Search found 99 matches
- Mon Mar 16, 2020 4:51 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: adding platinum
- Replies: 8
- Views: 575
Re: adding platinum
If there is no conductive solid already participating in the redox reaction, then you would need to [censored] an inert metal such as Pt(s)
- Mon Mar 16, 2020 4:50 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Voltaic Cells
- Replies: 3
- Views: 319
Re: Voltaic Cells
Yes, they are the same. The other kind of cell that was mentioned but not studied in class was the concentration cell.
- Mon Mar 16, 2020 4:49 pm
- Forum: Phase Changes & Related Calculations
- Topic: state functions
- Replies: 13
- Views: 1406
Re: state functions
State functions, such as enthalpy, entropy, and Gibb's free energy, are dependent on the initial and final values. However, in the case of heat (q) and work which are pathway functions, the 'path' taken or how much is done is important.
- Sun Mar 15, 2020 7:29 am
- Forum: Balancing Redox Reactions
- Topic: balancing h and o
- Replies: 7
- Views: 571
Re: balancing h and o
Start off by balancing the oxygen atoms by using H20. From there depending on the pH, you use H+ for acidic solutions and OH^- for basic solutions to balance out the Hydrogens.
- Sun Mar 15, 2020 7:26 am
- Forum: Balancing Redox Reactions
- Topic: OH vs H
- Replies: 12
- Views: 902
Re: OH vs H
No, H+ is only used to balance a redox rxn in acidic conditions. In the case of a basic solution, you MUST use OH^-
- Sun Mar 15, 2020 7:24 am
- Forum: Balancing Redox Reactions
- Topic: Pt in Cell Diagram
- Replies: 14
- Views: 937
Re: Pt in Cell Diagram
Pt(s) is used when there is no conductive solid participating in the reaction. It is an inert metal, therefore will not affect the redox rxn.
- Sun Mar 15, 2020 7:23 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: E cell
- Replies: 9
- Views: 635
Re: E cell
E° refers to standard conditions, such as 1M, 1atm, 25°C (This is found using the cathode-anode), while E is not under these same conditions and can be found using the Nernst equation. As long as you have one of them known, the Nernst equation can be used to find the other.
- Sun Mar 15, 2020 7:19 am
- Forum: Interesting Applications: Rechargeable Batteries (Cell Phones, Notebooks, Cars), Fuel Cells (Space Shuttle), Photovoltaic Cells (Solar Panels), Electrolysis, Rust
- Topic: Electrolysis
- Replies: 6
- Views: 483
Re: Electrolysis
It's on the outline, so it can appear on the final. However, I think as long as you understand the basics, you should be fine.
- Sun Mar 15, 2020 7:17 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Enthalpy and Heat
- Replies: 4
- Views: 434
Re: Enthalpy and Heat
enthalpy is the change in heat at constant pressure (at which is can be equal to ), while q is measure of heat under varying conditions.
- Sun Mar 15, 2020 7:15 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: calculating delta H for an expansion
- Replies: 3
- Views: 372
Re: calculating delta H for an expansion
You'll need to use the standard enthalpy of formation and the ∆Hr° = ∆H°  (products) - ∆H°
(products) - ∆H°  (reactants)
(reactants)
- Sun Mar 15, 2020 7:12 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Bond Enthalpies and Standard Enthalpies of Formation
- Replies: 2
- Views: 338
Re: Bond Enthalpies and Standard Enthalpies of Formation
If the values are not given, you might have to use the ∆G° = ∆H° - T∆S°equation to calculate enthalpy. If not, then the value might be zero. I don't believe there should be much confusion regarding this on the final.
- Sun Mar 15, 2020 7:09 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Lyndon Review: 1D
- Replies: 5
- Views: 524
Re: Lyndon Review: 1D
changing the size does not affect the cell potential
- Sun Mar 15, 2020 7:08 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Same charge
- Replies: 1
- Views: 255
Re: Same charge
First, make sure you balance the charges of each half reaction (for the most part you should need electrons to balance the half rxns). If then, you notice that both half rxns have the same charges then you should be fine as the electrons eventually cancel out.
- Sun Mar 15, 2020 7:05 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Flipping the anode
- Replies: 4
- Views: 377
Re: Flipping the anode
Since all the standard cell equations are written as reduction (which coincide with the cathodes), then the anode must be flipped to get the oxidation half reaction.
- Sun Mar 15, 2020 7:03 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Including H2O in Cell Diagram
- Replies: 3
- Views: 628
Re: Including H2O in Cell Diagram
Yes, H2O is not included in the cell diagram
- Sun Mar 15, 2020 7:02 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Adding H20 to solution
- Replies: 3
- Views: 275
Re: Adding H20 to solution
The effect of the dilution depends on where it is occurring. If the cathode is diluted then the cell potential decreased. However, if the anode is dilution there is an increase in cell potential.
- Sun Mar 15, 2020 7:00 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Electrode Mass
- Replies: 10
- Views: 4570
Re: Electrode Mass
Changing the concentration of the electrode might result in an increase, however for the mass it should not affect cell potential.
- Sat Mar 14, 2020 3:31 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: inert gases
- Replies: 6
- Views: 429
Re: inert gases
Kaylee Clarke 1G wrote:what of the elements such as platinum that seem to be attached to a cell diagram?
Pt(s) is used as a conductor when there is no conductive solid participating in the redox reaction. It is added to the cell diagram but does not participate.
- Sat Mar 14, 2020 3:28 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagram
- Replies: 11
- Views: 746
Re: Cell Diagram
Yes. Since Pt(s) is an inert metal, it is commonly used in the absence of a conductive solid.
- Sat Mar 14, 2020 3:27 pm
- Forum: Balancing Redox Reactions
- Topic: Redox Reaction in a Basic Solution
- Replies: 3
- Views: 383
Re: Redox Reaction in a Basic Solution
for basic solutions, you balance out the oxygens with H2O and then use OH^- to balance the hydrogens
- Sat Mar 14, 2020 3:08 pm
- Forum: Balancing Redox Reactions
- Topic: Oxidation number
- Replies: 12
- Views: 1230
Re: Oxidation number
the oxidation number helps identify whether a species is oxidized or reduced. Using the oxidation number, you can determine how many electrons are necessary to balance the charges of a half reaction.
- Sat Mar 14, 2020 3:06 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Endgame 1a
- Replies: 5
- Views: 516
Re: Endgame 1a
the anode is the side with the lower concentration as it is the side where oxidation occurs.
- Sat Mar 14, 2020 3:04 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: E vs Eo
- Replies: 6
- Views: 525
Re: E vs Eo
Eº refers to standard conditions while E is not and therefore is affected by changes such as temperature, concentration etc.
- Sat Mar 14, 2020 3:01 pm
- Forum: Balancing Redox Reactions
- Topic: Acid or base?
- Replies: 5
- Views: 516
Re: Acid or base?
Yes, we will be given that information as it is necessary to determine how to balance the redox rxns. In some cases, we might be given the pH or even pka or pkb to determine pH.
- Thu Mar 12, 2020 8:30 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6L.3
- Replies: 4
- Views: 366
Re: 6L.3
You get the half reactions by looking at the species used in the galvanic cell. It helps to think about AnOX RedCat. The left side or anode is where the oxidation reaction occurs (a loss of electrons) and the right is where the reduction occurs (gaining of electrons).
- Thu Mar 12, 2020 8:25 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Lavelle's review slides
- Replies: 3
- Views: 363
Re: Lavelle's review slides
Pt(s) is an inert metal, so it won't affect the reaction but is necessary to serve as conductor.
- Thu Mar 12, 2020 8:20 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: salt bridge
- Replies: 11
- Views: 663
Re: salt bridge
The salt bridge is essentially meant to neutralize solutions and balance the charges which occur due to the transfer of electrons in a redox reaction.
- Thu Mar 12, 2020 8:11 am
- Forum: Balancing Redox Reactions
- Topic: Determining the oxidizer and reducer
- Replies: 10
- Views: 741
Re: Determining the oxidizer and reducer
Familiarize yourself with the LEO (loss of electron, oxidization) and GER (gains electrons, reduction) acronyms, they help identify what is being reduced and oxidized. From there you also have to know that the species that is being reduced in the 'oxidizing agent' and the one being oxidized is the '...
- Thu Mar 12, 2020 8:07 am
- Forum: Balancing Redox Reactions
- Topic: Oxidation/Reduction
- Replies: 17
- Views: 1170
Re: Oxidation/Reduction
You take a look at the change in oxidation number. The molecule that is being reduce deceased in the oxidation number such as Cu2^+(aq)→ Cu(s), while the species being oxidized increases in the oxidation number Zn(s) → Zn2+(aq) *These examples can be found in RedOx reactions Part 1 sheet with furthe...
- Thu Mar 12, 2020 7:47 am
- Forum: Balancing Redox Reactions
- Topic: Half Reactions
- Replies: 15
- Views: 826
Re: Half Reactions
for half reactions you have to play attention on whether the solution is acidic or basic, this will tell you if you should use H+ and H2O (for acidic solutions) or OH- and H2O (for basic solutions). Start off with adding H2O to the side that needs it to balance out the O atoms. From there, depending...
- Thu Mar 12, 2020 7:36 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Finding k1 when given two temperatues
- Replies: 6
- Views: 479
Re: Finding k1 when given two temperatues
You don't really KNOW which is T1 and K1, you more assign given values. Despite this, whichever you choose to be T1 and K1 must correlate with one another. I usually label the initial temp and k as T1 and K1, then the second temp as T2 to find K2 (the unknown value). As long as you keep the related ...
- Thu Mar 12, 2020 7:22 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Combustion of gas
- Replies: 6
- Views: 544
Re: Combustion of gas
In the case of ∆G° = ∆H° - T∆S°, which indicates standard condition yes. The combustion of gas will be spontaneous due to the negative enthalpy value, the release of heat through an exothermic reaction, and a positive (increasing) entropy.
- Thu Mar 12, 2020 7:11 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: G=-nFe
- Replies: 7
- Views: 831
Re: G=-nFe
You have to look at the number of electrons transferred in the redox reaction. (Essentially, the number of electrons that balances the combined half-reactions.)
- Thu Mar 12, 2020 7:09 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: dilutions and Ecell
- Replies: 16
- Views: 3336
Re: dilutions and Ecell
It is important to note where the dilution is occurring as it will affect cell potential differently if it is in the cathode or anode. You can use E˚(cell) = E˚(cathode) - E˚(anode) to get a better understanding. When the cathode is diluted, cell potential decreases due to the lower cathode. Therefo...
- Thu Mar 12, 2020 7:03 am
- Forum: Phase Changes & Related Calculations
- Topic: delta G0 versus delta G
- Replies: 15
- Views: 2606
Re: delta G0 versus delta G
The "o" indicates standard conditions, (in temperature, pressure and concentration. ∆G° can be used to find ∆G and vice versa in the equation ∆G = ∆G° + RT ln Q
- Thu Jan 30, 2020 4:17 pm
- Forum: Phase Changes & Related Calculations
- Topic: State Functions
- Replies: 9
- Views: 474
Re: State Functions
Enthalpy is considered a state function as it is dependent on initial and final states.
- Thu Jan 30, 2020 4:16 pm
- Forum: Phase Changes & Related Calculations
- Topic: Calculating Work
- Replies: 6
- Views: 220
Re: Calculating Work
w=-P V
V
The equation for work has a negative. If the change in volume is also negative, then work will be positive. If not, then it will be negative.
The equation for work has a negative. If the change in volume is also negative, then work will be positive. If not, then it will be negative.
- Thu Jan 30, 2020 4:13 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Why can't qv equal delta H?
- Replies: 3
- Views: 444
Re: Why can't qv equal delta H?
qv is at constant volume, with changing pressure, while delta H is heat transfer at constant pressure.
- Thu Jan 30, 2020 4:11 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Delta H v. q
- Replies: 5
- Views: 264
Re: Delta H v. q
q refers to the energy, as heat, transferred due to a change in temperature.  is the heat transferred at constant pressure
is the heat transferred at constant pressure
- Thu Jan 30, 2020 4:08 pm
- Forum: Phase Changes & Related Calculations
- Topic: Cv and Cp
- Replies: 9
- Views: 447
Re: Cv and Cp
They are equal in the case of solids and liquids but change for gases due to compression and expansion.
- Thu Jan 30, 2020 4:05 pm
- Forum: Phase Changes & Related Calculations
- Topic: Molar Heat Capacity at Cp s. Cv
- Replies: 4
- Views: 221
Re: Molar Heat Capacity at Cp s. Cv
Both can be used for a gas, however they do not yield the same answer. The p subscript indicates constant pressure while the v is for volume.
- Thu Jan 30, 2020 4:02 pm
- Forum: Phase Changes & Related Calculations
- Topic: Units
- Replies: 16
- Views: 845
Re: Units
0°C= 273.15 K. When calculating change in temp, if you have 283.15K-276.15K or 10°C-3°C, the change in temperature is essentially the same.
- Thu Jan 30, 2020 3:52 pm
- Forum: Phase Changes & Related Calculations
- Topic: 4A. 1 Identifying open and closed system
- Replies: 11
- Views: 2729
Re: 4A. 1 Identifying open and closed system
C is a closed system as the clariometer does not allow the transfer of energy to the suroundings.
- Thu Jan 23, 2020 2:31 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Acids and Bases pka and pkb
- Replies: 8
- Views: 334
Re: Acids and Bases pka and pkb
A lower pKa indicates a stronger acid and a lower pKb is a stronger base, as pKa + pKb = 14
- Thu Jan 23, 2020 2:29 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: ice table
- Replies: 13
- Views: 563
Re: ice table
They aren't included, since you wouldn't include them in the K expression.
- Thu Jan 23, 2020 2:27 pm
- Forum: Ideal Gases
- Topic: ICE table approximation
- Replies: 10
- Views: 408
Re: ICE table approximation
You take a look at the K value. If it is less than 10^-3, then you can approximate. If the K value is 10^-3, it can get a bit tricky, so I would still do the full calculation.
- Thu Jan 23, 2020 2:25 pm
- Forum: Ideal Gases
- Topic: Equilibrium Constant
- Replies: 7
- Views: 265
Re: Equilibrium Constant
Since K=products/reactants. A small K value indicated that the numerator is being divided by a large denominator. So there are more reactants.
- Thu Jan 23, 2020 2:22 pm
- Forum: Ideal Gases
- Topic: PV=nRT
- Replies: 13
- Views: 696
Re: PV=nRT
This in relation to concentration (n/V). It's used mainly to calculate for the unknown variable.
- Thu Jan 23, 2020 2:19 pm
- Forum: Ideal Gases
- Topic: test 1
- Replies: 9
- Views: 423
Re: test 1
No, test one is only for chemical equilibrium and acids and bases
- Thu Jan 23, 2020 2:18 pm
- Forum: Ideal Gases
- Topic: Kc vs Kp
- Replies: 109
- Views: 4797
Re: Kc vs Kp
It depends on what asked and given. If you are calculating molar concentration then you would use Kc.
Re: naming
When it comes to alphabetizing, you look at the beginning of the compound itself, not the prefixes used.
- Thu Dec 05, 2019 5:48 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Ligand Names
- Replies: 2
- Views: 146
Re: Ligand Names
I believe that the ligands on the table are the principle ones that may come up during the final.
- Thu Dec 05, 2019 5:44 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Lone Pairs in this Compound
- Replies: 2
- Views: 193
Re: Lone Pairs in this Compound
The subscript of two shows that there is double of what is inside the parentheses, therefore 2 N. The third one is the N that is on the outside in the HN.
- Thu Dec 05, 2019 5:40 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: chelate
- Replies: 2
- Views: 208
Re: chelate
It also includes bidentate complexes, as chelating ligands refers to those that can make multiple (2 or more) bonds to the central metal atom.
Re: 9C 1A
iron is a naming exception when it comes to adding the -ate suffix, instead of ironate. We use the roman name of ferrum, then add the suffix.
- Thu Dec 05, 2019 5:33 pm
- Forum: Naming
- Topic: Cyanido vs cyano
- Replies: 5
- Views: 334
Cyanido vs cyano
I'm confused on these two terms. The textbook uses the form cyanido, but Lavelle wrote cyano for a naming example in lecture. Can they be used interchangeably, or are they different?
- Thu Dec 05, 2019 5:30 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: -ate
- Replies: 11
- Views: 727
Re: -ate
-ate is used with there is a negative charge on the complex and is added to the end of the TM
- Thu Dec 05, 2019 5:29 pm
- Forum: Naming
- Topic: sodium bisoxalato(diaqua)ferrate(III) (homework 9C.3D)
- Replies: 2
- Views: 219
Re: sodium bisoxalato(diaqua)ferrate(III) (homework 9C.3D)
3. I don't think there is a preference, but the textbook uses OH2 a lot, so i would stick with that format.
- Sat Nov 23, 2019 4:15 pm
- Forum: Hybridization
- Topic: Hybridization with lone pairs on central atom
- Replies: 6
- Views: 464
Re: Hybridization with lone pairs on central atom
Yes since the regions of electron density correlates with the hybridization. 2 regions=sp and so on like without lone pairs.
- Sat Nov 23, 2019 4:08 pm
- Forum: Hybridization
- Topic: hybridization
- Replies: 11
- Views: 571
Re: hybridization
You begin with the Lewis structure that will provide you with the regions of electron density of the center atom. Form their you can determine the hybridization. 2 regions corresponds with 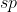 ; 3=
; 3= 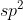 and so on.
and so on.
- Sat Nov 23, 2019 4:05 pm
- Forum: Hybridization
- Topic: 2.57
- Replies: 4
- Views: 409
Re: 2.57
For the right carbon you use 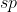 hybridized orbitals while the left carbon uses
hybridized orbitals while the left carbon uses 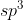
- Sat Nov 23, 2019 4:01 pm
- Forum: Hybridization
- Topic: Hybridization Structure
- Replies: 3
- Views: 257
Re: Hybridization Structure
I was a little confused about that too.
- Sat Nov 23, 2019 3:59 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Problem 3F10 b
- Replies: 6
- Views: 448
Re: Problem 3F10 b
Sydney Myers 4H wrote:Daniel Martinez 1k wrote:Si2F2
Is this due to the shape? because Si2F2 would have a more linear structure, compared with a round structure of SiF4, and higher surface area makes for higher intermolecular forces, specifically London Dispersion Forces.
I believe this question is dependent on polarity, no shape for size.
- Sat Nov 23, 2019 3:57 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: 3F 15
- Replies: 5
- Views: 947
Re: 3F 15
This question has to do with polarity, not size. Since AsF3 is polar, it has stronger dipole-dipole interaction than AsF5, a nonpolar molecule.
- Sat Nov 23, 2019 3:54 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Hydrogen Bonding Rules
- Replies: 6
- Views: 379
Re: Hydrogen Bonding Rules
A hydrogen bond can occur for one lone pair of N,O, and F. Therefore, a nitrogen atom with 2 lone pairs can form 2 hydrogen bonds.
- Sat Nov 23, 2019 3:50 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Homework 3F1
- Replies: 4
- Views: 325
Re: Homework 3F1
The question is asking what are the IMF of each molecule. For H2SeO4, due to its polarity, it has dipole-dipole and induced-induced. On top of that, it can also form hydrogen bonds.
- Sat Nov 23, 2019 3:47 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: london forces
- Replies: 9
- Views: 485
Re: london forces
London forces depends on the size of a molecule. Since larger molecules are more polarizable they form stronger London forces.
- Sat Nov 23, 2019 3:44 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: 3F.5
- Replies: 4
- Views: 303
Re: 3F.5
Butanol has a higher melting point, due to its ability to form hydrogen bonds.
- Sat Nov 23, 2019 3:42 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: dipole-dipole vs induced dipole
- Replies: 9
- Views: 589
Re: dipole-dipole vs induced dipole
Dipole-dipole interactions occur in polar molecules, while an induced dipole has to do with the uneven distribution of charges, resulting form a shift in electrons.
- Sat Nov 23, 2019 3:38 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Boiling Points
- Replies: 9
- Views: 609
Re: Boiling Points
I'm confused between the melting point and the boiling point of a compound? Is this referring to the same thing?
- Sat Nov 23, 2019 3:36 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Hydrogen Bonding Atoms
- Replies: 6
- Views: 323
Re: Hydrogen Bonding Atoms
Hydrogen bonds are formed between H and N,O,F atoms that have lone pairs.
- Sat Nov 23, 2019 3:35 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Best Approach to Find IMFs
- Replies: 11
- Views: 819
Re: Best Approach to Find IMFs
It's good to start off drawing the Lewis Structure. From there you can determine whether the molecule is polar or nonpolar and capable of forming a hydrogen. If a molecule is polar then there is a dipole-dipole interaction. All molecule tend to have a induced-induced interaction.
- Sat Nov 23, 2019 3:31 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: dipole-dipole in a solid phase vs gas phase
- Replies: 15
- Views: 1191
Re: dipole-dipole in a solid phase vs gas phase
The dipole-dipole interaction in the solid phase is stronger
- Fri Nov 01, 2019 1:44 pm
- Forum: Trends in The Periodic Table
- Topic: Calculating Ionization Energy
- Replies: 1
- Views: 136
Calculating Ionization Energy
In my discussion we did a problem that was essentially the following:
Calculate the ionization energy of RB given that a radiation with the wavelength of 58.4nm produces electrons with a velocity of 240km/s, when it hits RB.
Why do we solve for the work function?
Calculate the ionization energy of RB given that a radiation with the wavelength of 58.4nm produces electrons with a velocity of 240km/s, when it hits RB.
Why do we solve for the work function?
- Fri Nov 01, 2019 1:38 pm
- Forum: Trends in The Periodic Table
- Topic: Noble Gases
- Replies: 10
- Views: 692
Re: Noble Gases
The noble gasses do not follow the trends of IE and EA due to their full valence shell which makes them pretty much unreactive.
- Fri Nov 01, 2019 1:35 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: 1D.17
- Replies: 3
- Views: 207
Re: 1D.17
- Fri Nov 01, 2019 1:30 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: D orbital
- Replies: 5
- Views: 228
Re: D orbital
If you take a look at the magnetic quantum numbers 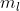 for the d, they are -2, -1, 0, +1, +2, which are 5 in total. Each orbital is able to hold 2 electrons, therefore the d-orbital can hold a total of 10 electrons.
for the d, they are -2, -1, 0, +1, +2, which are 5 in total. Each orbital is able to hold 2 electrons, therefore the d-orbital can hold a total of 10 electrons.
- Fri Nov 01, 2019 1:25 pm
- Forum: DeBroglie Equation
- Topic: Dino Nuggets Problem 8b
- Replies: 11
- Views: 971
Re: Dino Nuggets Problem 8b
I got that far, as to find the Energy of the ejected electron, but how do I cancel the mol^-1 of the work function to be able to add it to the 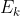 ?
?
- Fri Nov 01, 2019 1:20 pm
- Forum: *Shrodinger Equation
- Topic: shrodinger equation
- Replies: 4
- Views: 284
Re: shrodinger equation
I was confused about this too, since he went over it really quick. The textbook provides a bit more information on it.
- Fri Nov 01, 2019 1:18 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Accessing the E-textbook [ENDORSED]
- Replies: 125
- Views: 32047
Re: Accessing the E-textbook [ENDORSED]
The sampling isn't mandatory but it is highly recommended as it provides you with extra practice and questions that are useful to study for the exams.
- Fri Nov 01, 2019 1:16 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: What's the right equation?
- Replies: 7
- Views: 272
Re: What's the right equation?
I was confused about this too, but its the first equation with the 4pi.
- Fri Nov 01, 2019 1:07 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Electromagnetic Spectrum
- Replies: 3
- Views: 159
Re: Electromagnetic Spectrum
I don't believe we have to know what frequencies correspond to what, but i do think it's good to be familiar with the visible light spectrum. This questions was more based on vaguely knowing the Balmer and Lymen series.
- Fri Nov 01, 2019 1:04 pm
- Forum: *Black Body Radiation
- Topic: Info for Midterm
- Replies: 13
- Views: 1117
Re: Info for Midterm
No this will not be tested, since it was just briefly mentioned.
- Fri Nov 01, 2019 1:01 pm
- Forum: DeBroglie Equation
- Topic: Dino Nuggets Problem 8b
- Replies: 11
- Views: 971
Dino Nuggets Problem 8b
8. B) A newly designed laser pointer with a certain frequency is pointed at a sodium at a sodium metal surface. An electron is ejected from the metal surface with wavelength 1.10nm. What is the frequency of the light from the laser pointer? The work function of sodium is 150.6 kJmol^1. I understand ...
- Fri Oct 18, 2019 6:45 pm
- Forum: *Particle in a Box
- Topic: Particle in A box
- Replies: 8
- Views: 974
Re: Particle in A box
I think it's something that's meant to be more conceptual and help us understand what we are learning, not really something we have to focus a lot on since we didn't have questions on it.
- Fri Oct 18, 2019 6:42 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: heisenberg, calculating kinetic energy
- Replies: 3
- Views: 180
Re: heisenberg, calculating kinetic energy
It could be due because your velocity is not squared.
- Fri Oct 18, 2019 6:38 pm
- Forum: DeBroglie Equation
- Topic: De Broglie's Equation
- Replies: 17
- Views: 619
Re: De Broglie's Equation
A photon does not have mass which is required for the de Broglie's equation where 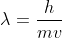
- Fri Oct 18, 2019 6:34 pm
- Forum: Einstein Equation
- Topic: HW 1B. 7
- Replies: 6
- Views: 446
Re: HW 1B. 7
The new formula is derived from solving for the frequency in 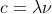 which gives you
which gives you 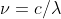 . You can then plug in this new value of
. You can then plug in this new value of 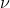 into E=h
into E=h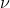 to get
to get 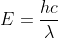
- Fri Oct 18, 2019 6:28 pm
- Forum: Properties of Light
- Topic: Unit for Wavelength
- Replies: 34
- Views: 2461
Re: Unit for Wavelength
Wavelength is measured in meters or other prefixes of meters such as nano and pico. It's easy to remember that units should cancel, c=λν which essentially is (m/s)=m x s^(-1)
- Fri Oct 11, 2019 12:55 pm
- Forum: Properties of Light
- Topic: Clarification on Frequency
- Replies: 8
- Views: 439
Re: Clarification on Frequency
Increasing the intensity of light will not change the frequency of a wave, it will only change the amplitude of the wave.
- Fri Oct 11, 2019 12:50 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Test Equation Sheets
- Replies: 5
- Views: 221
Re: Test Equation Sheets
An equation sheet will be provided during every test, so i don't think you have to worry much about memorizing the equations but more about how to apply them.
- Fri Oct 11, 2019 12:47 pm
- Forum: Properties of Light
- Topic: Electromagnetic Spectrum
- Replies: 5
- Views: 292
Re: Electromagnetic Spectrum
I don't believe we have to memorize the exact ranges, but possibly their placement based on wavelength. I do think you should know the range of visible light because it is common.
- Fri Oct 11, 2019 12:44 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Theoretical vs. Actual Yield
- Replies: 38
- Views: 14053
Re: Theoretical vs. Actual Yield
The actual yield is less than the theoretical yield due to impurities and side reactants. I don't believe it's a concept you'll have to justify in the lab. You just need to familiarize yourself with it.
- Fri Oct 11, 2019 12:39 pm
- Forum: SI Units, Unit Conversions
- Topic: How to express answers
- Replies: 13
- Views: 512
Re: How to express answers
I'm still a little confused as to when I should use scientific notation or not. For example, the textbook solution for Fundamentals E.23 part a is 0.0134 mol Cu^2+, while the solution for E.23 part b is 8.74x10^-3. Why did they decide not to use scientific notation for part a? I've noticed that sci...
- Fri Oct 04, 2019 1:37 pm
- Forum: Limiting Reactant Calculations
- Topic: General Limiting Reactant Question
- Replies: 4
- Views: 272
Re: General Limiting Reactant Question
When there is only one reactant in an equation, there is no limiting reactant. When calculating the limiting reactant of a reaction with multiple reactants, you need to use mass and molar mass. Then you find the theoretical/maximum yield.
- Fri Oct 04, 2019 1:32 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Units in Answer
- Replies: 18
- Views: 855
Re: Units in Answer
The question usually specifies which unit is preferred in the answer. If there is a certain question you are referencing, try posting it verbatim, in order to clear up the confusion.
- Fri Oct 04, 2019 1:28 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: Module: Molarity and Dilution of a solution
- Replies: 5
- Views: 392
Re: Module: Molarity and Dilution of a solution
This question requires the M1V1=M2V2 as it is a dilution problem. The 5.00g is necessary to find the moles of KMnO4. From there, you can calculate the M1, divide your answer by .15L (after unit conversion of mL). The 20.00mL or .02000L is your V1, with the new volume 250mL or .250L as your V2
- Fri Oct 04, 2019 1:17 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: Fundamentals G5
- Replies: 3
- Views: 153
Re: Fundamentals G5
I was confused on that question too, but you have to make sure to do a unit conversion of mmol to mol and to use the M=n/v. This question also required the use of the ratio of Na:Na2CO3, which is 2:1 and CO3:Na2CO3, 1:1.
- Fri Oct 04, 2019 1:11 pm
- Forum: SI Units, Unit Conversions
- Topic: Why do we always need grams when solving a problem?
- Replies: 6
- Views: 591
Re: Why do we always need grams when solving a problem?
The unit conversion is necessary for dimensional analysis. In a calculation that requires the use of a molecule's molecular weight, we cannot simply go from mg to moles. The mg nor the g would cancel out.

