if the metal will be oxidized? Do certain metals oxidize easier than others? Which metals are least likely to oxidize and dissolve into solution?
- Mon Feb 17, 2020 3:21 pm
- Forum: Balancing Redox Reactions
- Topic: Oxidation Number Rules
- Replies: 7
- Views: 515
Re: Oxidation Number Rules
Are there any oxidation numbers of specific compounds that would be helpful to know?
- Sun Feb 02, 2020 10:02 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Work done BY vs work done ON
- Replies: 9
- Views: 298
Re: Work done BY vs work done ON
When work is done by a system, the system is affecting the surroundings. ex. exothermic rxn, -
When work is done on a system, the surroundings are affecting the system. ex. endothermic rxn,
When work is done on a system, the surroundings are affecting the system. ex. endothermic rxn,
- Sun Feb 02, 2020 9:55 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Negative Enthalpy
- Replies: 3
- Views: 108
Re: Negative Enthalpy
Are you asking about spontaneous reactions?
- Sun Feb 02, 2020 8:58 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: degeneracy
- Replies: 3
- Views: 145
Re: degeneracy
What can we learn from the degeneracy of a system? when x=2 n=3 W=8, but what does that mean? Is it anything more than knowing that degeneracy is the number of ways of achieving a given state?
- Sun Feb 02, 2020 8:52 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: microstates
- Replies: 3
- Views: 130
Re: microstates
Because the different micro states all have the same energies, is there any instance we would have to use a micro state in an equation and specify the one we are using?
- Sun Feb 02, 2020 8:40 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Affect of temperature on entropy?
- Replies: 5
- Views: 256
Re: Affect of temperature on entropy?
Yes, as temperature increases, entropy increases due to the excited nature of the molecules within a system.
- Sun Feb 02, 2020 8:25 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Enthalpy of Rxn
- Replies: 9
- Views: 435
Re: Enthalpy of Rxn
As you can see here, you will want to multiply the values to cancel out the mol, so you are left with kJ. You are left with 15kJ.
- Sun Feb 02, 2020 8:12 pm
- Forum: Phase Changes & Related Calculations
- Topic: Relevance of Phase Changes
- Replies: 9
- Views: 478
Re: Relevance of Phase Changes
In many of the problems covered in thermo, you have to calculate the energies expelled/absorbed for each part of the phase change, and then add those up to find your enthalpy,  .
.
- Sun Feb 02, 2020 8:07 pm
- Forum: Phase Changes & Related Calculations
- Topic: Reversible and Irreversible
- Replies: 5
- Views: 255
Re: Reversible and Irreversible
Would irreversible reactions occur in open containers then? If the pressure is constant is it always a irreversible reaction? In open containers I believe you would use the irreversible equation since you are losing energy in the form of heat to the surroundings. According to the first comment, tha...
- Sun Feb 02, 2020 7:48 pm
- Forum: Phase Changes & Related Calculations
- Topic: phase change from liquid to vapor
- Replies: 8
- Views: 370
Re: phase change from liquid to vapor
Visible on the heating curve that we looked at in class, there is a significant amount of energy stored in vaporized water. When this vapor comes into contact with human skin (which is significantly cooler than the vapor) the vapor releases vast amounts of energy to return to its liquid state, which...
- Mon Jan 27, 2020 10:46 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: "Breaking bonds is always endothermic"
- Replies: 6
- Views: 1005
Re: "Breaking bonds is always endothermic"
In LS we learned that there is an input of energy to overcome the energy barrier, and that energy was released. But the energy put in or released depends on the type of bond that is broken. Determining enthalpy depends on the difference of energy between input to break and output to form these bonds.
- Mon Jan 27, 2020 10:35 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Signs for enthalpy
- Replies: 8
- Views: 518
Re: Signs for enthalpy
When a reaction has a negative enthalpy, it is considered ectothermic because it is releasing heat. You can think of how ecto sounds slightly like exit, and the negative sign as subtracting heat from a system and sending it into its surrounding environment. For endothermic, think of endo like enter....
- Mon Jan 20, 2020 11:51 am
- Forum: Ideal Gases
- Topic: Partial Pressures
- Replies: 4
- Views: 200
Re: Partial Pressures
In a chemical reaction, if one reactant is increased in pressure, the other will decrease to try and balance it, but ultimately the concentration then becomes higher and the equilibrium shifts to the products where pressure increases.
- Mon Jan 20, 2020 11:34 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Adding Inert Gas
- Replies: 9
- Views: 586
Re: Adding Inert Gas
Adding an inert gas, a noble gas, has no change on the chemical system.
- Mon Jan 20, 2020 11:32 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Le Chatelier Principle
- Replies: 4
- Views: 264
Re: Le Chatelier Principle
When considering temperature, pressure, and concentration, it is important to note that pressure and concentration both only change where equilibrium lies on the equilibrium constant. If you were to increase pressure or concentration in one part of the equation, pressure or concentration would decre...
- Mon Jan 20, 2020 11:18 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Weak acids & bases
- Replies: 7
- Views: 356
Re: Weak acids & bases
then compare your % to 5%.
- Mon Jan 20, 2020 11:12 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5% rule
- Replies: 3
- Views: 124
Re: 5% rule
The coefficients are taken into account when calculating the concentration of the reactants and the products. Once you have done that, I don't think you need to consider the coefficients again. For example, if you have calculated that there was x amount of product at equilibrium and y amount initia...
- Sun Jan 12, 2020 10:03 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Concentration
- Replies: 5
- Views: 150
Re: Concentration
A change in concentration does not cause Kc to change because equilibrium adjusts until the standard Kc rate is met. However, an increase in temperature will cause Kc to shift towards the reactants and vice versa.
- Sun Jan 12, 2020 9:44 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Situations in which Q=K
- Replies: 7
- Views: 373
Re: Situations in which Q=K
When Q=K the reaction is at equilibrium meaning that the forward and reverse reactions are happening at the same rate. This does not mean that they are not occurring, but rather [R] and [P] are being formed at the same time, and neither is being formed more than the other.
- Sun Jan 12, 2020 9:31 pm
- Forum: Ideal Gases
- Topic: PV = nRT
- Replies: 16
- Views: 1986
Re: PV = nRT
P- Pressure
V- Volume
n- Amount in moles
R- The ideal gas constant (depends on the units you are using)
T- Temperature
P and V are inversely related. Assuming a constant temperature, when pressure increases volume decreases and so forth.
V- Volume
n- Amount in moles
R- The ideal gas constant (depends on the units you are using)
T- Temperature
P and V are inversely related. Assuming a constant temperature, when pressure increases volume decreases and so forth.
- Sun Jan 12, 2020 12:17 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Q and relation of [R] to [P]
- Replies: 5
- Views: 383
Re: Q and relation of [R] to [P]
For this problem we are only looking at the change in the P or R values of the Q equation, using K as a fixed ratio. Your K value is the fixed ratio between the left and right sides when at equilibrium. While Q is also a ratio between the [R] and [P] but the ratio might not be at equilibrium. This m...
- Sat Jan 11, 2020 10:55 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Le Chatelier's Principle
- Replies: 19
- Views: 1726
Re: Le Chatelier's Principle
Is it appropriate to consider Le Chatelier's Principle as a ratio?
I.e. determining the change of one value would aid in determining the other values and thus, the equilibrium?
I.e. determining the change of one value would aid in determining the other values and thus, the equilibrium?

