Search found 50 matches
- Sun Dec 08, 2019 11:03 am
- Forum: Polyprotic Acids & Bases
- Topic: Polyproptic Acids/Bases
- Replies: 4
- Views: 418
Polyproptic Acids/Bases
How can you tell which acids and bases are polyproptic? Are there any common examples?
- Sun Dec 08, 2019 10:48 am
- Forum: Biological Examples
- Topic: en and edta
- Replies: 6
- Views: 653
en and edta
For en and EDTA are we only supposed to know that en is bidentate and EDTA is hexadentate?
- Sun Dec 08, 2019 10:44 am
- Forum: Biological Examples
- Topic: Hemoglobin and Myoglobin
- Replies: 4
- Views: 451
Re: Hemoglobin and Myoglobin
Sydney Pell 3E wrote:What is the function of hemoglobin and myoglobin?
Myoglobin transports oxygen in muscle cells ( heme complex + protein -> myoglobin -> Fe binds to oxygen)
Hemoglobin transports O2 in the blood by binding 4 O2
- Sun Dec 08, 2019 10:40 am
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Hydrogen bonding
- Replies: 3
- Views: 300
Hydrogen bonding
To form a hydrogen bond, do both molecules have to have a hydrogen connected to a F, O or N?
- Sun Dec 08, 2019 10:37 am
- Forum: Lewis Acids & Bases
- Topic: Strong Acids vs weak
- Replies: 2
- Views: 337
Strong Acids vs weak
Should we assume acids that aren’t one of the string ones are weak or should we also memorize the neutral ones?
- Sun Dec 01, 2019 8:46 pm
- Forum: Naming
- Topic: -bis, -tris, etc
- Replies: 5
- Views: 457
-bis, -tris, etc
I'm still confused on when we would use -bis, -tris, -tetrakis etc. Are there any examples of this that we'd be more likely to see on the final?
- Sun Dec 01, 2019 8:44 pm
- Forum: Amphoteric Compounds
- Topic: Amphoteric Compound
- Replies: 2
- Views: 244
Amphoteric Compound
What exactly does it mean for a compound to be amphoteric and how can you figure this out?
- Sun Dec 01, 2019 8:39 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Homework question 9C.7
- Replies: 2
- Views: 333
Homework question 9C.7
9C.7 Which of the following isomers of diaminobenzene can form chelating complexes? Explain your reasoning.
How do you know which isomers can form chelating complexes?
How do you know which isomers can form chelating complexes?
- Sun Dec 01, 2019 8:25 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: 9C.1
- Replies: 1
- Views: 198
9C.1
9C.1 Name each of the following complex ions and identify the oxidation number of the metal: (a) [Fe(CN)6]42; (b) [Co(NH3)6]31; (c) [Co(CN)5(OH2)]22; (d) [Co(NH3)5(SO4)]1.
How would we identify the oxidation number for say a?
How would we identify the oxidation number for say a?
- Sun Dec 01, 2019 8:22 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Hydrogen Bonds
- Replies: 2
- Views: 225
Hydrogen Bonds
Why would we consider lone pairs on N or O possible hydrogen bonding sites?
- Sun Nov 24, 2019 11:02 pm
- Forum: Bronsted Acids & Bases
- Topic: Bronsted vs Lewis acids
- Replies: 5
- Views: 364
Re: Bronsted vs Lewis acids
Lewis acids accept electron pairs, meanwhile Bronsted acids donate proton.
- Sun Nov 24, 2019 9:51 pm
- Forum: Biological Examples
- Topic: Cisplatin [ENDORSED]
- Replies: 19
- Views: 1338
Cisplatin [ENDORSED]
What did Dr. Lavelle say cisplatin does to normal, healthy cells?
- Sun Nov 24, 2019 6:48 pm
- Forum: Lewis Acids & Bases
- Topic: HCl vs HF
- Replies: 19
- Views: 1402
HCl vs HF
Why is HCl a stronger acid than HF?
- Sun Nov 24, 2019 6:45 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Chelating Ligand
- Replies: 1
- Views: 132
Chelating Ligand
How do chelating ligands work?
- Sun Nov 24, 2019 6:33 pm
- Forum: Hybridization
- Topic: Hybrid Orbitals
- Replies: 2
- Views: 146
Hybrid Orbitals
How do you determine hybrid orbitals ?
- Sun Nov 17, 2019 10:26 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: size and intermolecular forces
- Replies: 1
- Views: 158
size and intermolecular forces
Why do bigger atoms have stronger intermolecular forces?
- Sun Nov 17, 2019 10:20 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: dipole-induced dipoles
- Replies: 2
- Views: 186
dipole-induced dipoles
When do dipole-induced dipoles occur? Any examples?
- Sun Nov 17, 2019 10:19 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: SF4
- Replies: 3
- Views: 244
SF4
In Friday's lecture, what did Lavelle mean when he said that one of the equatorial (?) positions is where we place the lone pairs? What does that mean, and what does that do in terms of the electrons and the bond angles?
- Sun Nov 17, 2019 10:10 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: CH2Cl2
- Replies: 1
- Views: 146
CH2Cl2
Why is CH2Cl2 polar and not non polar like other tetrahedrals?
- Sun Nov 17, 2019 10:06 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Molecular Shape
- Replies: 3
- Views: 205
Molecular Shape
What's the difference between trigonal pyramidal and t-shape? For example, why is BrF3 t-shape and NH3 trigonal pyramidal?
- Sun Nov 10, 2019 11:04 pm
- Forum: Coordinate Covalent Bonds
- Topic: Coordinate Covalent Bond
- Replies: 3
- Views: 272
Coordinate Covalent Bond
What is a coordinate covalent bond? How are they different from a covalent bond?
- Sun Nov 10, 2019 10:59 pm
- Forum: Dipole Moments
- Topic: Dipole Moments
- Replies: 6
- Views: 374
Re: Dipole Moments
So can dipole moments be temporary or all they all permanent?
- Sun Nov 10, 2019 10:45 pm
- Forum: Bond Lengths & Energies
- Topic: Bond lengths and strength
- Replies: 4
- Views: 187
Bond lengths and strength
Why are double bonds stronger than single bonds?
- Sun Nov 10, 2019 10:40 pm
- Forum: Dipole Moments
- Topic: Dipole Moments
- Replies: 6
- Views: 374
Dipole Moments
What exactly is a dipole moment?
- Sun Nov 10, 2019 10:36 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Polarizability
- Replies: 4
- Views: 359
Polarizability
Why is it that larger atoms have higher polarizability?
- Sun Nov 03, 2019 11:30 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Differences between polarizing power and polarizablity
- Replies: 2
- Views: 157
Differences between polarizing power and polarizablity
What’s the difference between polarizability and polarizing power? And how exactly can you tell who unions have higher polarizing power?
- Sun Nov 03, 2019 11:28 pm
- Forum: Bond Lengths & Energies
- Topic: Short bond lengths vs long bond lengths
- Replies: 6
- Views: 477
Short bond lengths vs long bond lengths
Why are shorter bond lengths stronger than longer bond lengths?
- Sun Nov 03, 2019 11:27 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Formal charge
- Replies: 16
- Views: 935
Formal charge
What if we can’t get all the atoms to have a formal charge of 0? Is there a “second best” option?
- Sun Nov 03, 2019 11:25 pm
- Forum: Dipole Moments
- Topic: Dipole moments on Lewis structures
- Replies: 3
- Views: 269
Dipole moments on Lewis structures
how do we draw dipole moments on Lewis structures?
- Sun Nov 03, 2019 11:24 pm
- Forum: Bond Lengths & Energies
- Topic: Bond lengths
- Replies: 4
- Views: 184
Bond lengths
How do you predict bond lengths? I know Dr. Lavelle showed us an example in lecture, but I’m still confused as to how you’d figure that out.
- Sun Oct 27, 2019 8:40 pm
- Forum: Trends in The Periodic Table
- Topic: Electronegativity vs. Electron affinity
- Replies: 8
- Views: 631
Electronegativity vs. Electron affinity
Is there a difference between electronegativity and electron affinity?
- Sun Oct 27, 2019 8:38 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Chromium and Copper
- Replies: 5
- Views: 422
Chromium and Copper
Why are Chromium and Copper exceptions to the Aufbau principle?
- Sun Oct 27, 2019 8:28 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: 1E.23 (unpaired electrons)
- Replies: 2
- Views: 1490
1E.23 (unpaired electrons)
1E.23. The elements Ga, Ge, As, Se, and Br lie in the same period in the periodic table. Write the electron configuration expected for the ground-state atoms of these elements and predict how many unpaired electrons, if any, each atom has. How exactly do you know how many unpaired electrons an atom ...
- Sun Oct 27, 2019 8:22 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Electron Configurations (3d-orbital and 4s-orbital)
- Replies: 2
- Views: 167
Electron Configurations (3d-orbital and 4s-orbital)
Is there a specific reason we fill the 4s-orbital before the 3d-orbital when doing the electron configurations? And does that mean when we’re taking electrons away, we take them from 4s first?
- Sun Oct 27, 2019 8:21 pm
- Forum: Trends in The Periodic Table
- Topic: Ionization Energy
- Replies: 4
- Views: 243
Ionization Energy
How can you tell which atom has the lowest ionization energy when constructing a Lewis structure? Or just in general?
- Sun Oct 20, 2019 9:39 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Electron configurations of ions
- Replies: 4
- Views: 183
Electron configurations of ions
For ions, how do you know when you need to take away electrons from the configuration, and when you need to add them?
- Sun Oct 20, 2019 9:35 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Uncertainty value in equation
- Replies: 9
- Views: 390
Uncertainty value in equation
For the uncertainty value, you add up the total uncertainty right? So for example, if you have 105 m +/- 3 m, the uncertainty value is 6 m? Or is it 3?
- Sun Oct 20, 2019 5:01 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Rydberg's Constant
- Replies: 3
- Views: 158
Rydberg's Constant
For Rydberg's constant, we should only use the one provided in the equations and constants sheet Dr. Lavelle has on his website, right? And we should stick to E=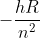 rather than Rydberg's equation?
rather than Rydberg's equation?
- Thu Oct 17, 2019 11:29 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Quantum Numbers
- Replies: 3
- Views: 157
Quantum Numbers
Why is 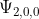 not possible? I did not understand Dr. Lavelle's explanation in lecture.
not possible? I did not understand Dr. Lavelle's explanation in lecture.
- Thu Oct 17, 2019 11:09 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Lyman vs Balmer Series
- Replies: 2
- Views: 137
Lyman vs Balmer Series
How do we know when a line is in either the Lyman Series or the Balmer series? I'm still confused as to how you can tell.
- Sun Oct 13, 2019 11:08 pm
- Forum: Properties of Electrons
- Topic: Quantum Mechanic Description of Atoms
- Replies: 1
- Views: 135
Quantum Mechanic Description of Atoms
Dr. Lavelle's explanation of this towards the end of Friday's lecture confused me. Can someone explain what he meant when he showed the two diagrams ("Allowed Energy Level" vs "Not allowed") and why the Allowed Energy Level diagram is the correct one when talking about electrons?
- Sun Oct 13, 2019 10:57 pm
- Forum: DeBroglie Equation
- Topic: Using the De Broglie equation
- Replies: 1
- Views: 113
Using the De Broglie equation
What did Dr. Lavelle say was the minimum wavelike properties that could be detected?
- Sun Oct 13, 2019 10:55 pm
- Forum: Properties of Light
- Topic: Atomic Spectra
- Replies: 3
- Views: 197
Atomic Spectra
Will it be enough to just memorize that 400nm-700nm is visible light, and anything less than 400nm is UV, and anything above 700nm is infrared? Can we just assume we don't have to worry about the other ones (e.g., x-rays, microwaves, etc.) right now?
- Sun Oct 13, 2019 10:23 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Atomic Spectroscopy
- Replies: 1
- Views: 104
Atomic Spectroscopy
Is there a reason that the distance between n=1 and n=2 is greater than say n=2 and n=3?
- Sun Oct 13, 2019 9:53 pm
- Forum: Properties of Electrons
- Topic: Diffraction
- Replies: 2
- Views: 105
Diffraction
What is the difference between constructive interference and destructive interference?
- Sun Oct 06, 2019 8:02 pm
- Forum: Significant Figures
- Topic: When do zeros count?
- Replies: 5
- Views: 215
When do zeros count?
I'm still unclear as to when zeros are counted in the number of sig figs and when they aren't. Why does 1.00 have 3, but 0.01 only have 1? Can someone explain the difference?
- Sun Oct 06, 2019 7:45 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: M1V1=M2V2
- Replies: 11
- Views: 86733
Re: M1V1=M2V2
You could use this formula in a question like: If you dilute 300. mL of a 4.00 M solution of CuCl_{2} to 900. mL, what is the new concentration of the solution? (from a step-up session) First, you'd use your given to figure out what parts of the equation you already have and then just plugging it in...
- Sun Oct 06, 2019 7:32 pm
- Forum: Significant Figures
- Topic: Number of significant figures
- Replies: 5
- Views: 422
Re: Number of significant figures
It depends on the problem, I think. You should look at what is given in each problem and then use the lowest number of sig figs.
- Sun Oct 06, 2019 7:24 pm
- Forum: Balancing Chemical Reactions
- Topic: Steps of question L35
- Replies: 1
- Views: 109
Steps of question L35
I'm unclear as to why in the solution it goes  \times (\frac{3 mol FeBr_{2}}{1 mol Fe_{3}Br_{8} })...)
More specifically, why do we multiply those two together?
More specifically, why do we multiply those two together?
- Sun Oct 06, 2019 7:05 pm
- Forum: Limiting Reactant Calculations
- Topic: Unclear on Law of Conservation of Mass
- Replies: 1
- Views: 137
Unclear on Law of Conservation of Mass
The Law of Conservation of Mass makes sense to me, but in lecture the example used was 2Na(s) + 2H_{2}O(l) \rightarrow 2NaOH(aq) +H_{2}(g) and he put that it's basically 2 moles of sodium + 2 moles of water = 2 moles of NaOH + 1 mole of hydrogen. Does there not have t...

