Search found 105 matches
- Sat Mar 14, 2020 6:25 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Slow and Fast Step
- Replies: 7
- Views: 546
Re: Slow and Fast Step
Does it matter if the order of the rates? For example if the first step rate is slow and the second step is fast does the slow step control the rate? What happens if the first step is fast and the second step is slow?
- Fri Mar 13, 2020 11:53 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Lavelle's review slides
- Replies: 3
- Views: 288
Re: Lavelle's review slides
Nvm! i just realized that Cl2 is the intermediate and not O2
- Fri Mar 13, 2020 11:51 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Lavelle's review slides
- Replies: 3
- Views: 288
Re: Lavelle's review slides
I am confused on step 2 of student B. The rate limiting reaction rate in step 2 is k 2 [Cl 2 (g)][O 2 (g)] 2 . Since O 2 (g) is an intermediate, we use K1 to replace it. However, I am confused since [O 2 (g)]=K1[ClO 2 ] 2 /[Cl 2 (g)]. How come when [O 2 (g)] in the rate law is replaced with that val...
- Tue Mar 10, 2020 1:14 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: 7D.7
- Replies: 1
- Views: 127
7D.7
For the reversible, one-step reaction A + A \leftrightharpoons B + C, the forward rate constant for the formation of B is 265 L/mol*min and the rate constant for the reverse reaction is 392 L/mol*min. The activation energy for the forward reaction is 39.7 kJ/mol and that of the reverse reaction is 2...
- Mon Mar 09, 2020 8:47 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Homework 7C11
- Replies: 2
- Views: 161
Re: Homework 7C11
For part a of 7C.11 it states: For a reaction with a very large equilibrium constant, the rate constant of the forward reaction is much larger than the rate constant of the reverse reaction.
Why is this true?
Why is this true?
- Mon Mar 09, 2020 8:23 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: 7C.7
- Replies: 1
- Views: 212
7C.7
The following mechanism has been proposed for the reaction between nitric oxide and bromine: Step 1 NO + Br2 --> NOBr2 (slow) Step 2 NOBr2 + NO --> NOBr + NOBr (fast) Write the rate law for the formation of NOBr implied by this mechanism I am unsure how to find the rate law. do we use step 1 or 2 an...
- Sat Mar 07, 2020 6:54 pm
- Forum: First Order Reactions
- Topic: First Order Integrated Rate Laws
- Replies: 1
- Views: 176
First Order Integrated Rate Laws
When the problem asks for the time it takes for a reaction to decay why do we use the equation t= (1/kr)*ln([A]0/[A]t)? Isnt there supposed to be a negative sign if we solve for t using ln([A]0/[A]t)= -krt?
- Sat Mar 07, 2020 5:08 pm
- Forum: General Rate Laws
- Topic: 7A.17
- Replies: 1
- Views: 251
7A.17
The following data were obtained for the reaction A + B + C --> products A B C Initial Rate 1 1.25 1.25 1.25 8.7 2 2.5 1.25 1.25 17.4 3 1.25 3.02 1.25 50.8 4 1.25 3.02 3.75 457 5 3.01 1.00 1.15 ? d) Use the data to predict the reaction rate for Experiment 5. I am having trouble getting the correct a...
- Wed Mar 04, 2020 6:30 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Hw 6N.3 a)
- Replies: 2
- Views: 295
Hw 6N.3 a)
Predict the potential of each of the following cells: a) Pt(s) I H2(g, 1.0 bar) I HCl(aq, 0.075 mol?L21) II HCl(aq, 1.0 mol/L) I H2(g, 1.0 bar) I Pt(s) I am having trouble writing out the reaction and half reactions from the cell diagram. Can someone pls explain the process of using the cell diagram...
- Wed Mar 04, 2020 12:40 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6M.13
- Replies: 1
- Views: 197
6M.13
Identify the reactions with K > 1 in the following list and, for each such reaction, identify the oxidizing agent and calculate the standard cell potential. Does K>1 indicate that there are more products than reactants so the reaction should favor products? How do I determine whether the reaction fa...
- Wed Mar 04, 2020 11:35 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6M.11
- Replies: 1
- Views: 217
6M.11
Suppose that each of the following pairs of redox couples is combined to form a galvanic cell that generates a current under standard conditions. Identify the oxidizing agent and the reducing agent, write a cell diagram, and calculate the standard cell potential from the standard potentials of the e...
- Sun Mar 01, 2020 8:07 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6M.5
- Replies: 4
- Views: 365
6M.5
For each reaction that is spontaneous under standard conditions (that is, K > 1), write a cell diagram, determine the standard cell potential, and calculate \Delta G ^{\circ} for the reaction: a) 2NO3 - (aq) + 8H + (aq) + 6Hg(l) --> 3Hg2 2+ (aq) + 2NO(g) + 4H2O(l) I am unsure how to split the equati...
- Sat Feb 29, 2020 4:38 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Self Check 6M.1A
- Replies: 1
- Views: 120
Self Check 6M.1A
Which is the stronger reducing agent for species in aqueous solution, lead or aluminum under standard conditions? (a) Evaluate the standard potential of the appropriate cell; (b) write the net ionic equation for the spontaneous reaction; (c) state your answer to the question. I am confused and do no...
- Mon Feb 24, 2020 11:32 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6L.5 c
- Replies: 1
- Views: 157
6L.5 c
Write the half-reactions, the balanced equation for the cell reaction, and the cell diagram for each of the following skeletal equations: c) Cl2(g) + H2(g) ---> HCl(aq) I am confused on writing the cell diagram. How do we know to add an inert electrode? I am also confused in the order that we should...
- Mon Feb 24, 2020 8:37 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6L.3 b
- Replies: 1
- Views: 157
6L.3 b
Write the half-reactions and the balanced equation for the cell reaction for each of the following galvanic cells: b) C(gr) I H2(g) I H+(aq) II Cl-(aq) I Cl2(g) I Pt(s) To find the half reaction, do we ignore C(gr) and Pt(s) and only H2(g) I H+ and Cl-(aq) I Cl2(g)? If so why do we not use C(gr) or ...
- Sun Feb 23, 2020 7:41 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6L.7
- Replies: 2
- Views: 261
6L.7
Write the half-reactions and devise a galvanic cell (write a cell diagram) to study each of the following reactions: (a) AgBr(s) \rightleftharpoons Ag1(aq) + Br2(aq), a solubility equilibrium (b) H1(aq) + OH - (aq) \rightleftharpoons H2O(l), the Brønsted I am having trouble solving this problem. How...
- Sun Feb 23, 2020 7:28 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Self Test 6L.2A
- Replies: 1
- Views: 270
Self Test 6L.2A
Write the diagram for a cell with a hydrogen electrode on the left and an iron(III)/iron(II) electrode on the right. The two electrode compartments are connected by a salt bridge, and platinum is used as the conductor at each electrode. I am unsure how to approach this problem. Can someone explain s...
- Sat Feb 22, 2020 6:45 pm
- Forum: Balancing Redox Reactions
- Topic: Reducing agent/oxidizing agent
- Replies: 5
- Views: 406
Reducing agent/oxidizing agent
What is the method for determining whether a reactant is a reducing or an oxidizing agent? I am having trouble determining the oxidation number. For the hw problems I determined whether the reactant is an oxidizing or reducing agent by writing the half reactions and seeing which side the electrons a...
- Sat Feb 22, 2020 4:55 pm
- Forum: Balancing Redox Reactions
- Topic: Hw problem 6k.5 b)
- Replies: 2
- Views: 240
Hw problem 6k.5 b)
Balance each of the following skeletal equations by using oxidation and reduction half-reactions. All the reactions take place in basic solution. Identify the oxidizing agent and reducing agent in each reaction. b) Br2(l) ---> BrO3 - (aq) + Br - (aq) For this problem, I am unsure how to find the hal...
- Thu Feb 20, 2020 12:15 am
- Forum: Balancing Redox Reactions
- Topic: 6K.3 Part c
- Replies: 1
- Views: 178
6K.3 Part c
Balance each of the following skeletal equations by using oxidation and reduction half-reactions. All the reactions take place in acidic solution. Identify the oxidizing agent and reducing agent in each reaction. c) reaction of chlorine in water: Cl2(g) ---> HClO(aq) + Cl2(aq) I am unsure how to sol...
- Sat Feb 15, 2020 9:59 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Hw 5G.19
- Replies: 1
- Views: 171
Hw 5G.19
Calculate the standard Gibbs free energy of each of the following reactions:
(b) Ag2CrO4(s)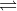 2 Ag+ (aq) + CrO42-(aq), K = 1.1 x 10^12 at 298 K
2 Ag+ (aq) + CrO42-(aq), K = 1.1 x 10^12 at 298 K
I am having trouble getting the right answer for part b. Can someone explain how to solve this step by step?
(b) Ag2CrO4(s)
I am having trouble getting the right answer for part b. Can someone explain how to solve this step by step?
- Wed Feb 12, 2020 10:46 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: What to exclude in K
- Replies: 9
- Views: 623
Re: What to exclude in K
Yes, if H2O is denoted as a gas then you would include it in the calculation for K
- Tue Feb 11, 2020 2:46 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Change in enthalpy (4C.3)
- Replies: 1
- Views: 192
Change in enthalpy (4C.3)
Calculate the final temperature and the change in enthalpy when 765 J of energy is transferred as heat to 0.820 mol Kr(g) at 298 K and 1.00 atm (a) at constant pressure; (b) at constant volume. Treat the gas as ideal. I know how to find the final temperature but I am having trouble finding the chang...
- Mon Feb 10, 2020 10:08 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5.61
- Replies: 1
- Views: 161
5.61
The overall photosynthesis reaction is 6 CO2(g) + 6 H2O(l) --> C6H12O6(aq) + 6 O2(g) State the effect that each of the following changes will have on the equilibrium composition: b) The system is compressed I am having trouble understanding this problem. At first I thought the reaction will shift to...
- Mon Feb 10, 2020 9:24 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Hw 5.39
- Replies: 1
- Views: 147
Hw 5.39
In an experiment, 0.020 mol NO2 was introduced into a flask of volume 1.00 L and the reaction 2 NO2(g) Δ N2O4(g) was allowed to come to equilibrium at 298 K. (a) Using information in Table 5E.2, calculate the equilibrium concentrations of the two gases. (b) The volume of the flask is reduced to half...
- Sat Feb 08, 2020 11:06 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: 4J.5 Part c
- Replies: 2
- Views: 163
4J.5 Part c
Write a balanced chemical equation for the formation reaction of (c) CO(g)
I am confused as to how to know that the chemical equation for CO(g) is C(s, graphite) + (1/2)O2 --> CO(g)
How do we know C should be solid graphite? why can't it be C(g)?
I am confused as to how to know that the chemical equation for CO(g) is C(s, graphite) + (1/2)O2 --> CO(g)
How do we know C should be solid graphite? why can't it be C(g)?
- Sat Feb 08, 2020 9:52 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: 4J.1
- Replies: 1
- Views: 93
4J.1
Why are so many exothermic reactions spontaneous?
Looking at the equation G =
G =  H - T
H - T S, in order for a reaction to be spontaneous,
S, in order for a reaction to be spontaneous,  G must be negative. What does an exothermic reaction tell us about the enthalpy change or entropy change?
G must be negative. What does an exothermic reaction tell us about the enthalpy change or entropy change?
Looking at the equation
- Sat Feb 08, 2020 11:55 am
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: 4H.7
- Replies: 2
- Views: 140
4H.7
For the reaction Cl2(g) + H2O(l) ---> HCl(aq) + HClO(aq) is there an increase or decrease in entropy?
I am confused as to why there is an increase in entropy. How do aqueous compare in entropy to solids, gases and liquids?
I am confused as to why there is an increase in entropy. How do aqueous compare in entropy to solids, gases and liquids?
- Tue Feb 04, 2020 8:55 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 4E.7
- Replies: 2
- Views: 152
4E.7
Use the data in Tables 4E.2 and 4E.3 to estimate the reaction enthalpy for
b) CH3CHCH2(g) + H2O(g) --> CH3CH(OH)CH3(g)
I am really confused on how to determine which bonds are broken/formed. I looked at all the bonds but the solution only used a few. how do we know which ones to use?
b) CH3CHCH2(g) + H2O(g) --> CH3CH(OH)CH3(g)
I am really confused on how to determine which bonds are broken/formed. I looked at all the bonds but the solution only used a few. how do we know which ones to use?
- Tue Feb 04, 2020 7:56 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 4E.5
- Replies: 1
- Views: 99
4E.5
Use the bond enthalpies in Tables 4E.2 and 4E.3 to estimate the reaction enthalpy for a) 3C 2 H 2 (g) --> C 6 H 6 (g) I am confused on how to solve this problem. For the reactants the solution says there are 3 moles of (C \equiv C) and for the reactants there are 6 moles of (C---C). How come you do ...
- Mon Feb 03, 2020 8:14 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: HW 4D.9
- Replies: 2
- Views: 164
Re: HW 4D.9
The enthalpy of the reaction was found to be -13168.48 KJ/mol. How come the answer is not negative?
- Sun Feb 02, 2020 7:25 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Textbook question 4C.3
- Replies: 3
- Views: 175
Re: Textbook question 4C.3
How would you find the change in enthalpy? Would you use the equation Cp= H/
H/  T and solve for
T and solve for  H?
H?
- Sun Feb 02, 2020 6:03 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: 4B.5
- Replies: 1
- Views: 92
4B.5
An ideal gas in a cylinder was placed in a heater and gained 5.50 kJ of energy as heat. If the cylinder increased in volume from 345 mL to 1846 mL against an atmospheric pressure of 750. Torr during this process, what is the change in internal energy of the gas in the cylinder? I know to solve this ...
- Sun Feb 02, 2020 5:10 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Self test 4B.3A
- Replies: 1
- Views: 101
Self test 4B.3A
Estimate the contribution of motion to the molar internal energy of water vapor at 25 C.
I am having trouble getting the write answer which is 7.44 kJ/mol. Is water vapor translational, rotational and linear or rotational and nonlinear?
I am having trouble getting the write answer which is 7.44 kJ/mol. Is water vapor translational, rotational and linear or rotational and nonlinear?
- Sun Feb 02, 2020 2:57 pm
- Forum: Calculating Work of Expansion
- Topic: Hw 4A.13
- Replies: 2
- Views: 117
Hw 4A.13
A constant-volume calorimeter was calibrated by carrying out a reaction known to release 3.50 kJ of heat in 0.200 L of solution in the calorimeter (q 5 23.50 kJ), resulting in a temperature rise of 7.32 8C. In a subsequent experiment, 100.0 mL of 0.200 m HBr(aq) and 100.0 mL of 0.200 m KOH(aq) were ...
- Sun Feb 02, 2020 1:44 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Extensive vs. intensive property
- Replies: 3
- Views: 205
Extensive vs. intensive property
What is the difference between and extensive and intensive property? What are some examples of the two? For example, why is heat capacity an extensive property?
- Tue Jan 21, 2020 11:41 pm
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: 6D.15 part b
- Replies: 3
- Views: 274
6D.15 part b
Calculate the pH of (b) 0.055 M AlCl3(aq) I am really confused on how to solve this problem. I looked at the solutions to see how they solved it and the chemical reaction was Al(H20) 6 + H2O \rightleftharpoons H30+ + Al(H2O) 5 OH2 2+ How did they get this? Where did H2O come from and what happened t...
- Tue Jan 21, 2020 10:51 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6D. 13
- Replies: 2
- Views: 137
Re: 6D. 13
How do you tell whether it is an acid or a base? And how do you tell whether it is a strong or weak acid?
- Tue Jan 21, 2020 10:29 pm
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: Hw 6D.9
- Replies: 1
- Views: 324
Hw 6D.9
The percentage deprotonation of benzoic acid in a 0.110 m solution is 2.4%. What is the pH of the solution and the Ka of benzoic acid? To solve this problem first I found the concentration of [H3O+] which is (0.024*0.110)= 2.6x10^-3 mol/L and then the pH= -log (2.6x10^-3)= 2.58. However, I am confus...
- Mon Jan 20, 2020 5:32 pm
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: Self Check 6D.4A
- Replies: 1
- Views: 238
Self Check 6D.4A
Use Tables 6D.1 and 6D.2 to decide whether aqueous solutions of the salts (a) Ba(NO2)2, (b) CrCl3, and (c) NH4NO3 are acidic, neutral, or basic.
I am confused on how to solve this problem. How do you know whether the solution of the salts will be acidic, neutral or basic? Thank you!
I am confused on how to solve this problem. How do you know whether the solution of the salts will be acidic, neutral or basic? Thank you!
- Mon Jan 20, 2020 12:02 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Hw 6C.1
- Replies: 2
- Views: 118
Hw 6C.1
Write (a) the chemical equation for the proton transfer equilibrium in water and the corresponding expression for Ka and (b) the chemical equation for the proton transfer equilibrium of the conjugate base and the corresponding expression for Kb for each of the following weak acids: (iii) C6H5OH For ...
- Sat Jan 18, 2020 9:54 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Decreasing pressure
- Replies: 4
- Views: 172
Decreasing pressure
When you decrease the total pressure of the total equilibrium reaction, does the reaction shift towards the side with more or less moles? why?
- Fri Jan 17, 2020 11:50 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6B.11 Part b
- Replies: 1
- Views: 44
6B.11 Part b
A student added solid Na2O to a volumetric flask of volume 200.0 mL, which was then filled with water, resulting in 200.0 mL of NaOH solution. Then 5.00 mL of the solution was transferred to another volumetric flask and diluted to 500.0 mL. The pH of the diluted solution is 13.25 b) What mass of Na2...
- Wed Jan 15, 2020 2:36 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6B.5
- Replies: 1
- Views: 85
6B.5
Calculate the pH and pOH of each of the following aqueous solutions of a strong acid or base:
c) 0.0092 M Ba(OH)2(aq)
In the answer key they multiplied [Ba(OH)2] by 2 and calculated pOH as -log(2*0.0092)
Why do we have to multiply by 2?
c) 0.0092 M Ba(OH)2(aq)
In the answer key they multiplied [Ba(OH)2] by 2 and calculated pOH as -log(2*0.0092)
Why do we have to multiply by 2?
- Tue Jan 14, 2020 8:29 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: 5I.29
- Replies: 2
- Views: 181
5I.29
At 25 C, K 5 3.2 x 10^-34 for the reaction 2HCl(g) \rightleftharpoons H2(g) + Cl2(g). If a reaction vessel of volume 1.0 L is filled with HCl at 0.22 bar, what are the equilibrium partial pressures of HCl, H2, and Cl2? In the answer key, they said to assume that x << 0.22. Why do they do this?
- Tue Jan 14, 2020 7:16 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Hw 5J.11
- Replies: 2
- Views: 119
Hw 5J.11
Predict whether each of the following equilibria will shift toward products or reactants with a temperature increase: a) N2O4(g) \rightleftharpoons 2NO2(g), \Delta H°= +57 kJ c) Ni(s) + 4CO(g) \rightleftharpoons Ni(CO)4(g), \Delta H° -161 kJ I am confused on how to determine whether the reaction is ...
- Sat Jan 11, 2020 2:05 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Table 5G.2
- Replies: 5
- Views: 155
Table 5G.2
In the table, it gives us both the k and kc values for the reactions. What is the difference between the two and how do we know which one to use? For example in problem 5I.13 how do we know whether to use K or Kc when finding the reaction composition?
- Fri Jan 10, 2020 10:53 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Hw 5I.1
- Replies: 4
- Views: 222
Hw 5I.1
At 500. K, the equilibrium constant for the reaction Cl2(g) + Br2(g) \rightleftharpoons 2 BrCl(g) is Kc 5 0.031. If the equilibrium composition is 0.495 mol/L Cl2 and 0.145 mol/L BrCl, what is the equilibrium molar concentration of Br2? I am unsure how to solve this problem. Do I create the equilibr...
- Wed Jan 08, 2020 9:27 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5G.11
- Replies: 2
- Views: 113
5G.11
Write the reaction quotient Q for (a) 2 BCl3(g) + 2 Hg(l) \rightleftharpoons B2Cl4(s) + Hg2Cl2(s) At first I got Q= \frac{1}{[BCl_{3}]^2[H_{2}]^2} but the answer is actually \frac{1}{(P_{BCl_{3}})^2} How come Hg is not included? Also how do you know whether to use concentration or partial pr...
- Wed Jan 08, 2020 11:12 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Chemical Equilibrium Part 2 #19
- Replies: 2
- Views: 171
Chemical Equilibrium Part 2 #19
Edit: Sorry i wrote the wrong problem. Here is number 19 Calculate the reaction quotient, Qc, from . the following equilibrium data collected in a 3.00 L sealed reaction vessel for the reaction: 2AsH 3 (g) \rightleftharpoons 2As(s) + 3H 2 (g) AsH 3 = 5.55x10^-4 As= 3.31x10^-3 Hs= 1.23x10^-3 I calcul...
- Tue Jan 07, 2020 10:16 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Chemical Equilibrium Part 2 Post Module #12
- Replies: 2
- Views: 181
Chemical Equilibrium Part 2 Post Module #12
Consider the following reaction at 1200 K, for which you know Kc = 1.7 x 10-3. Br2 (g) ⇌ 2 Br (g) Your experimental setup is able to measure the equilibrium concentration of Br2 based on its color, but you are unable to measure the concentration of Br directly. If you measure at equilibrium [Br2] to...
- Fri Dec 06, 2019 12:54 am
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: 6C.19
- Replies: 3
- Views: 195
6C.19
Which is the stronger base, the hypobromite ion, BrO2, or morphine, C17H19O3N? Justify your answer.
I am unsure of how to approach this problem. Pls provide a detailed explanation thanks!
I am unsure of how to approach this problem. Pls provide a detailed explanation thanks!
- Fri Dec 06, 2019 12:45 am
- Forum: Polyprotic Acids & Bases
- Topic: Electron withdrawing
- Replies: 1
- Views: 166
Electron withdrawing
Why does electron withdrawing increase the acidity?
- Thu Dec 05, 2019 11:29 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: 6C. 19 f)
- Replies: 1
- Views: 72
6C. 19 f)
Decide which acid in each of the following pairs is the stronger and explain why: f) H2CO3 or H2GeO3 Initially I thought H2GeO3 was the stronger acid since Ge and C are both in the same group, I thought you would choose the one that has the weaker bonds. However, the answer key says H2CO3 is the str...
- Thu Dec 05, 2019 10:51 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Strong vs. weak acids and bases
- Replies: 3
- Views: 332
Strong vs. weak acids and bases
In the textbook, it states that "Acid strengths of binary acids across a period correlate with electron affinities; acid strengths down a group correlate with bond strength."
What does this mean? I am having trouble understanding this
What does this mean? I am having trouble understanding this
- Thu Dec 05, 2019 5:42 pm
- Forum: Conjugate Acids & Bases
- Topic: 6A.4
- Replies: 1
- Views: 549
6A.4
Write the chemical equations for the proton transfer equilibria of the following bases in aqueous solution and identify the
conjugate acid–base pairs in each case: CO(NH2)2, urea
I am having trouble writing out the chemical equation for urea and water. How does urea lose an H?
conjugate acid–base pairs in each case: CO(NH2)2, urea
I am having trouble writing out the chemical equation for urea and water. How does urea lose an H?
- Tue Dec 03, 2019 9:17 pm
- Forum: Bronsted Acids & Bases
- Topic: J.9 calcium hydroxide and bromous acid
- Replies: 1
- Views: 276
J.9 calcium hydroxide and bromous acid
Identify the salt that is produced from the acid–base neutralization reaction between: calcium hydroxide and bromous acid
I am having trouble figuring out the solution to this problem.
What is the complete ionic equation (the 6th edition solutions manual doesn't show the answer to this problem)
I am having trouble figuring out the solution to this problem.
What is the complete ionic equation (the 6th edition solutions manual doesn't show the answer to this problem)
- Tue Dec 03, 2019 8:18 pm
- Forum: Bronsted Acids & Bases
- Topic: J.7
- Replies: 1
- Views: 157
J.7
Select an acid and a base for a neutralization reaction that results in the formation: d) potassium phosphate For this problem I got H3PO4(aq) + 3KOH(aq) --> K3PO4(aq) + 3H2O(l) but the solutions writes the answer as 3KOH(aq) + H3PO4(aq) --> K3PO4(aq) + 3H2O(l) Does it matter what oder you write the...
- Tue Nov 26, 2019 4:35 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: HW Question 9C.5
- Replies: 4
- Views: 308
Re: HW Question 9C.5
Just to clarify, even though oxalate has 4 oxygen with lone pairs, oxalate can only bind through 2 oxygen. Is it because oxalate has two double bonds between O and C so only the single bonds count?
- Tue Nov 26, 2019 3:28 pm
- Forum: Naming
- Topic: Polydentate
- Replies: 4
- Views: 200
Polydentate
Problem 9C.5 asks: Which of the following ligands can be polydentate? If the ligand can be polydentate, give the maximum number of places on
the ligand that can bind simultaneously to a single metal center: (a) HN(CH2CH2NH2)2
I am unsure how to approach this problem. What is a polydentate?
the ligand that can bind simultaneously to a single metal center: (a) HN(CH2CH2NH2)2
I am unsure how to approach this problem. What is a polydentate?
9C.3
Use the information in Table 9C.1 to write the formula for each of the following coordination compounds: d) sodium bisoxalato(diaqua)ferrate(III) For this problem I got Na[Fe(C2O4)2(H2O)2] but the correct answer is Na[Fe(H2O)2(C2O4)2]. Why is this the correct order? I know it has something to do wit...
9C.1 c)
For problem 9C.1 it asks you to name each of the following complex ions and determine the oxidation number of the metal: c) [Co(CN) 5 (OH 2 )] 2- I found the oxidation number of cobalt to be +3 but I am confused about the naming of the complex. At first I put aquapentacyanidocobalt(III) ion but the ...
- Mon Nov 25, 2019 7:35 pm
- Forum: Hybridization
- Topic: 2F.15
- Replies: 3
- Views: 182
2F.15
Noting that the bond angle of an sp 3 hybridized atom is 109.5 8 and that of an sp 2 hybridized atom is 120 8 , do you expect the bond angle between two hybrid orbitals to increase or decrease as the s-character of the hybrids is increased? I am confused on what it means for an s-character to increa...
- Thu Nov 21, 2019 1:38 pm
- Forum: Dipole Moments
- Topic: Lewis Structure and Polarity
- Replies: 3
- Views: 262
Lewis Structure and Polarity
2E.27 asks you to predict whether C5H5N is polar or nonpolar. I started by drawing the lewis structure but it was different than the solution. I drew the 5 carbons connected together with alternating single and double bonds except for one carbon which has single bonds with the two C and one double b...
- Wed Nov 20, 2019 11:24 pm
- Forum: Lewis Structures
- Topic: I3- Lewis Structure
- Replies: 2
- Views: 284
I3- Lewis Structure
The solutions says the lewis structure should have the I connected by single bonds, all with 3 lone pairs. However, I drew the Lewis structure as three I in a linear line connected by a triple bond with the two I on the sides with two lone pairs and the I in the middle with one lone pair. Is this al...
- Wed Nov 20, 2019 8:46 pm
- Forum: Dipole Moments
- Topic: Cancelling Dipole Moments
- Replies: 4
- Views: 374
Re: Cancelling Dipole Moments
CH2Cl2 is a tetrahedral so the atoms are not directly opposite of each other. For example, you would think that the H are on opposite sides which would cancel out the dipole moment. However, since in a tetrahedral, the angle between the atoms are 109.5 degrees, no atom would be directly opposite to ...
- Wed Nov 20, 2019 5:01 pm
- Forum: Dipole Moments
- Topic: Hydrogen Bonds
- Replies: 2
- Views: 109
Hydrogen Bonds
I am a bit confused on determining whether a molecule forms hydrogen bonds. I know it only occurs when the hydrogen is attached to either N, O, or F. What are the other requirements? For example, hydrogen bonds between different molecules. Can the H only bond to N,O,F on other molecules? Or can it b...
- Wed Nov 20, 2019 4:33 pm
- Forum: Dipole Moments
- Topic: 3F.5
- Replies: 2
- Views: 120
3F.5
Suggest, giving reasons, which substance in each of the following pairs is likely to have the higher normal melting point: CHI3 or CHF3. The solution says CHI3 will have the higher melting point since it has larger london forces. However, I am unsure why. How do you determine which has the stronger ...
- Thu Nov 14, 2019 5:12 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: 2E.25
- Replies: 2
- Views: 115
2E.25
Draw the Lewis structure and predict whether each of the following molecules is polar or nonpolar (d) SF4
I drew the Lewis structure which had S as the central atom and 4 F single bonded to S. There is also a lone pair on S. How do I know if this is polar or nonpolar?
I drew the Lewis structure which had S as the central atom and 4 F single bonded to S. There is also a lone pair on S. How do I know if this is polar or nonpolar?
- Thu Nov 14, 2019 12:09 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Difference between molecular shape
- Replies: 3
- Views: 305
Difference between molecular shape
What is the difference between trigonal pyramidal and T-shaped? Similarly, what is the difference between seesaw and tetrahedral? I keep choosing one but the answer is actually the other.
- Wed Nov 13, 2019 11:31 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: 2E.11
- Replies: 1
- Views: 125
2E.11
Use Lewis structures and the VSEPR model to give the VSEPR formula for each of the following species and predict its shape: (b) iodine trichloride I am confused on why the shape of iodine trichloride is t-shaped. Initially I thought it would be a trigonal pyramidal but the solution says that the nam...
- Wed Nov 13, 2019 8:21 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Self Check 2E.3A
- Replies: 2
- Views: 123
Self Check 2E.3A
The problem asks you to give the electron arrangement for NH3 and its shape. How do you determine electron arrangement and how does it differ from molecular shape?
- Tue Nov 12, 2019 10:16 pm
- Forum: Dipole Moments
- Topic: 3F.5
- Replies: 6
- Views: 254
Re: 3F.5
How do you determine that buthanol has h-bonds? How come diethyl ether doesn't have h bonds?
- Tue Nov 05, 2019 11:42 pm
- Forum: Electronegativity
- Topic: How to compare electronegativity difference
- Replies: 2
- Views: 290
Re: How to compare electronegativity difference
Electronegativity increases as you move to the right and up the periodic table. For CH4 and CF4, we can compare the electronegativity of H and F. Since F is further to the right than H, its electronegativity is higher (also keep in mind F is known to have a really high electronegativity). Since for ...
- Tue Nov 05, 2019 11:24 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Polarizing Strength of Cations
- Replies: 8
- Views: 353
Re: Polarizing Strength of Cations
From my understanding, I think smaller cations have higher polarizing powers since they have less valence electrons to block the pull of the nucleus. Therefore, when a small cation is next to another atom, it can exert a stronger pull on the neighboring atom's electrons.
- Tue Nov 05, 2019 11:19 pm
- Forum: Empirical & Molecular Formulas
- Topic: Number 1 in Dino Nuggets
- Replies: 3
- Views: 315
Re: Number 1 in Dino Nuggets
First find the moles of C by dividing the grams of CO2 by the molar mass. (0.561g CO2)/(44.01 g/mol) which will give you the moles of CO2. However, you are interested in the moles of C. Since there is (1 mol of C)/(1 mol of CO2), you multiply this by the moles of CO2 to get 0.0127 mol C. Similarly, ...
- Tue Nov 05, 2019 11:02 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: Midterm Review Problem 5
- Replies: 1
- Views: 261
Re: Midterm Review Problem 5
First I would find the mol of KMnO4 by dividing the grams (5.00g) by molar mass (158.04g/mol). Then I would find the molarity (M=mol/L) but before I use this equation, I first have to convert 150.00 mL to L. After plugging the values into mol/L, I have the initial Molarity. Next I will use the equat...
- Sun Nov 03, 2019 2:57 pm
- Forum: DeBroglie Equation
- Topic: Dino Nugget Problem 8c
- Replies: 1
- Views: 157
Dino Nugget Problem 8c
Hello I'm having trouble with 8c which asks if students can see the laser pointer color. First I know you have to use the frequency of the laser (which we found in part a to be 6.50 x 10^33 Hz) to find the wavelength. Using the equation I got 4.62 x 10^-26 m. For visible light , the wavelength falls...
- Sat Nov 02, 2019 6:54 pm
- Forum: DeBroglie Equation
- Topic: Dino Nuggets Problem 8b
- Replies: 11
- Views: 971
Re: Dino Nuggets Problem 8b
Hello I'm having trouble understanding the next part of the problem which asks if students can see the laser pointer color. First I know you have to use the frequency of the laser to find the wavelength. Using the equation \lambda =c/\gamma I got 4.62 x 10^-26 m. For visible light , the wavelength f...
- Wed Oct 30, 2019 11:56 pm
- Forum: Octet Exceptions
- Topic: 2C.5
- Replies: 4
- Views: 332
2C.5
This problem asks us to draw the lewis structure for the reactive species. I am having trouble understanding the lewis structure for chlorine nitrate (ClONO2). In the solution, N has a double bond with O, a single bond with another O and another single bond with another O. One of the O with a single...
- Wed Oct 30, 2019 11:00 pm
- Forum: Octet Exceptions
- Topic: Octet Rule Exception (More than 8 electrons)
- Replies: 2
- Views: 139
Octet Rule Exception (More than 8 electrons)
I am confused on the exception to the octet rule. In the text book it says that only p block atoms in period 3 or later periods can expand their valence shells. Why is this the exception? For example, phosphorous is in period 3 but does not have any electrons in the d orbital. How can it expand the ...
- Sun Oct 27, 2019 9:17 pm
- Forum: Ionic & Covalent Bonds
- Topic: 2B.5
- Replies: 1
- Views: 135
2B.5
For part a of this problem, it asks to draw the lewis structure for tetrahydridoborate ion, BH 4 - . I started by counting how many valence electrons there are, which is 3 from B, 4(1) and one extra electron because of the oxidation number so the total number of valence electrons is 8. I think the a...
- Sun Oct 27, 2019 4:13 pm
- Forum: Ionic & Covalent Bonds
- Topic: 2A.23
- Replies: 5
- Views: 193
Re: 2A.23
I'm confused on part d of the problem. Why is hydrogen telluride H2Te? I don't know how to get this since i thought hydrogen would want to gain an electron so it would also be an anion?
- Sun Oct 27, 2019 12:52 pm
- Forum: Ionic & Covalent Bonds
- Topic: 2A.15
- Replies: 4
- Views: 200
2A.15
For problem 2A.15 it asks us to write the most likely charge for the ions formed by each of the following elements. I am having trouble understanding why for Ga, the most likely charge is 3+. I initially thought it would be 1+ since it would lose the one electron in the 4p orbital. Why does it prefe...
- Wed Oct 23, 2019 10:51 pm
- Forum: Trends in The Periodic Table
- Topic: Electron Affinity
- Replies: 2
- Views: 488
Electron Affinity
In the self check problem 1F.3a, it asks to account for the large decrease in electron affinity between lithium and beryllium. I'm not sure which one has the higher electron affinity. I'm confused on how to tell which atom has a higher or lower affinity and what an electron affinity tells us.
- Wed Oct 23, 2019 9:09 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Order of electron configuration
- Replies: 6
- Views: 264
Order of electron configuration
Hello! I'm confused on how to correctly write electron configurations. For example, in the homework problem 1E.23, it asks for the electron configuration of Ga, Ge, As, Se and Br. For Ga, I wrote the electron configuration as [Ar] 4s 2 3d 10 4p 1 . However, in the solution manual, they wrote it as [...
- Wed Oct 23, 2019 9:03 pm
- Forum: Photoelectric Effect
- Topic: Hw Help 1B.15
- Replies: 2
- Views: 153
Re: Hw Help 1B.15
Hello! To start, they gave us velocity as 3.6x10 3 km/s which we can convert to 3.6x10 6 m/s. a) Asks for the wavelength so we can use the formula wavelength=h/p where h is Planck's constant 6.626x10^-34 Js and p is mass x velocity (mass of an electron is 9.1x10 -31 and the velocity is given). This ...
- Wed Oct 23, 2019 10:37 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: 1E.13
- Replies: 2
- Views: 119
1E.13
Write the ground-state electron configuration for silver. What I got initially was [Kr] 5s24d9.
However, the answer is actually [Kr] 4d105s. Can someone explain why.
However, the answer is actually [Kr] 4d105s. Can someone explain why.
- Tue Oct 22, 2019 2:43 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: 1D.13
- Replies: 3
- Views: 225
1D.13
How many values of ml are allowed for an electron in a 6d-subshell?
I am not sure how to determine how many values of ml
I am not sure how to determine how many values of ml
- Wed Oct 16, 2019 11:08 pm
- Forum: DeBroglie Equation
- Topic: 1B15
- Replies: 3
- Views: 232
1B15
The velocity of an electron that is emitted from a metallic surface by a photon is 3.6 x 10 3 km/s (a) What is the wavelength of the ejected electron? I initially solved this problem by finding the kinetic energy using the equation E k = (1/2)mv 2 and got 5.9 x 10 -18 J. I then found the wavelength ...
- Wed Oct 16, 2019 10:50 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: General question 1B.27
- Replies: 4
- Views: 218
Re: General question 1B.27
+ or - represents the uncertainty so you would use 5.0 in the equation delta x >= h/(4pi)(5.0 m/s)(mass). In this equation, 5 is the uncertainty in the velocity.
- Tue Oct 15, 2019 2:17 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: #20 Post Module
- Replies: 3
- Views: 213
#20 Post Module
Use the above uncertainty in velocity to calculate the electron's uncertainty in kinetic energy. Then calculate the uncertainty in kinetic energy per mole of electrons (that is, per mole of hydrogen atoms). In the previous problem I solved for delta velocity and got 3.4x10^10 m/s. I then used the eq...
- Sat Oct 12, 2019 3:45 pm
- Forum: Properties of Electrons
- Topic: Atomic Spectra Post Module
- Replies: 4
- Views: 171
Atomic Spectra Post Module
The transition from n = 4 to n = 2 emits radiation of longer wavelength than the transition from n = 5 to n = 1. This statement is true, however, I am confused as to why this is true. I thought the wavelength of the transition from n=5 to n=1 would be a longer wavelength. Are there any calculations ...
- Sat Oct 12, 2019 12:35 pm
- Forum: Einstein Equation
- Topic: Atomic Spectra
- Replies: 3
- Views: 259
Atomic Spectra
Write the equation that allows one to calculate the electronic energy levels for the hydrogen atom. Do the calculated energies compare favorably with empirical observation (spectroscopic results)? I know the equation is E=-hR/n^2, however, I am confused on what the second part of the question is ask...
- Fri Oct 11, 2019 6:51 pm
- Forum: Limiting Reactant Calculations
- Topic: Exercise M.1
- Replies: 3
- Views: 428
Exercise M.1
Hydrazine, N2H4, is an oily liquid used as a rocket fuel. It can be prepared in water by oxidizing ammonia with hypochlorite ions: 2 NH3+ ClO --> N2H4+ Cl+ H2O. When 35.0 g of ammonia reacted with an excess of hypochlorite ion, 25.2 g of hydrazine was produced. What is the percentage yield of hydraz...
- Fri Oct 11, 2019 4:15 pm
- Forum: Photoelectric Effect
- Topic: Post Module Assessment
- Replies: 1
- Views: 58
Post Module Assessment
Hello, for #28 in the Module the problem is : Light hits a sodium metal surface and the velocity of the ejected electron is 6.61x10^5 m/s. The work function for sodium is 150.6 KJ/mol. What is the kinetic energy of the ejected electrons? To solve this problem I know I have to use the equation E k =(...
- Wed Oct 09, 2019 10:35 pm
- Forum: Limiting Reactant Calculations
- Topic: Determining an empirical formula by combustion analysis
- Replies: 2
- Views: 203
Determining an empirical formula by combustion analysis
I'm confused on how to solve Self Test M.4A: When 0.528 g of sucrose (a compound of carbon, hydrogen, and oxygen) is burned, 0.306 g of water and 0.815 g of carbon dioxide are formed. Deduce the empirical formula of sucrose. I started by finding the mass of H, C and got 0.0342g H, 0.222g C and then ...
- Tue Oct 08, 2019 8:07 pm
- Forum: Photoelectric Effect
- Topic: How much energy to remove one electron?
- Replies: 8
- Views: 263
Re: How much energy to remove one electron?
Jk what i did was on the right track. Since the work function is the same as the threshold, I converted 150.6 kJ/mol to 1.506x10^5 J/mol. However, since the units are J/mol, I need to divide by Avogadro's constant, (6.022x10^23 electrons/mol) which would cancel out the moles and leave me with just J...
- Tue Oct 08, 2019 7:22 pm
- Forum: Photoelectric Effect
- Topic: How much energy to remove one electron?
- Replies: 8
- Views: 263
Re: How much energy to remove one electron?
Hello, I'm also confused about this problem. Is the work function the same thing as the threshold energy? I approached this problem with this in mind and just converted 150.6 kJ/mol to 1.506x10^5 J which is incorrect. However, I am unsure why this is wrong
- Tue Oct 08, 2019 2:27 pm
- Forum: Photoelectric Effect
- Topic: Photoelectric Effect Module
- Replies: 1
- Views: 95
Photoelectric Effect Module
For question 27 on the module it asks us to calculate the energy per photon of ultraviolet radiation of frequency 3.00 x 10^15 Hz.
To solve this I used the equation E=hv to find the energy. I multiplied (6.626x10^-34 Js)(3.00x10^15 Hz) but I am unsure of how the units will cancel out to become J.
To solve this I used the equation E=hv to find the energy. I multiplied (6.626x10^-34 Js)(3.00x10^15 Hz) but I am unsure of how the units will cancel out to become J.

