Search found 49 matches
- Sat Mar 14, 2020 6:56 pm
- Forum: *Nucleophiles
- Topic: FInal
- Replies: 11
- Views: 1768
Re: FInal
If you are able to do the in text problems then you should be good. He often wouldn't cover certain topics that we ended up needing to know in lecture but everything you need to know is in the text book.
- Sat Mar 14, 2020 6:54 pm
- Forum: Student Social/Study Group
- Topic: Final Jitters
- Replies: 457
- Views: 424008
Re: Final Jitters
Treat it like the SAT. Every question is weighted roughly the same so do a once over where you answer everything you know off the top of your head. Then go back over and spend some time with the ones you skipped. Then go over it one last time to check that you did them all correctly and didn't make ...
- Sat Mar 14, 2020 6:08 pm
- Forum: General Science Questions
- Topic: Who makes the Final
- Replies: 23
- Views: 1535
Re: Who makes the Final
Jose Robles 1D wrote:Wait is the whole test going to be on CCLE now right?
I believe so yes
- Sat Mar 14, 2020 6:01 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: determining a catalyst
- Replies: 5
- Views: 378
Re: determining a catalyst
The catalyst is not created by the reaction but is also not present in the final balance equation because it gets canceled out. An intermediate is essentially the same except it is created by the reaction and is then used up and canceled out by the next mechanism. So it too is not in the final balan...
- Sat Mar 14, 2020 5:58 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: A- frequency factor
- Replies: 3
- Views: 330
A- frequency factor
What are the units for A? Also is it a numerical value? All I really know is that it relates to the frequency and orientation of collisions
- Sat Mar 14, 2020 5:57 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: arrhenius equation
- Replies: 4
- Views: 353
Re: arrhenius equation
A is the frequency factor so it includes the frequency and orientation of collisions
- Sat Mar 14, 2020 5:54 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Catalysts on Final
- Replies: 7
- Views: 574
Re: Catalysts on Final
probably when given some sort of mechanism you would need to be able to tell the difference between a catalyst and an intermediate. So the catalyst is not created but the intermediate is created and then is then used.
- Sat Mar 14, 2020 5:53 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Arrhenius equation
- Replies: 2
- Views: 261
Arrhenius equation
I've seen a version of the Arrhenius equation that looks like: =-E_{A}/R(1/T_{2}-1/T_{1})) . I'm guessing it's used for finding one temp or the other but wouldn't that mean you would have to be given both ks?
. I'm guessing it's used for finding one temp or the other but wouldn't that mean you would have to be given both ks?
- Sat Mar 14, 2020 4:01 pm
- Forum: General Rate Laws
- Topic: integrals and derivatives
- Replies: 7
- Views: 626
integrals and derivatives
Will have to actually do integrals and derivatives or was that just used as showing us conceptual why the equation works?
- Sat Mar 14, 2020 3:58 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3908236
Re: Post All Chemistry Jokes Here
How everyone is about to be with spring quarter online classes
- Sat Mar 14, 2020 8:21 am
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3908236
Re: Post All Chemistry Jokes Here
we're all gonna be this cool after we finish this final
- Fri Mar 13, 2020 5:32 pm
- Forum: Student Social/Study Group
- Topic: Tips for Staying Focused
- Replies: 64
- Views: 4673
Re: Tips for Staying Focused
I've been putting my phone on do not disturb and setting a timer for an hour at a time and so for that I hour I sit and study and then when the timer goes off I give myself a 10 minute break and then I restart the timer for another hour. You just have to get into a groove. The hardest part is starti...
- Thu Mar 12, 2020 12:03 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: translational and rotational contribution from molecular motion
- Replies: 3
- Views: 331
translational and rotational contribution from molecular motion
Will translational and rotational contribution from molecular motion be on the final? I don't ever remember covering it in class but it is in some of the 4C homework problems
- Thu Mar 12, 2020 11:20 am
- Forum: Administrative Questions and Class Announcements
- Topic: Take Home Final
- Replies: 16
- Views: 1067
Re: Take Home Final
I think he said he is going to send a more detailed email about logistics later this week
- Thu Mar 12, 2020 8:20 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Lavelle's review slides
- Replies: 3
- Views: 288
Lavelle's review slides
On the last slide of Lavelle's review it asks to choose which students' mechanism is valid based on the observed rate law. I'm not sure where the K1,k1, and k2 are coming from and how they are used to determine the rate limiting step. If someone doesn't mind walking me through that whole problem, it...
- Thu Mar 12, 2020 7:57 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Lavelle's review slides
- Replies: 3
- Views: 368
Lavelle's review slides
In Lavelle's review slides there is a problem asking what the problem is with the cell diagram and the correction is the addition of Pt(s) to the right side. Is this because the right side only had Br(aq) and Br(g) and there needs to be a solid conductor in a galvanic cell?
- Thu Mar 12, 2020 7:51 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Lavelle's review slides
- Replies: 1
- Views: 235
Lavelle's review slides
In Lavelle's review there is a problem talking about calculating the Gibbs free energy under non equilibrium condition and in the end \Delta G is positive. The slide says that because it is positive the reaction is spontaneous. I thought in order for a reaction to be spontaneous \Delta G had to be n...
- Wed Mar 11, 2020 2:54 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: text problem 4A.9
- Replies: 2
- Views: 332
text problem 4A.9
What equation is the solution manual using to solve what the final temp of 50.7 g of water at 22C is after adding 20g of copper at 100C? assuming no energy is lost to surroundings
- Wed Mar 11, 2020 1:59 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: test problem 4A.5 continued
- Replies: 3
- Views: 401
test problem 4A.5 continued
Why does this problem use 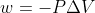 for an irreversible expansion but w=-nRTln(V2/V1) for a reversible expansion?
for an irreversible expansion but w=-nRTln(V2/V1) for a reversible expansion?
- Wed Mar 11, 2020 1:54 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: text problem 4A.5
- Replies: 3
- Views: 328
text problem 4A.5
A piston confines 0.200 mol Ne(g) in 1.20L as 25C. The gas is allowed to expand through an additional 1.20 L against a constant pressure of 1.00 atm
How do we know that the expansion of the piston is irreversible expansion?
How do we know that the expansion of the piston is irreversible expansion?
- Tue Mar 10, 2020 1:55 pm
- Forum: Administrative Questions and Class Announcements
- Topic: 14B acids and bases section
- Replies: 2
- Views: 231
14B acids and bases section
Should we use the 14A section on Acids and Bases for our questions? I am confused about Q3D of the midterm where it asks: at pH 6, what is the net charge of acetic acid, pKa= 4.75? At the review the TA said that you can tell that it will be a -1 charge because the pKa is smaller than the pH. I am co...
- Sun Mar 08, 2020 5:17 pm
- Forum: General Rate Laws
- Topic: derivations?
- Replies: 5
- Views: 441
derivations?
Will we have to know how to do the derivations of integrated rate laws for the test?
- Sat Mar 07, 2020 1:04 pm
- Forum: Experimental Details
- Topic: determining order of reaction
- Replies: 6
- Views: 740
determining order of reaction
Will we always be given the order of the reaction because it has to be determined experimentally?
- Tue Mar 03, 2020 8:02 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: text problem 6m.1
- Replies: 1
- Views: 215
text problem 6m.1
The stated short hand for the reaction in the text is M\left | M^{+} \left \| Cu^{2+}\left | Cu But in the solution manual says it's Cu\left | Cu^{2+}\left \| M^{2+}\left |M This affects which element is considered the anode and which is considered the cathode but I'm unclear as to why they reversed...
- Mon Mar 02, 2020 3:29 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: text problem 6L.1
- Replies: 1
- Views: 152
text problem 6L.1
given the equation 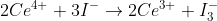 how can you tell how many moles of electrons are being used?
how can you tell how many moles of electrons are being used?
- Mon Mar 02, 2020 2:07 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: text problem 4J.7
- Replies: 2
- Views: 237
Re: text problem 4J.7
they also omit oxygen from the calculation of  but not from the calculation of
but not from the calculation of 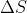
- Mon Mar 02, 2020 2:05 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: text problem 4J.7
- Replies: 2
- Views: 237
text problem 4J.7
when finding the \Delta H of the reaction 2H_{2}O_{2}(l)\rightarrow 2H_{2}O_{2}(l)+O_{2}(g) is oxygen not included because it is a gas? Are gases never included in enthalpy formations? Or am I just misinterpreting the solution manual's omission of oxygen in the enthalpy forma...
- Mon Mar 02, 2020 1:49 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: text problem 4J.5
- Replies: 2
- Views: 319
text problem 4J.5
it is asking to write the balanced formation reaction for NH3. The solution manual says 1/2N2 + 3/2H2 --> NH3. Is there a formula for how to arrive at these numbers or is it just guess and check? I'm confused as to how 2 Ns goes to only 1 N and how H2 gains another H
- Mon Mar 02, 2020 12:11 pm
- Forum: Balancing Redox Reactions
- Topic: Sapling Assignment 5 problem 9
- Replies: 2
- Views: 119
Re: Sapling Assignment 5 problem 9
the two equations are: a) MnO_{4}^{-}+S^{2-}\rightarrow S+MnO_{2} b) [Pb(OH)_{4}]^{2-}+ClO^{-}\rightarrow PbO_{2}+Cl^{-} it asks for the oxidizing agent, the reducing agent, and the balanced equation of each. I'm mostly confused on how to determine the oxidation states so that I know which e...
- Sat Feb 29, 2020 1:58 pm
- Forum: Balancing Redox Reactions
- Topic: Sapling Assignment 5 problem 9
- Replies: 2
- Views: 119
Sapling Assignment 5 problem 9
I'm having trouble determining the oxidation numbers in order to determine how many electrons are being exchanged for both a and b. I know this is like step 1 and pretty basic stuff but I haven't taken chemistry in 5 years and I was never totally comfortable with this topic anyway. Any tips would be...
- Tue Feb 25, 2020 10:02 am
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3908236
Re: Post All Chemistry Jokes Here
for all your extermination needs
- Fri Feb 21, 2020 10:52 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3908236
Re: Post All Chemistry Jokes Here
chem final outfit ideas
- Fri Feb 21, 2020 4:14 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: midterm Q6A
- Replies: 2
- Views: 316
midterm Q6A
why is D correct if it is +23 and the given enthalpy is negative? Even when you divide by the number of moles shouldn't it still be negative?
- Fri Feb 21, 2020 4:12 pm
- Forum: Identifying Acidic & Basic Salts
- Topic: midterm Q4
- Replies: 1
- Views: 255
midterm Q4
given the ratio of concentrations how do we know that the hydrogen bromide salt remains pronated?
- Fri Feb 21, 2020 4:10 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Midterm question 3D
- Replies: 1
- Views: 313
Midterm question 3D
how to do you know the net charge of an acid given the pH and the pKa?
- Mon Feb 17, 2020 5:59 pm
- Forum: Balancing Redox Reactions
- Topic: 6k3 part d
- Replies: 4
- Views: 212
Re: 6k3 part d
I had the same question. but I guess if it's a typo then it makes sense. If there were to be Cl2 on both sides of the equation would it be solvable?
- Thu Feb 13, 2020 3:18 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Lavelle's Office Hours
- Replies: 5
- Views: 480
Lavelle's Office Hours
does anyone know when Lavelle himself holds office hours? I can't find it on the course website. im sure it's somewhere in plain sight but Im just having trouble finding it
- Thu Feb 13, 2020 1:23 pm
- Forum: Administrative Questions and Class Announcements
- Topic: grading curve
- Replies: 10
- Views: 702
grading curve
does Lavelle grade on a curve?
- Tue Feb 11, 2020 2:08 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Pizza Rolls REVIEW Session DOWNLOAD HERE
- Replies: 67
- Views: 5973
Re: Pizza Rolls REVIEW Session DOWNLOAD HERE
for number 7 I understand how to solve for q but how do you solve for w with so many missing variables from the pv=nrt equation?
- Tue Feb 11, 2020 1:42 pm
- Forum: Student Social/Study Group
- Topic: set up of the midterm
- Replies: 4
- Views: 336
set up of the midterm
does anyone know how many questions there will be on the midterm? or what the general set up will be?
- Tue Feb 11, 2020 12:58 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Pizza Rolls REVIEW Session DOWNLOAD HERE
- Replies: 67
- Views: 5973
Re: Pizza Rolls REVIEW Session DOWNLOAD HERE
what is the difference between extensive and intensive properties?
- Tue Feb 11, 2020 12:57 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Pizza Rolls REVIEW Session DOWNLOAD HERE
- Replies: 67
- Views: 5973
Re: Pizza Rolls REVIEW Session DOWNLOAD HERE
as long as our units are consistent does it matter if we use kJ or J when talking about enthalpy or entropy?
- Tue Feb 11, 2020 12:23 pm
- Forum: Student Social/Study Group
- Topic: just confirming there is no class Wednesday of the midterm?
- Replies: 5
- Views: 420
just confirming there is no class Wednesday of the midterm?
just confirming there is no class Wednesday of the midterm?
- Tue Feb 11, 2020 12:19 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: how to find a heat capacity when not given
- Replies: 3
- Views: 194
how to find a heat capacity when not given
Does anyone know the formula for finding the heat capacity or the specific heat capacity when it is not specifically given in the question?
- Fri Feb 07, 2020 5:44 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: GFE dividing by temp
- Replies: 3
- Views: 181
GFE dividing by temp
Does anyone have a deeper explanation for why we would divide ΔG by T for the equation -ΔG/T= -ΔH/T + ΔSsys? In lecture I didn't understand what the purpose was for doing this.
- Thu Feb 06, 2020 5:13 pm
- Forum: Administrative Questions and Class Announcements
- Topic: entropy of state changes on the midterm
- Replies: 1
- Views: 94
Re: entropy of state changes on the midterm
or Gibbs free energy, because it is also assigned as reading but we haven't covered it in class yet
- Thu Feb 06, 2020 5:09 pm
- Forum: Administrative Questions and Class Announcements
- Topic: entropy of state changes on the midterm
- Replies: 1
- Views: 94
entropy of state changes on the midterm
does anyone know potentially how much about entropy of state changes we will have to know for the midterm? It's part of the textbook readings but we haven't talked about much in class yet.
- Thu Feb 06, 2020 11:24 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: sapling problems assignment 3
- Replies: 1
- Views: 100
sapling problems assignment 3
Does anyone know why in some sapling problems of assignment 3 the equation for q is q=nCdeltaT and then other times its just q=CdeltaT? They remove the quantity of the substance in some problems but im having trouble finding out why they do that.
Thanks!
Thanks!
- Sun Jan 26, 2020 12:31 pm
- Forum: Phase Changes & Related Calculations
- Topic: Heat vs temperature
- Replies: 6
- Views: 363
Re: Heat vs temperature
I think this was in reference to the heating curves. My understanding is that if you to take the temperature of the boiling water it would remain the same temperature until there was a phase change even though you are continuing to add heat to the substance in order to induce said phase change.

