"Balancing Redox Reactions in Acidic and Basic Conditions" by Professor Dave Explains had a nice and simplified explanation that really helped. I'll paste the link. Hope this helps and good luck!!
https://www.youtube.com/watch?v=N6ivvu6xlog&t=390s
Search found 102 matches
- Sat Mar 14, 2020 7:53 pm
- Forum: Balancing Redox Reactions
- Topic: YouTube videos for Redox Reactions
- Replies: 4
- Views: 522
- Sat Mar 14, 2020 7:50 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Cvm/Cpm
- Replies: 3
- Views: 409
Re: Cvm/Cpm
they are typically used when using the 2 of the 3 different forms of the entropy equation:
1. Constant volume (isochoric): dS = n*Cv*ln(T2/T1)
2. Constant pressure (isobaric): dS = n*Cp*ln(T2/T1)
1. Constant volume (isochoric): dS = n*Cv*ln(T2/T1)
2. Constant pressure (isobaric): dS = n*Cp*ln(T2/T1)
- Sat Mar 14, 2020 7:39 pm
- Forum: Phase Changes & Related Calculations
- Topic: expansion
- Replies: 5
- Views: 533
Re: expansion
it is also important to know the difference between gradual expansion and sudden expansion, in which you would apply the appropriate formula. The questions I have seen typically state whether the process is occurring gradually or suddenly. Hope this helps!
- Sat Mar 14, 2020 7:33 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: k
- Replies: 4
- Views: 416
Re: k
specifically, if temperature increases, then so does the rate constant. If temperature decreases, the rate constant decreases as well. In short, there is a direct relationship between temperature and the rate constant.
- Sat Mar 14, 2020 7:31 pm
- Forum: Balancing Redox Reactions
- Topic: oh
- Replies: 11
- Views: 828
Re: oh
OH- is only added when balancing a redox reaction in a basic solution. You add the same number of OH- as you have H+ to both sides of the equation.
- Sun Mar 08, 2020 8:53 am
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: factors that affect k
- Replies: 8
- Views: 717
Re: factors that affect k
if you are referring to the reaction rate constant (lowercase k), then I believe that varying the temperature and solvent would affect its value.
- Sun Mar 08, 2020 8:46 am
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Terminology for reaction rate constant
- Replies: 3
- Views: 381
Re: Terminology for reaction rate constant
The only other name I can think of is reaction rate coefficient, but oftentimes it's just presented as k in a question.
- Sun Mar 08, 2020 8:44 am
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: determine n
- Replies: 16
- Views: 1439
Re: determine n
remember when given a table with the concentration of reactants and rates of different reactions, the order can be found by setting up a ratio of the concentration from (ex.) (Conc. Experiment 1 / Conc. Experiment 2)^n = (Rate Experiment 1 / Rate Experiment 2). Remember that the variables that repre...
- Sat Mar 07, 2020 9:30 pm
- Forum: Balancing Redox Reactions
- Topic: Basic vs Acidic Conditions.
- Replies: 6
- Views: 481
Re: Basic vs Acidic Conditions.
I believe so. If I'm understanding your question correctly, the balancing of a redox reaction in basic and acidic conditions follows the same steps. The only difference is that for a basic reaction you add OH- as you did H+ to both sides. The side with both H+ and OH- combines to H2O.
- Sat Mar 07, 2020 8:52 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: intermediates
- Replies: 4
- Views: 433
Re: intermediates
also make sure not to confuse intermediates for catalysts, which are first consumed then produced in a later elementary step, as opposed to intermediates, which are first produced then consumed during a later elementary step.
- Sun Mar 01, 2020 10:04 am
- Forum: Balancing Redox Reactions
- Topic: Redox Reactions
- Replies: 2
- Views: 360
Re: Redox Reactions
I've learned you balance in order of... Acidic: any element besides O and H, balance O by adding H2O, balance H by adding H+, add electrons to appropriate side Basic: any element besides O and H, balance O by adding H2O, balance H by adding H+, add the same number of OH- (as you did H+ in the previo...
- Sun Mar 01, 2020 9:48 am
- Forum: Balancing Redox Reactions
- Topic: Redox Reactions and Acid/Base Reactions
- Replies: 9
- Views: 763
Re: Redox Reactions and Acid/Base Reactions
No they are not always acid and base reactions. For a reaction to be considered redox, the number of electrons an element has must change, which can occur in the absence of acids and bases.
- Sun Mar 01, 2020 9:40 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: homework question 6L.1
- Replies: 3
- Views: 381
Re: homework question 6L.1
Keep in mind, however, that in order to answer the question correctly, you must write out the half-reactions for both part a and part b. This will allow you to determine how many moles of electrons were transferred, n. Otherwise, all the other needed pieces of information are given.
- Sun Mar 01, 2020 9:36 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Calculating the value of n (6L.1)
- Replies: 2
- Views: 246
Re: Calculating the value of n (6L.1)
I think the half reactions should be...
Reduction: 2Ce(4+) + 2e- --> 2Ce(3+)
Oxidation: 3I(1-) --> I3(1-) + 2e-
From this we know that the moles of electrons transferred, n, is 2. Hope this helps!
Reduction: 2Ce(4+) + 2e- --> 2Ce(3+)
Oxidation: 3I(1-) --> I3(1-) + 2e-
From this we know that the moles of electrons transferred, n, is 2. Hope this helps!
- Sun Mar 01, 2020 9:29 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Values of Standard Electrode Potentials
- Replies: 4
- Views: 378
Re: Values of Standard Electrode Potentials
In regards to the first part of your question, I believe that a negative value for standard electrode potentials indicates that the element or compound in question forms ions easily.
- Sun Feb 23, 2020 4:10 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: salt bridge
- Replies: 10
- Views: 709
Re: salt bridge
a salt bridge is a device used to neutralize the charges of each half-cell in a galvanic cell. Because it prevents the buildup of charge in both the anode and cathode, the transfer of electrons can continue.
- Sun Feb 23, 2020 4:02 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: electrode
- Replies: 4
- Views: 421
Re: electrode
I believe an electrode is the solid metal that is placed into the solutions. They function to provoke electrical conductivity (can someone confirm this please).
- Sun Feb 23, 2020 3:52 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Salt Bridge
- Replies: 9
- Views: 661
Re: Salt Bridge
Without a salt bridge, because the anode becomes more positive overtime and the cathode becomes more negative overtime, the cell would no longer cause a transfer of electrons. Electrons would have no reason to leave the anode to the cathode, which is negatively charged (like charges repel each other...
- Sun Feb 23, 2020 1:23 am
- Forum: Balancing Redox Reactions
- Topic: Finding moles of the reaction
- Replies: 4
- Views: 533
Re: Finding moles of the reaction
In short, what is lost by one half reaction (oxidation) is gained by the other reaction (reduction), therefore representing a transfer of electrons. This is the number that should be inputted for n. in the equation: G = -nFE
- Sun Feb 23, 2020 1:14 am
- Forum: Balancing Redox Reactions
- Topic: writing redox equations
- Replies: 3
- Views: 224
Re: writing redox equations
For example: if given two reduction reactions, the higher the voltage, the more likely the reaction is to occur. Therefore, we know that the lower number is the reaction that we must flip to become an oxidation reaction (because it is less likely to occur as a reduction reaction relative to the othe...
- Sat Feb 15, 2020 8:02 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Balanced Chemical Equations
- Replies: 4
- Views: 438
Re: Balanced Chemical Equations
If you have fractional coefficients, multiplying to yield whole number coefficients would affect every component of the reaction and therefore the ratio should remain the same and so should your answers. I think you should be okay.
- Sat Feb 15, 2020 7:55 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Unit for Pressure in delta G equation
- Replies: 2
- Views: 242
Re: Unit for Pressure in delta G equation
I'm not completely sure, but I think for this question, since the given pressures would be used to find Qp, the units of bars would cancel out since Q has no units (and because it's products/reactants, so units would cancel) , so as long as the two pressures have the same units, I don't think it mat...
- Sat Feb 15, 2020 7:33 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Isothermal
- Replies: 9
- Views: 546
Re: Isothermal
Additionally, I believe that a system being isothermal suggests that the change in internal energy for the system is 0, meaning that
dU = q + w ==> q = -w
dU = q + w ==> q = -w
- Sat Feb 15, 2020 7:30 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Isothermal
- Replies: 9
- Views: 546
Re: Isothermal
To add to the statement above, many questions in the textbook describe systems as isothermally reversible and irreversible, so it is not necessarily correct to consider an isothermal system to be only reversible.
- Sat Feb 15, 2020 7:12 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Isobaric systems
- Replies: 16
- Views: 851
Re: Isobaric systems
During Lyndon and Matt's Pizza Rolls review session, Matt made a comment that might help with remembering the meaning of isobaric. He emphasized the BAR in isoBARic and compared it to BARometer, which is an instrument used to measure atmospheric pressure. Hope this helps!
- Sun Feb 09, 2020 1:28 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Calorimeters
- Replies: 17
- Views: 994
Re: Calorimeters
To answer the question above, I believe it's safe to expect to see both variations. We should know the different conditions that correspond to each type of calorimeter because both types show up in the homework problems. Hope this helps!
- Sun Feb 09, 2020 1:24 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Calorimeter
- Replies: 5
- Views: 409
Re: Calorimeter
In regards to calorimeters, it is also important to remember that -q = qcal. In other words, heat that is lost from the system is gained by the calorimeter. Hope this helps.
- Sun Feb 09, 2020 1:20 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: reversible vs irreversible work
- Replies: 7
- Views: 481
Re: reversible vs irreversible work
Oftentimes, the question will also state whether the gas in question "expands reversibly" or "expands irreversibly", so be on the look out for key terms when reading the question!
- Sun Feb 09, 2020 1:14 am
- Forum: Calculating Work of Expansion
- Topic: 4A.3 part c
- Replies: 3
- Views: 147
Re: 4A.3 part c
dU = w whenever there is no each exchanged with the surroundings (a.k.a q = 0). This condition is known as adiabatic.
- Sun Feb 09, 2020 1:05 am
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Microstates
- Replies: 7
- Views: 219
Re: Microstates
Entropy is associated with maximization of the number of microstates or number of arrangements. Spontaneous processes are also characterized by maximizing microstates, and therefore maximizing entropy.
- Sun Feb 02, 2020 10:55 pm
- Forum: Calculating Work of Expansion
- Topic: Integrals
- Replies: 4
- Views: 208
Re: Integrals
I'm pretty sure it's just to understand what is going on conceptually. I think the function of the integral was to clearly show the derivation of the reversible isothermal expansion equation: w = -nRT ln(Vf - Vi) .... (I would focus more on knowing how and when to use this equation. Essentially, the...
- Sun Feb 02, 2020 10:38 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Adiabatic Wall
- Replies: 3
- Views: 199
Re: Adiabatic Wall
Since no heat is exchanged with the surroundings in the presence of an adiabatic wall, q=0.
If we apply this fact to the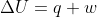 equation, we find that
equation, we find that 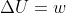 when adiabatic
when adiabatic
If we apply this fact to the
- Sun Feb 02, 2020 10:26 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Energy Transfer of An Isolated System
- Replies: 1
- Views: 97
Re: Energy Transfer of An Isolated System
Good question! I'm not entirely sure, but I understand that a closed system has a constant internal energy, since there is no gain or loss of energy/heat. Since internal energy is the heat plus work, I am assuming the definition accounts for work as well. Can someone please double check this? Thanks...
- Sat Feb 01, 2020 1:35 pm
- Forum: Phase Changes & Related Calculations
- Topic: Internal Energy, U
- Replies: 6
- Views: 316
Re: Internal Energy, U
A counter example would be in an isolated system, where neither heat nor matter can be exchanged, dU = q + w.
- Sat Feb 01, 2020 12:59 pm
- Forum: Phase Changes & Related Calculations
- Topic: Calorimeter
- Replies: 8
- Views: 581
Re: Calorimeter
Although both are structured differently, it is important to remember that they serve the same function-- to measure specific heat capacity (often referred to as specific heat).
- Sun Jan 26, 2020 8:37 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Reaction Enthalpy vs Formation Enthalpy??
- Replies: 2
- Views: 138
Re: Reaction Enthalpy vs Formation Enthalpy??
I'm not too sure about entropy, but some of the enthalpy unit terms we have are: enthalpy itself, which refers to the study of heat released or absorbed in chemical reins and physical changes...it can also refer to the amount of heat released or absorbed at a constant rate if reaction gives a net re...
- Sun Jan 26, 2020 8:26 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Hess's Law
- Replies: 2
- Views: 141
Re: Hess's Law
According to my notes, the reaction was: N2 +O2 --> 2 NO dHrxn = 180 kJ + 2 NO + O2 --> 2 NO2 dHrxn = -112 kJ as you can see, the product of the first reaction is found in the reactants of the second reactions. That means that it is an intermediate component and can be cancelled out. Additionally, t...
- Sun Jan 26, 2020 8:19 am
- Forum: Phase Changes & Related Calculations
- Topic: State Function
- Replies: 7
- Views: 360
Re: State Function
Examples of other state properties include: energy, pressure, volume, temperature, density, and heat capacity
In contrast, work and heat both depend on the path taken to from its initial value to its final value, preventing them from being considered state functions
In contrast, work and heat both depend on the path taken to from its initial value to its final value, preventing them from being considered state functions
- Sun Jan 26, 2020 8:15 am
- Forum: Phase Changes & Related Calculations
- Topic: Hess's Law
- Replies: 3
- Views: 113
Re: Hess's Law
This is very similar to how when reactions took multiple steps to reach equilibrium we would multiply the equilibrium (K) values from each step. When you put products over reactants of each step and multiplied, any intermediate components were cancelled out. Kind of similar to Hess's law, so we've s...
- Sun Jan 26, 2020 8:00 am
- Forum: Phase Changes & Related Calculations
- Topic: Steam vs Water
- Replies: 6
- Views: 230
Re: Steam vs Water
All of the above in addition to the fact that water vapor is at a higher temperature when it is in the form of steam can be visualized by looking at the cooling/heating chart that professor Lavelle went over during lecture. Hope this helps with understanding!
- Sat Jan 18, 2020 3:38 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: 5J.1 a)
- Replies: 5
- Views: 138
Re: 5J.1 a)
It's also important to understand that partial pressure is different from total pressure, in which the total number of moles on each side of the reaction would be considered.
- Sat Jan 18, 2020 2:44 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Partial Pressure
- Replies: 19
- Views: 749
Re: Partial Pressure
How do we know when to use K_c and when to use Partial Pressure Notation? Kc notation is used when the reactants and products of a homogeneous reaction are in the aqueous phase, while partial pressure notation is used when the reactants and products of a homogeneous reaction are in the gas phase. K...
- Sat Jan 18, 2020 2:39 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Partial Pressure
- Replies: 19
- Views: 749
Re: Partial Pressure
Why are solids and liquids not included when calculating K values? I believe solids and liquids are not included when calculating K values because their concentrations don't change significantly over the course of a reaction. Can anyone confirm this? I'm not exactly sure if this is the right explan...
- Sat Jan 18, 2020 2:34 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Partial Pressure
- Replies: 19
- Views: 749
Re: Partial Pressure
In regards to what the difference is between Kc and Kp:
Kc represents the equilibrium concentrations of reactants and products, while Kp suggests the equilibrium partial pressures of the reactants and products
Kc represents the equilibrium concentrations of reactants and products, while Kp suggests the equilibrium partial pressures of the reactants and products
- Sat Jan 18, 2020 2:17 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Q < K
- Replies: 16
- Views: 852
Re: Q < K
A method I use to remember the meanings of when Q>K and Q<K is to think of Q wanting to approach K. This can be visualized as a number line with K placed in the center. If Q is less than K, then the reaction must proceed in the forward direction in order to reach K. Conversely, if Q is greater than ...
- Sun Jan 12, 2020 12:22 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Dissociated Ionic Compounds
- Replies: 5
- Views: 179
Re: Dissociated Ionic Compounds
In regards to the activity of each ion, I believe they are referring to the ion's respective concentration. I hope this helps!
- Sun Jan 12, 2020 12:18 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: appliction of principle
- Replies: 5
- Views: 256
Re: appliction of principle
Essentially, this principle applies to changes in all physical parameters, as stated in lecture
- Sun Jan 12, 2020 12:07 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Le Chatelier's Principle
- Replies: 5
- Views: 212
Re: Le Chatelier's Principle
Le Chatelier's Principle tells us that chemical reactions adjust to minimize the effect of changes, but because solids and liquids are negligible in equilibrium equations, adding or removing them will not have an effect on the equilibrium.
- Sun Jan 12, 2020 11:53 am
- Forum: Ideal Gases
- Topic: Textbook Help
- Replies: 4
- Views: 348
Re: Textbook Help
Try searching for a free (or even cheaper) download for the textbook PDF. It would likely be easier to use and you'd save money!
- Sun Jan 12, 2020 11:37 am
- Forum: Ideal Gases
- Topic: Topics on Test 1
- Replies: 37
- Views: 1414
Re: Topics on Test 1
He usually will let us know in lecture. I assume everything in outline 1 and all other material covered during lecture up until the test, unless otherwise stated. Your TAs might also be able to provide a more definite answer. Hope this helps!
- Sun Jan 12, 2020 11:32 am
- Forum: Ideal Gases
- Topic: PV = nRT
- Replies: 16
- Views: 1986
Re: PV = nRT
Knowing these comes in handy when given their values and you have to rearrange them in order to find a solutions concentration, for example. Notably, concentration (or n/V) = P/RT.
- Sun Dec 08, 2019 9:42 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: boiling point
- Replies: 9
- Views: 961
Re: boiling point
It is also important to remember that boiling does not break bonds. So when explaining the effect that boiling point has, my TA suggested avoiding using the phrase "break bonds" and use "overcome intermolecular forces" instead.
- Sun Dec 08, 2019 9:34 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Strength
- Replies: 6
- Views: 493
Re: Strength
Yes, it is the weakest relative to the others and its strength (compared to other molecules with only LDFs present) increases with size.
- Sun Dec 08, 2019 9:15 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: H bonding
- Replies: 4
- Views: 398
Re: H bonding
Yes, it is also important to understand that hydrogen atoms bonded to carbon atoms are not counted when determining possible hydrogen bonding sites.
- Sun Dec 08, 2019 9:01 pm
- Forum: Significant Figures
- Topic: Logarithm sigfigs with O
- Replies: 2
- Views: 377
Re: Logarithm sigfigs with O
^I understand that this is true for conventional numbers, however, for pH specifically, my TA mentioned that each number after the decimal point is a significant figure. Please double check with another source, though.
- Sun Dec 08, 2019 8:54 pm
- Forum: SI Units, Unit Conversions
- Topic: HELP WITH UNITS
- Replies: 9
- Views: 2349
Re: HELP WITH UNITS
Yeah! This video (https://youtu.be/7N0lRJLwpPI) helped me with this topic. Hope this is what you're looking for!
- Sat Nov 30, 2019 6:47 pm
- Forum: Bond Lengths & Energies
- Topic: Melting Points
- Replies: 8
- Views: 767
Re: Melting Points
I think in terms of this unit, we just need to know that boiling point and melting point are when a molecule changes its phase. If the intermolecular forces are stronger in one molecule as opposed to another, it will have a higher boiling point and higher melting point relative to the other molecule...
- Sat Nov 30, 2019 6:22 pm
- Forum: Dipole Moments
- Topic: boiling point
- Replies: 6
- Views: 653
Re: boiling point
To add to this, among molecules that contain only London dispersion forces, the way to tell whether one is stronger than another is by the size of the entire molecule. The larger the molecule is, the greater the strength of LDFs.
- Sat Nov 30, 2019 6:12 pm
- Forum: Dipole Moments
- Topic: Polar or nonpolar?
- Replies: 4
- Views: 9080
Re: Polar or nonpolar?
My TA told me that generally if there is at least one atom that is not the same (amongst the outside 4 atoms of a tetrahedral) then the molecule as a whole is polar.
- Sat Nov 30, 2019 5:57 pm
- Forum: Sigma & Pi Bonds
- Topic: Question on Test 2
- Replies: 11
- Views: 934
Re: Question on Test 2
In response to Matthew ILG 1L, I believe the term "hydrogen-bonding sites" refers to the places where hydrogen bonds can potentially form as well as where they already exist, which is why you would include the H-bonds already present in order to find the total number of bonding sites.
- Sat Nov 30, 2019 5:28 pm
- Forum: Sigma & Pi Bonds
- Topic: Sigma & Pi Bonds
- Replies: 9
- Views: 637
Re: Sigma & Pi Bonds
I believe the bond formed between an s-orbital and an s-orbital is a sigma bond.
- Sat Nov 23, 2019 12:12 pm
- Forum: Naming
- Topic: transition metals
- Replies: 2
- Views: 175
Re: transition metals
I believe this only applies to transition metals, as the central atom of a coordination compound is a metal and the surrounding atoms are nonmetals/ligands. I think it would be different for other compounds. Can someone please confirm this though?
- Sat Nov 23, 2019 11:59 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Double/Triple Bonds in Coordination Compounds
- Replies: 2
- Views: 192
Re: Double/Triple Bonds in Coordination Compounds
I believe this is because you are counting the number of atoms directly bonded to the central atom, not necessarily the number of bonds they contain. Hope this makes sense!
- Sat Nov 23, 2019 11:49 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Coordination Number
- Replies: 2
- Views: 164
Re: Coordination Number
^I agree. It may help to know that ligands directly attached to the central atom make up the coordination sphere, so it makes sense that the number of bonds attached to the central atom are what comprise the molecule's coordination number (this is how I try to remember it).
- Sat Nov 23, 2019 11:44 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Naming Ligands
- Replies: 2
- Views: 262
Re: Naming Ligands
I also believe that we have to use these principles for naming ligands in 14B, so it would be helpful to have them memorized, as later chapters likely build upon this knowledge
- Sat Nov 23, 2019 11:39 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: [Fe(CN)6]4-
- Replies: 5
- Views: 487
Re: [Fe(CN)6]4-
I believe it is CN-, meaning it can form the coordinate covalent bond. However, I'm not exactly sure why the total charge is 4-.
- Sun Nov 17, 2019 12:34 pm
- Forum: Lewis Structures
- Topic: Exceptions
- Replies: 5
- Views: 199
Re: Exceptions
I believe that H, He, Be, and Li are also exceptions in that they are content with having less than 8 valence electrons.
- Sun Nov 17, 2019 12:03 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Polar Molecules
- Replies: 4
- Views: 239
Re: Polar Molecules
I believe so, this is according to the example he gave during lecture regarding cis-DICHLOROETHENE (polar) v.s. trans-DICHLOROEHTENE (non-polar). The arrangement of the Cl atoms influences the polarity it seems.
- Sun Nov 17, 2019 11:54 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Shapes We Are Expected to Know
- Replies: 6
- Views: 445
Re: Shapes We Are Expected to Know
Sorry I put square pyramid twice, I meant square planar for that last one
- Sun Nov 17, 2019 11:51 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Shapes We Are Expected to Know
- Replies: 6
- Views: 445
Re: Shapes We Are Expected to Know
To add on to these responses, I believe this includes: linear, trigonal planar, bent/angular (<120 degrees), tetrahedral, trigonal pyramid, bent/angular (<<109 degrees), trigonal bipyramid, seesaw, T-shape, octahedral, square pyramid, square pyramid (So about 12 shapes, double check just in case)
- Sun Nov 17, 2019 11:44 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Van Der Waals Interaction
- Replies: 11
- Views: 641
Re: Van Der Waals Interaction
In terms of molecules containing only Van Der Waals interactions: the larger the size of the molecule, the more interactions there are, meaning that the interactions in that given molecule are stronger relative to one with a smaller size/less interactions.
- Sun Nov 17, 2019 11:24 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Ions
- Replies: 3
- Views: 336
Re: Ions
It is also important to keep in mind that in addition to all of this, the number of protons remains the same in the given cation or anion. Isoelectronicity means that the number of valence electrons is the same between two different elements, not the number of protons.
- Tue Nov 05, 2019 11:22 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Orbital angular momentum
- Replies: 4
- Views: 420
Re: Orbital angular momentum
I don't believe that knowing the equation is necessary. I think it's just important to know that angular momentum is denoted by the letter "l" and that it can be calculated by subtracting the principle quantum number, n, by 1.
In other words, l = n-1
In other words, l = n-1
- Tue Nov 05, 2019 11:12 pm
- Forum: Photoelectric Effect
- Topic: Best way to study for this topic?
- Replies: 8
- Views: 436
Re: Best way to study for this topic?
I find watching videos very helpful. It's a change of pace from lecture since we usually have to copy down stuff. Videos give me a chance to just listen. If the modules that are provided aren't enough, then I suggest watching Crash Course or Bozeman Science videos on YouTube.
- Tue Nov 05, 2019 11:07 pm
- Forum: Photoelectric Effect
- Topic: binding energy
- Replies: 3
- Views: 400
Re: binding energy
Yes, but this should not be confused with the ionization energy. Ionization energy is the energy required to remove an electron from a different state of matter, which is not the same as removing it form a metal surface.
- Tue Nov 05, 2019 11:00 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Heisenberg Indeterminacy Equation
- Replies: 6
- Views: 534
Re: Heisenberg Indeterminacy Equation
The delta x is the uncertainty in position and the delta p is uncertainty in momentum. The product of these two are greater than or equal to Planck's constant divided by 4*pi.
If we know the position, then the momentum is uncertain, and vice versa.
If we know the position, then the momentum is uncertain, and vice versa.
- Tue Nov 05, 2019 10:53 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: +- vs Uncertainty
- Replies: 3
- Views: 300
Re: +- vs Uncertainty
I believe the rule is to multiply the uncertainty by two
- Sun Nov 03, 2019 9:28 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Uncertainty in Speed [ENDORSED]
- Replies: 31
- Views: 17640
Re: Uncertainty in Speed [ENDORSED]
In response to Brennayoung's question, I believe that we will only be applying this to hydrogen for now. This principle is used when measuring position and momentum of a particle.
- Sun Nov 03, 2019 9:19 pm
- Forum: Properties of Electrons
- Topic: Photoelectric effect
- Replies: 5
- Views: 473
Re: Photoelectric effect
Crash Course has a quantum mechanics series out on YouTube, which is pretty helpful as well!
- Sun Nov 03, 2019 8:55 pm
- Forum: Empirical & Molecular Formulas
- Topic: Rounding
- Replies: 6
- Views: 641
Re: Rounding
I believe .9 and above is a safe range for rounding up. Anything lower I would multiply to get the numbers behind the decimal point to 0.9 or higher. Hope this helps.
- Sun Nov 03, 2019 8:37 pm
- Forum: SI Units, Unit Conversions
- Topic: Do you always convert to SI units for calculations?
- Replies: 3
- Views: 302
Re: Do you always convert to SI units for calculations?
Yes, I agree. SI units are a great way of checking whether or not your are on the right track. If the units, when cancelled out, leave you with the desired units, you can assume that your answer is right (given the math is done correctly).
- Sat Nov 02, 2019 8:44 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: using indeterminancy
- Replies: 3
- Views: 206
Re: using indeterminancy
I believe so, as that's what Lyndon did during Dino Nuggets
- Sun Oct 27, 2019 1:52 am
- Forum: Electronegativity
- Topic: Molecular Geometry
- Replies: 4
- Views: 226
Re: Molecular Geometry
I wouldn't rule it out completely, especially if we are learning hybridization soon, as it is known to have an influence on molecular geometry. But for now I can assume it's a no.
- Sun Oct 27, 2019 1:42 am
- Forum: Resonance Structures
- Topic: Resonance
- Replies: 4
- Views: 349
Re: Resonance
That being said, some structures are more stable than others, even though the substance is the same. This is where the calculation of formal charges comes into play. Substances that have more atoms with a formal charge of 0 will be more stable.
- Sun Oct 27, 2019 1:40 am
- Forum: Lewis Structures
- Topic: Lewis Structure bonds
- Replies: 4
- Views: 440
Re: Lewis Structure bonds
MichelleRamirez_2f, yes. The formal charge helps us indicate which structure is the most stable (more formal charges of 0), which is favored.
- Sun Oct 27, 2019 1:36 am
- Forum: Ideal Gases
- Topic: Reading the textbook
- Replies: 262
- Views: 152460
Re: Reading the textbook
From what I have seen, the topics covered in lecture correlate to what appears on the test. If anything, the textbook may go into more detail than need, which isn't necessarily a bad thing. However, in terms of maximizing studying, I would rely more on the notes from lecture. Hope this helps!
- Sun Oct 27, 2019 1:34 am
- Forum: General Science Questions
- Topic: Rusty on High School Chem [ENDORSED]
- Replies: 347
- Views: 444772
Re: Rusty on High School Chem [ENDORSED]
I'm not sure what the likelihood of this is, but if you still have your notes from chemistry in high school (perhaps in a binder), revisiting those may help clear up any misunderstandings
- Sat Oct 19, 2019 10:12 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Lyman and Balmer
- Replies: 3
- Views: 181
Re: Lyman and Balmer
In addition to these, it may be relevant for you to know about the paschen (n=3), brackett (n=4), and pfund (n=5) series.
- Sat Oct 19, 2019 9:48 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: how to prepare
- Replies: 22
- Views: 912
Re: how to prepare
Also, if none of the above works, you can always resort to YouTube! Hearing it repeatedly could help retain information. Plus, if you're a visual learner, YouTube is a nice and convenient method that doesn't require access to a textbook or notes. Hope this helps!
- Sat Oct 19, 2019 9:24 pm
- Forum: SI Units, Unit Conversions
- Topic: Practice Problems?
- Replies: 11
- Views: 617
Re: Practice Problems?
The AAP program also provides peer learning facilitators who function the same. If you are eligible to join, I would recommend doing so, as they tend to consist of less people (typically around 10 max).
- Sat Oct 19, 2019 9:19 pm
- Forum: Balancing Chemical Reactions
- Topic: balancing chemical reactions
- Replies: 7
- Views: 2586
Re: balancing chemical reactions
To add to this most recent comment, an example would be in combustion reactions, where oxygen gas is always by itself on the reactant side of the chemical equation.
- Sat Oct 19, 2019 2:39 pm
- Forum: General Science Questions
- Topic: Rusty on High School Chem [ENDORSED]
- Replies: 347
- Views: 444772
Re: Rusty on High School Chem [ENDORSED]
Personally, simply reviewing the tables and charts in the textbook helped me sharpen up on high school chemistry. YouTube videos are also a great resource!
- Fri Oct 11, 2019 10:34 am
- Forum: Significant Figures
- Topic: Significant Figures
- Replies: 10
- Views: 809
Re: Significant Figures
Yes, your last question was correct. For example, if you were adding 3.5 and 2.11, you'd get 5.61; however, in order for the answer to be rounded to the correct number of significant figures, you'd have to make your final answer 5.6. This is because the final answer must take the same amount of numb...
- Fri Oct 11, 2019 10:26 am
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Word Problem Efficiency
- Replies: 7
- Views: 450
Re: Word Problem Efficiency
I typically read each sentence and underline as I go. Although it sounds pretty elementary, I've found that it helps me better pick out the relevant information, while also ensuring that I don't miss anything that can be used to help solve the problem. Breaking it down sentence by sentence helps tre...
- Fri Oct 11, 2019 10:20 am
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Theoretical vs. Actual Yield
- Replies: 38
- Views: 14113
Re: Theoretical vs. Actual Yield
If given the values of the other terms, you could simply solve the equation for the missing value. For example: if solving for actual yield, the question would likely provide the values of theoretical yield and percent yield, so you can solve for actual yield by multiplying the theoretical yield by ...
- Fri Oct 11, 2019 10:15 am
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Test 1 [ENDORSED]
- Replies: 107
- Views: 23325
Re: Test 1 [ENDORSED]
No, all compounds will be given in terms of their formulas.
- Tue Oct 08, 2019 11:11 pm
- Forum: SI Units, Unit Conversions
- Topic: Are there going to be any questions on this week's test regarding Quanta and Photons?
- Replies: 5
- Views: 347
Re: Are there going to be any questions on this week's test regarding Quanta and Photons?
Yeah, this week's test only consists of everything up until Quanta and Photons; however, for the homework we are able to turn in problems relating to the Quanta and Photons lessons in addition to those relating to the high school chemistry review lessons.
- Fri Oct 04, 2019 12:17 am
- Forum: SI Units, Unit Conversions
- Topic: Memorizing Metric Conversions
- Replies: 8
- Views: 1025
Re: Memorizing Metric Conversions
Knowing the prefixes will be useful when using conversion factors to get to a final answer, so I do think memorizing prefixes will be necessary. In regards to fento, atto, and angstrom, I'm not too sure. I would focus more on the angstrom, since that's what he touched on the most during lecture. Hop...
- Thu Oct 03, 2019 5:03 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: Delta
- Replies: 2
- Views: 193
Re: Delta
I don't think the delta has anything to do with the removal of products, it just signifies that the reaction requires a high temperature in order to occur. For example, the conversion of limestone into quicklime takes place at about 800 degrees Celsius (found in the textbook).
- Thu Oct 03, 2019 12:30 am
- Forum: Administrative Questions and Class Announcements
- Topic: Test #1
- Replies: 3
- Views: 249
Re: Test #1
I'm going to assume that the questions will resemble the practice we do during discussion, so free response. That way they can [censored] our ability to work through the problems, use sig figs etc..

