Search found 83 matches
- Tue Mar 10, 2020 8:25 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Identifying Catalysts and intermediates
- Replies: 4
- Views: 347
Re: Identifying Catalysts and intermediates
Intermediates are formed as products in one elementary reaction and immediately used in the following elementary reaction. They can be cancelled out from 2 successive reactions. Catalysts are used for a reaction (kind of like a reactant) but are not consumed in the following reaction as a reactant.
- Tue Mar 10, 2020 8:12 pm
- Forum: Second Order Reactions
- Topic: 7B.13 Numerator
- Replies: 2
- Views: 258
Re: 7B.13 Numerator
This is because, for example, part a is asking for 1/16 of the original concentration. In the solution manual, they are setting [A]=(1/16)[A]0. Plugging this value into the 2nd order linear equation, you get 1/([A]0/16)=kt+(1/[A]0). The left side of this equation written in simpler terms is 16/[A]0....
- Tue Mar 10, 2020 8:08 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: 7A.17
- Replies: 2
- Views: 267
Re: 7A.17
The book converts mmol to mol and work with these units throughout the entire problem. If you convert to mol from the beginning and work with these values throughout, you should get the same answer.
- Tue Mar 10, 2020 8:04 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: 7D.5
- Replies: 3
- Views: 317
Re: 7D.5
Im pretty sure the book meant an equal sign instead of a subtraction sign. If you carry out the right side of the equation and neglect the -0.59, you actually get 0.59 as the answer. You use the 0.59 for the rest of the problem.
- Tue Mar 10, 2020 8:04 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: 7D.5
- Replies: 3
- Views: 317
Re: 7D.5
Im pretty sure the book meant an equal sign instead of a subtraction sign. If you carry out the right side of the equation and neglect the -0.59, you actually get 0.59 as the answer. You use the 0.59 for the rest of the problem.
- Tue Mar 10, 2020 7:59 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: 7D.7
- Replies: 2
- Views: 248
Re: 7D.7
For this question, you should look at the activation energies. It helps to draw it out. If it takes 39.7kj/mol for the forward reaction and only takes 25.4kj/mol for the reverse reaction, you can determine that the end of the forward reaction has higher energy than the start of the forward reaction,...
- Tue Mar 10, 2020 7:54 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: 7C.1
- Replies: 1
- Views: 161
Re: 7C.1
yes, and part b is rate=k[Cl2] and unimolecular because there is only 1 molecule reacting in the reactant side
- Sat Mar 07, 2020 3:26 pm
- Forum: General Rate Laws
- Topic: 7.23b
- Replies: 1
- Views: 216
7.23b
When do you know if you have to change a rate law into a different for using other elementary reactions? I dont understand why they changed it here. Their goal was to express it in terms of reactants and products of the overall reaction but OH- isn't in the overall reaction
- Tue Mar 03, 2020 11:05 pm
- Forum: Balancing Redox Reactions
- Topic: Balancing Redox reactions
- Replies: 4
- Views: 477
Re: Balancing Redox reactions
First balance the Cr by making the Cr on the right have a 3 as a coefficient. Then balance the oxygen by adding 7 H2O to the right. Then balance the Hydrogen by adding 14 H+ to the left. Then balance the charge by adding 6 electrons to the left. The book recommends balancing the main element first t...
- Tue Mar 03, 2020 5:39 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagrams
- Replies: 3
- Views: 258
Re: Cell Diagrams
The cathode is always written on the right side and the anode is always written on the left. And does the flow of electrons go from the anode to the cathode? Yes the flow of electrons goes from anode to cathode. Anode is where oxidation takes place (loss of electrons). Cathode is where reduction ta...
- Tue Mar 03, 2020 5:38 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6N3 a) Concentrations?
- Replies: 1
- Views: 200
Re: 6N3 a) Concentrations?
You would look at the half reactions. At the anode, it is H2-->2H+ +2e-. At the cathode, it is 2H+ +2e- -->H2. Q is always product over reactant. So you take the products of both equations and multiply those values as the numerator. Then you take the reactants of both equations and multiply those va...
- Tue Mar 03, 2020 5:33 pm
- Forum: Balancing Redox Reactions
- Topic: Problem 6K.3
- Replies: 1
- Views: 205
Re: Problem 6K.3
yes, it should be Cl-
- Tue Mar 03, 2020 5:32 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: delta G=-nFE
- Replies: 1
- Views: 157
Re: delta G=-nFE
Look at the half reactions for full reaction. See how many electrons are transferred in this reaction. If reduction reaction has 2 electrons gained but oxidation reaction has 3 electrons lost, n=6 (because you have to balance electrons when you combine them). If both reduction and oxidation reaction...
- Tue Mar 03, 2020 5:29 pm
- Forum: Van't Hoff Equation
- Topic: 5J.15
- Replies: 1
- Views: 261
Re: 5J.15
Because G=-RTlnK, you can rearrange this equation to solve for K so K=e^(-G/RT). For the 25 degree part, find G of the reaction by taking the standard Gibbs energy of products minus that of reactants. After finding G of reaction, plug that into the equation solving for K where R is ideal gas constan...
- Tue Mar 03, 2020 5:25 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagrams
- Replies: 3
- Views: 258
Re: Cell Diagrams
The cathode is always written on the right side and the anode is always written on the left.
- Tue Mar 03, 2020 5:23 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: G=-nFE equation [ENDORSED]
- Replies: 4
- Views: 474
G=-nFE equation [ENDORSED]
does the equation G=-nFE still apply to nonstandard values? It only specifies the standard values on the equation sheet.
- Sun Mar 01, 2020 3:38 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: cell diagram order
- Replies: 3
- Views: 241
cell diagram order
Should the order in the cell diagrams be "product|reactant||product|reactant" or should it be "s|g|aq||aq|g|s" ?
- Sun Mar 01, 2020 3:33 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: precipitation reactions
- Replies: 3
- Views: 304
precipitation reactions
How can you tell when a reaction is a precipitation reaction by looking at the equation?
- Sun Mar 01, 2020 2:58 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6L.5b
- Replies: 1
- Views: 181
6L.5b
For part b, Why is there a Pt(s) on the left/anode side of this cell diagram? I thought this was only used when there was no solid but there is an Iodine solid on this side. Is this because Iodine is not a metal?
- Sat Feb 29, 2020 3:31 pm
- Forum: Interesting Applications: Rechargeable Batteries (Cell Phones, Notebooks, Cars), Fuel Cells (Space Shuttle), Photovoltaic Cells (Solar Panels), Electrolysis, Rust
- Topic: 6O.3a
- Replies: 1
- Views: 170
6O.3a
For this question, if I choose the water reaction to occur at the cathode and the Mn2+ reaction to happen at the anode, wouldn't this yield a positive Ecell value? Since Ecell=-0.42V-(-1.18V)=+0.76V. But we are dealing with electrolytic cells which are supposed to be negative Ecell right? How does t...
- Sat Feb 29, 2020 2:32 pm
- Forum: Interesting Applications: Rechargeable Batteries (Cell Phones, Notebooks, Cars), Fuel Cells (Space Shuttle), Photovoltaic Cells (Solar Panels), Electrolysis, Rust
- Topic: 6O.1
- Replies: 3
- Views: 313
6O.1
In the solution manual, it says to choose the reduction reaction with most positive standard reduction potential for the cathode and choose reduction reaction with most negative standard reduction potential for the anode. But they choose Ni2+ reaction for cathode which has E=-0.23V and choose H2O re...
- Sat Feb 29, 2020 1:53 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: E potentials
- Replies: 5
- Views: 443
E potentials
Will E potentials always be given as reduction potentials, even on tests? Or could it possibly be given as an oxidation potential? Some sources online use oxidation potentials instead. Is this something I should carefully pay attention to on tests?
- Wed Feb 26, 2020 11:32 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 6N.9
- Replies: 1
- Views: 241
6N.9
A tin electrode in 0.015 M Sn(NO3)2(aq) is connected to a hydrogen electrode in which the pressure of H2 is 1.0 bar. If the cell potential is 0.061 V at 25 °C, what is the pH of the electrolyte at the hydrogen electrode? How do we determine which is the cathode and which is the anode? Is there a gen...
- Wed Feb 26, 2020 12:09 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6L.3(b)
- Replies: 1
- Views: 198
Re: 6L.3(b)
The reaction on the right is always going to be a reduction reaction and the reaction on the left is always going to be an oxidation reaction. For the question, you would flip the right side to get the equation Cl2+2e- ---> 2Cl- which is a reduction reaction.
- Wed Feb 26, 2020 12:05 am
- Forum: Balancing Redox Reactions
- Topic: Acidic vs basic solution
- Replies: 2
- Views: 186
Re: Acidic vs basic solution
The question will usually specify which one it is. I have seen some questions where they will tell you the pH too and you can determine yourself how to balance the equation based on the pH.
- Mon Feb 24, 2020 9:41 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Galvanic Cells
- Replies: 2
- Views: 203
Re: Galvanic Cells
Yes, galvanic cells always have a positive cell potential.
- Mon Feb 24, 2020 9:40 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Anode/ Cathode
- Replies: 3
- Views: 218
Re: Anode/ Cathode
Notice that they use the word oxidizing "agent" and reducing "agent." You are correct in your statement. The oxidizing agent is actually being reduced and the reducing agent is actually being oxidized. So the oxidizing agent is the one taking the electron from the one being oxidi...
- Mon Feb 24, 2020 9:36 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: nernst equation
- Replies: 3
- Views: 279
Re: nernst equation
It relates the potential of an electrochemical cell to concentrations in the cell reaction. It also is useful because it can be used for nonstandard conditions too.
- Mon Feb 24, 2020 9:31 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 6N.1b
- Replies: 1
- Views: 145
6N.1b
In the answer key they say that the reduction half reaction involves two electrons transferred and therefore n would be equal to 2. However, shouldn't the reaction only be involving 1 electron transfer since it is going from In3+ to In2+ which would also make lnK=4.67 and K=107?
- Sun Feb 23, 2020 6:24 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6M.5a Cell diagram
- Replies: 1
- Views: 102
6M.5a Cell diagram
for part a, the answer key doesn't put a Pt(s) on the left side of the cell but it does on the right. Why does the left side not need it?
- Sun Feb 23, 2020 5:37 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6M.1
- Replies: 4
- Views: 362
6M.1
A student was given a standard Cu(s)|Cu2+(aq)Cu(s)|Cu2+(aq) half-cell and another half-cell containing an unknown metal M in 1.00 M (NO3)2(aq) and formed the cell M(s)|M+(aq)||Cu2+(aq)|Cu(s). The cell potential was found to be −0.689 V. What is the value of E°(M2+/M)? Can someone explain this one to...
- Sun Feb 23, 2020 4:40 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: chemistry community posts
- Replies: 12
- Views: 661
chemistry community posts
what days make up 1 week of chemistry community posts? Is it corresponding to the week of the quarter?
- Sun Feb 23, 2020 4:38 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Electrochemical series diagram
- Replies: 2
- Views: 198
Electrochemical series diagram
In figure 6M.5, it uses the terms "can reduce H+" for strongly reducing and "cannot reduce H+" for strongly oxidizing. What does it mean to reduce H+?
- Wed Feb 19, 2020 8:42 pm
- Forum: Balancing Redox Reactions
- Topic: balancing redox reactions where the oxidizing agent and reducing agent are the same
- Replies: 2
- Views: 787
Re: balancing redox reactions where the oxidizing agent and reducing agent are the same
In these cases, you would have two separate half-reactions with the same reactant for both the oxidation half-reaction and reduction half-reaction. In the case of Br2 that you gave, the oxidation half-reaction would be Br_{2} (l)+12OH^{-}(aq)\rightarrow 2BrO_{3}^{-}(aq)+6H_{...
- Wed Feb 19, 2020 8:35 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6L.5b cell diagrams
- Replies: 1
- Views: 92
6L.5b cell diagrams
With the information we are given, how do we know when to put the Pt(s) as the outermost component of each electrode? The answer key puts Pt(s) in the cell diagrams for part b and c but not a or d. But this is never given in any of the equations.
- Wed Feb 19, 2020 4:56 pm
- Forum: Balancing Redox Reactions
- Topic: balancing redox reactions where the oxidizing agent and reducing agent are the same
- Replies: 2
- Views: 787
balancing redox reactions where the oxidizing agent and reducing agent are the same
I'm having trouble balancing redox reactions where the oxidizing agent and reducing agent are the same. Is there a different process for these types of reactions? For example, Br2(l)→BrO3−(aq)+Br−(aq) has Br2 as both reducing and oxidizing agents. Also P4(s)→H2PO2−(aq)+PH3(aq) has P4 as both reducin...
- Wed Feb 19, 2020 1:03 am
- Forum: Balancing Redox Reactions
- Topic: 6K.3d
- Replies: 1
- Views: 668
6K.3d
Balance each of the following skeletal equations by using oxidation and reduction half-reactions. All the reactions take place in acidic solution. Identify the oxidizing agent and reducing agent in each reaction. (d) Reaction of chlorine in water: Cl2(g)→HClO(aq)+Cl2(g) I understand how to get the o...
- Mon Feb 17, 2020 10:07 pm
- Forum: Van't Hoff Equation
- Topic: 5.55b [ENDORSED]
- Replies: 1
- Views: 462
5.55b [ENDORSED]
A reaction used in the production of gaseous fuels from coal, which is mainly carbon, is C(s)+H2O(g)⇌CO(g)+H2(g) (a) Evaluate K At 900 K, given that the standard Gibbs free energies of formation of CO(g) and H2O(g)at 900 K are −191.28kJ⋅mol−and −198.08kJ⋅mol, respectively. (b) A sample of graphite o...
- Thu Feb 13, 2020 5:00 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Delta S
- Replies: 8
- Views: 671
Re: Delta S
Delta S is the change in the entropy of the system. Delta S (surroundings) is the change in entropy of the surroundings. Delta S (total) is Delta S plus Delta S (Surroundings). In a reversible reaction, delta S (surr) is equal to negative Delta S. In an irreversible reaction, Delta S(surr)=0.
- Thu Feb 13, 2020 4:58 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: enthalpy and entropy
- Replies: 4
- Views: 326
Re: enthalpy and entropy
Rearranging the equation for Gibbs free energy (G=H-TS), we can get TS=H-G. Using this equation, we can see that entropy (S) change can be positive if the change in Gibbs free energy (G) is more negative than H. If G is more negative than H, we are adding on a value that is greater than H, therefore...
- Thu Feb 13, 2020 4:53 pm
- Forum: Phase Changes & Related Calculations
- Topic: change in entropy
- Replies: 7
- Views: 684
Re: change in entropy
In general, if the change in anything is less than 0, it indicates a decrease in this property. It's the same concept as for change in enthalpy, Gibbs free energy, temperature, etc.
- Thu Feb 13, 2020 4:49 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Entropy Changes
- Replies: 3
- Views: 280
Re: Entropy Changes
Also remember that entropy increases with the complexity of a molecule and also increasing molar mass. It also increases from solid to gas phases.
- Thu Feb 13, 2020 4:46 pm
- Forum: Calculating Work of Expansion
- Topic: Work
- Replies: 5
- Views: 318
Re: Work
delta S equations are the same for both reversible and irreversible. The only difference is that for the irreversible one, S(surr)=0 which makes S(tot)=S(sys) while in the reversible one, S(sys)=-S(surr) which means S(tot)=0
- Sun Feb 09, 2020 10:09 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: 4A3.c
- Replies: 1
- Views: 111
4A3.c
Air in a bicycle pump is compressed by pushing in the handle. The inner diameter of the pump is 3.0 cm and the pump is depressed 20. cm with a pressure of 2.00 atm. (c) What is the change in internal energy of the system? How would you calculate the change in internal energy if you only know the cha...
- Sat Feb 08, 2020 9:55 am
- Forum: Calculating Work of Expansion
- Topic: Midterm equation sheet
- Replies: 16
- Views: 732
Midterm equation sheet
Will the same exact equation/constant sheet on the course website also be given to us to use on the midterm?
- Thu Feb 06, 2020 12:31 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Textbook question 4B.9
- Replies: 4
- Views: 187
Re: Textbook question 4B.9
in an adiabatic process, energy can only be transferred as work, not heat. a) Because deltaU=q+w, and we know q=0 (because no heat is transferred), deltaU can only be 0 if w is also 0. d) Using similar reasoning, deltaU can only equal q if the other component of the equation (w) is set to 0, giving ...
- Thu Feb 06, 2020 12:21 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Textbook question 4B.3
- Replies: 3
- Views: 132
Re: Textbook question 4B.3
I believe I asked a question on this earlier. I also got 490J and someone who replied to my question also got the same answer. I think the textbook's answer is wrong and it should be 490J.
- Thu Feb 06, 2020 12:18 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: calculating entropy of irreversible and reversible expansion reactions
- Replies: 1
- Views: 129
calculating entropy of irreversible and reversible expansion reactions
I know for calculating work, there are different equations used for reversible vs irreversible reactions. What are the different equations used to calculate entropy of reversible and irreversible reactions?
- Thu Feb 06, 2020 12:16 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: 4F.17
- Replies: 1
- Views: 229
4F.17
Calculate the standard entropy of vaporization of ammonia at 210.0 K, given that the molar heat capacities at constant pressure of liquid ammonia and ammonia vapor are 80.8J⋅K -1 ⋅mol -1 and 35.1J⋅K -1 ⋅mol -1 , respectively, in this range (see Table 4C.1). Can anyone give me guidance on how to star...
- Thu Feb 06, 2020 11:59 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: micro states
- Replies: 1
- Views: 82
micro states
I'm confused on how to determine the number of microstates as you get to more complicated molecules (such as ones that take on octahedral shape)
- Wed Feb 05, 2020 6:38 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Temperature effect on Entropy
- Replies: 1
- Views: 180
Temperature effect on Entropy
Using the equation 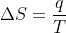 , entropy would decrease as temperature increases. Why is this? Wouldn't you expect entropy to increase as temperature increases because the molecules are in a more disorderly state as temperature increases?
, entropy would decrease as temperature increases. Why is this? Wouldn't you expect entropy to increase as temperature increases because the molecules are in a more disorderly state as temperature increases?
- Fri Jan 31, 2020 9:05 pm
- Forum: Calculating Work of Expansion
- Topic: 4.7b
- Replies: 1
- Views: 120
4.7b
(a) Calculate the work that must be done against the atmosphere for the expansion of the gaseous products in the combustion of 1.00mol C6H6 (l) at 25°C and 1.00 bar. (b) Using data in Appendix 2A, calculate the standard enthalpy of the reaction. (c) Calculate the change in internal energy, ΔU, of th...
- Thu Jan 30, 2020 10:28 pm
- Forum: Calculating Work of Expansion
- Topic: 4.7
- Replies: 1
- Views: 129
4.7
(a) Calculate the work that must be done against the atmosphere for the expansion of the gaseous products in the combustion of 1.00 mol C6H6(l) at 25°C and 1.00 bar.
I'm not really sure which work equation to use for this question.
I'm not really sure which work equation to use for this question.
- Wed Jan 29, 2020 11:20 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: 4b.3b
- Replies: 3
- Views: 167
4b.3b
The internal energy of a system increased by 982 J when it was supplied with 492 J of energy as heat. (b) How much work was done? Wouldn't you just calculate the work by taking the total energy of the system and subtracting the energy from heat (since internal energy is equal to heat plus work)? Whe...
- Wed Jan 29, 2020 11:15 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: 4A.7
- Replies: 3
- Views: 120
Re: 4A.7
Yes, you set up 2 equations: one for changing the temperature of copper and one for changing the temperature of water. Solve for heat and add these two together to get the total heat required.
- Wed Jan 29, 2020 11:13 pm
- Forum: Calculating Work of Expansion
- Topic: 4A.3
- Replies: 2
- Views: 144
Re: 4A.3
Pa x m^3 is equal to one Joule. You have to convert the atm to Pascals to make the units equivalent to a Joule. 1 atm=101325 Pascals.
- Wed Jan 29, 2020 11:12 pm
- Forum: Phase Changes & Related Calculations
- Topic: 4B.1
- Replies: 6
- Views: 242
Re: 4B.1
It is positive because the force exerted is in the same direction as the displacement. And when work is done on the system, it is positive.
- Mon Jan 27, 2020 11:22 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 4E.5c
- Replies: 1
- Views: 84
4E.5c
Why wouldn't the answer for this question be 0? The same bonds that are being broken on the reactants side as the bonds that are being formed on the products side.
- Mon Jan 27, 2020 8:57 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 4C.3
- Replies: 4
- Views: 214
4C.3
This question has 2 parts but the answer key only gives one answer. What did you guys get for these parts?
- Mon Jan 27, 2020 8:53 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 4D.21c
- Replies: 1
- Views: 146
4D.21c
Use standard enthalpies of formation from Appendix 2A to calculate the standard reaction enthalpy for: (c) the formation of a sulfide by the action of hydrogen sulfide on an aqueous solution of a base: H2S(aq)+2KOH(aq)→K2S(aq)+2H2O(l). I calculated this by taking sums of standard enthalpies of forma...
- Mon Jan 27, 2020 8:42 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 4C.13
- Replies: 3
- Views: 129
4C.13
An ice cube of mass 50.0 g at 0.0°C is added to a glass containing 400.0 g of water at 45.0°C. What is the final temperature of the system (see Tables 4A.2 and 4C.1)? Assume that no heat is lost to the surroundings. I went about this problem by using the equation q=mCdeltaT and setting two of these ...
- Mon Jan 27, 2020 8:29 pm
- Forum: Phase Changes & Related Calculations
- Topic: #4C11
- Replies: 2
- Views: 135
Re: #4C11
This is the standard enthalpy of fusion for water from table 4C.1
- Wed Jan 22, 2020 7:19 pm
- Forum: Phase Changes & Related Calculations
- Topic: Thermochemistry homework problems
- Replies: 2
- Views: 79
Thermochemistry homework problems
Since we are starting this unit in the middle of the readings in the book, what sections should we do homework on this week?
- Wed Jan 22, 2020 7:16 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: HW 5I.35
- Replies: 2
- Views: 101
Re: HW 5I.35
If you make an ice table, the initial value for NO is 1.0 bar. The change would be -2x because the mole ratio. And the equilibrium value of NO would therefore be 1.0-2x. In this case, we are told p is the equilibrium value of N2. Since N2 starts with 0 concentration, p would be equal to x. So we can...
- Wed Jan 22, 2020 7:12 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6B.9
- Replies: 1
- Views: 83
Re: 6B.9
The answer key is wrong. The calculated pH should be negative.
- Wed Jan 22, 2020 7:10 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6A.21
- Replies: 1
- Views: 149
Re: 6A.21
Yes for both a and b, the molar concentrations are 1.4 x 10^-7 M
- Mon Jan 20, 2020 4:32 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5J.5
- Replies: 1
- Views: 72
5J.5
State whether reactants or products will be favored by an increase in the total pressure (resulting from compression) on each of the following equilibria. If there is no change, explain why that is so. (d) 2HD(g)+H2(g)⇌D2(g) Why is the answer no change? The reactants have more moles of gas so I woul...
- Mon Jan 20, 2020 3:06 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5I.25
- Replies: 2
- Views: 101
5I.25
Is there a simpler way to solve this without using the quadratic equation? The problem gets messy when using quadratic.
- Sun Jan 19, 2020 3:31 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6D.13
- Replies: 1
- Views: 83
6D.13
Just to check my answers, did someone record their calculated pH's from this question? if so, what were they?
- Wed Jan 15, 2020 2:33 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6B.9a
- Replies: 1
- Views: 86
6B.9a
For the first line, when I type in -log(1.50) to try to get the pH, I keep getting -0.176 but the answer is supposed to be positive 0.176. Also, when I multiply the OH- and H3O+ concentrations in the answer key, I don't get 1.0 x 10^-14. Is this an error in the book?
- Tue Jan 14, 2020 10:58 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Equilibrium Constant
- Replies: 4
- Views: 133
Re: Equilibrium Constant
solids and liquids are always excluded. Aqueous solutions are included though.
- Tue Jan 14, 2020 2:37 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6B.5 C
- Replies: 2
- Views: 151
Re: 6B.5 C
for every one mole of Ba(OH)2, there is 2 moles of OH- produced. Remember to take the concentration of Ba(OH)2 and multiply by 2 before taking the -log.
- Mon Jan 13, 2020 10:00 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: 5J.5
- Replies: 3
- Views: 148
Re: 5J.5
Yes, the composition will want to change in the direction that minimizes the result of increasing pressure and restores equilibrium. There will be less pressure on the side of the reaction with less moles so the composition shifts towards that side.
- Mon Jan 13, 2020 9:54 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: K vs Kc
- Replies: 2
- Views: 88
Re: K vs Kc
I use the Kc for the concentration and treat K as I would for Kp.
- Mon Jan 13, 2020 9:53 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6B.5d
- Replies: 2
- Views: 93
6B.5d
Calculate the pH and pOH of each of the following aqueous solutions of a strong acid or base:
(d) 2.00 mL of 0.175M KOH(aq) after dilution to 0.500 L
I'm not really sure how to approach this problem.
(d) 2.00 mL of 0.175M KOH(aq) after dilution to 0.500 L
I'm not really sure how to approach this problem.
- Sun Jan 12, 2020 2:07 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: 5J:5d
- Replies: 2
- Views: 112
5J:5d
State whether reactants or products will be favored by an increase in the total pressure (resulting from compression) on each of the following equilibria. If there is no change, explain why that is so. d. 2HD(g)+H2(g)⇌D2(g) The answer is no change. I would expect the products to be favored since the...
- Tue Jan 07, 2020 5:04 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5I.11
- Replies: 3
- Views: 145
Re: 5I.11
convert from millimoles to moles (1000 mmol = 1 mol ). Also remember to convert the value so it corresponds to 1 Liter instead of the 0.5L.
- Tue Jan 07, 2020 5:02 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5I.13
- Replies: 2
- Views: 147
5I.13
I'm not really sure how to approach this question. I used a different method than the book's answer key but still got the right answer. Can anyone explain the book's method? Also how can we use these answers to determine which is more thermodynamically stable?
- Tue Jan 07, 2020 4:52 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5G.1(d)
- Replies: 3
- Views: 208
Re: 5G.1(d)
If one increases, the other has to increase with it to maintain the constant K. If products were to increase and the reactants stayed the same, K would increase. But it has to be constant.
- Tue Jan 07, 2020 4:49 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kc vs K
- Replies: 6
- Views: 260
Re: Kc vs K
In the book, they seem to use K for pressure. While the "c" in Kc stands for concentration. So it is used when we use concentration as opposed to pressure to calculate K.
- Tue Jan 07, 2020 4:48 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Q vs K
- Replies: 13
- Views: 496
Re: Q vs K
Q is calculated the same as K. However, Q is not necessarily at equilibrium while K is.
- Tue Jan 07, 2020 4:43 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: How to interpret reactions based on quotient in relation to equilibrium constant
- Replies: 5
- Views: 214
Re: How to interpret reactions based on quotient in relation to equilibrium constant
Q is equal to the products raised to their coefficients divided by the reactant raised to their coefficients. It is the reaction quotient (not necessarily at equilibrium). K is the constant at equilibrium. So at equilibrium, K=Q. If Q is smaller than K, that means that there are too many reactants i...
- Mon Jan 06, 2020 10:31 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Textbook question 5G.9
- Replies: 2
- Views: 124
Re: Textbook question 5G.9
It would be different because the equilibrium constant is equal to (PO2)^3/(PO3)^2 , not PO2/PO3. The statement in part C doesn't take into account the coefficients of the reaction.

