Search found 105 matches
- Wed Mar 11, 2020 5:14 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Molecularity
- Replies: 4
- Views: 414
Re: Molecularity
Molecularity describes the number of reactant molecules that are required to collide in each elementary step of a reaction. Elementary steps that involve only a single reactant molecule are unimolecular, those that involve two are bimolecular, three termolecular, and so on. Note that molecularity de...
- Wed Mar 11, 2020 5:05 pm
- Forum: General Rate Laws
- Topic: intermediates
- Replies: 12
- Views: 744
Re: intermediates
Use the same concept behind the pre-equilibrium approximation to derive expressions for the intermediates in your rate laws. For example, for the reaction 2\textup{NO} +{\textup O_{2}} \rightarrow 2\textup{N}{\textup O_{2}} with elementary steps 2 \textup{NO} \rightleftharpoons \textup N_{2} \textup...
- Wed Mar 11, 2020 4:05 pm
- Forum: General Rate Laws
- Topic: Rate limiting step
- Replies: 13
- Views: 798
Re: Rate limiting step
If you are given a reaction mechanism and an overall rate law, sometimes it is possible to intuitively determine the slow step. This is because the overall reaction rate is determined by the rate of the slow step (which is why it is also called the rate-limiting step). For example, the reaction NO2 ...
- Wed Mar 11, 2020 3:55 pm
- Forum: General Rate Laws
- Topic: 7A.3
- Replies: 5
- Views: 412
Re: 7A.3
0.44, in this case, is not your k value, but rather the actual observed rate so you cannot put it into a rate law the way that you did. The unique rate refers to the -1/a*(d[R]/dt) or 1/b*(d[P]/dt) for the general reaction aR --> bP. Thus, to get the actual rate at which a specific product is produc...
- Wed Mar 11, 2020 3:50 pm
- Forum: General Rate Laws
- Topic: 7A.9
- Replies: 4
- Views: 418
Re: 7A.9
Am I missing something here? I believe they tell you in the very first sentence that the decomposition reaction is first-order.
- Wed Mar 11, 2020 3:37 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: calculating Q
- Replies: 12
- Views: 777
Re: calculating Q
Q is a reaction quotient, which means that for most redox reactions, as long as you have the balanced equation, it should look identical to the equilibrium expression (with appropriate exponents to match the coefficients in the balanced equation). The equilibrium expression always puts products in t...
- Sun Mar 08, 2020 5:16 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Calculating ln Q
- Replies: 20
- Views: 1616
Re: Calculating ln Q
Q is easy enough to find for typical redox reactions. It gets a little weirder for concentration cells, but writing out the half-reactions should immediately make it clear which concentration to use for the products and which to use for the reactants. What I don't understand is how one is supposed t...
- Fri Mar 06, 2020 10:48 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: K in 6.65
- Replies: 2
- Views: 308
Re: K in 6.65
Since I'm confused as well, the full question is, "What range (in volts) does a voltmeter need to have to measure pH in the range of 1 to 14 at 25C if the voltage is zero when pH is 7?" Can someone point out the mistakes I'm making in the reasoning below? Since the two physical metal elect...
- Wed Mar 04, 2020 12:50 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Rate Constant, k
- Replies: 4
- Views: 381
Re: Rate Constant, k
You can easily determine the units of k for any order of reaction by looking at a generic rate law of that order. For example, a possible fifth-order rate law is rate = k*[A]^5. Rate always has the units M/s and concentration always has the units M. Therefore k, in this case, must have the units M/s...
- Wed Mar 04, 2020 12:44 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 6.65
- Replies: 3
- Views: 355
Re: 6.65
Can somebody post a full solution to this problem? Like the original poster, I don't understand where the expression for Q comes from. Why is Q not something like [H+ in solution]/[H+ inside pH electrode]?
- Wed Mar 04, 2020 11:25 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: 6M.11
- Replies: 3
- Views: 387
Re: 6M.11
The standard convention is to list standard reduction potentials, meaning the E values for the reduction reactions. If you are dealing with an oxidation half-reaction (the reverse of the reduction half-reaction), you typically have to negate the given E value.
- Wed Mar 04, 2020 11:22 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Work and delta G
- Replies: 1
- Views: 232
Re: Work and delta G
You can think of delta G as the amount of energy released by a system that is free (hence the name Gibbs' free energy) to do work. Naturally, the maximum amount of work can be done must then be equal to delta G (i.e. all released free energy is used to do work). Delta G is not the same as delta U (c...
- Wed Mar 04, 2020 11:13 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Strongest Reducing Agent
- Replies: 5
- Views: 534
Re: Strongest Reducing Agent
The potentials listed in most tables are standard reduction potentials, with higher values indicating that reduction is more likely to occur. The strongest reducing agents (i.e. the species most likely to give up electrons and become oxidized) are the ones that are the least likely to be reduced. Th...
- Wed Feb 26, 2020 11:25 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Work and Battery system
- Replies: 6
- Views: 435
Re: Work and Battery system
Yes, work (max) equals delta G under constant temperature and pressure. What is the exact formula? He basically already wrote out the exact formula. At constant temperature and pressure, w_{max} = \Delta G . You can think of delta G as the amount of energy that is released that is free to do work. ...
- Wed Feb 26, 2020 11:21 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Cell Diagram to Redox Reactions
- Replies: 2
- Views: 202
Re: Cell Diagram to Redox Reactions
The standard convention is to put the oxidation reaction (anode) on the left and the reduction reaction (cathode) on the right. In this particular example, the Cl- is being oxidized to Cl2 on the left and the H+ is being reduced to H2 on the right.
- Wed Feb 26, 2020 11:18 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 6N3c
- Replies: 2
- Views: 245
Re: 6N3c
Yeah, it bothers me as well that their reaction quotient appears to mix partial pressures and molar concentrations. When someone asked this question during my discussion section, the TA said something about how equilibrium constants are ideally calculated using activities that can be estimated using...
- Wed Feb 26, 2020 11:07 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: K and Cell potential
- Replies: 3
- Views: 299
Re: K and Cell potential
Your reasoning is correct but, to be precise, a high K indicates a positive E naught and a low K indicates a negative E naught (which is measured at standard conditions of 298.15K, 1M, 1 atm).
- Wed Feb 26, 2020 11:05 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: log or ln
- Replies: 6
- Views: 512
Re: log or ln
Strictly speaking, you can use either as long as you are able to calculate the Q for the reaction. It is often more convenient to use log for problems involving pH because pH = -log[H+]. Do note that that you must multiply the logarithmic term by a factor of 1/log(e) = ~2.303 when you use the log eq...
- Fri Feb 21, 2020 9:18 am
- Forum: Balancing Redox Reactions
- Topic: Homework 6K.3
- Replies: 3
- Views: 321
Re: Homework 6K.3
I had no clue how to do this one either. I just assumed that the Cl2(g) on the products side was a typo and balanced the equation assuming they meant to put Cl-(aq) there. The question just didn't make any sense to me otherwise.
- Fri Feb 21, 2020 9:15 am
- Forum: Balancing Redox Reactions
- Topic: 6K.5 part a
- Replies: 2
- Views: 215
Re: 6K.5 part a
After determining the coefficients for the species being reduced (in this case O3) and the species being oxidized (in this case Br-) by balancing the electron transfer (# of e- lost = # of e- gained), you must ensure that your final equation has balanced charges as well (total charge of product side...
- Fri Feb 21, 2020 9:07 am
- Forum: Balancing Redox Reactions
- Topic: balancing half reactions in a basic solution
- Replies: 7
- Views: 449
Re: balancing half reactions in a basic solution
Yes, for redox reactions in basic solutions, add OH- to both sides when balancing out the H+. On the side that has H+, the two species will combine to form neutral H2O.
- Fri Feb 21, 2020 9:06 am
- Forum: Balancing Redox Reactions
- Topic: oxidation numbers
- Replies: 8
- Views: 589
Re: oxidation numbers
Here is a website with some common rules that one should follow when assigning oxidation numbers: https://www.thoughtco.com/rules-for-assigning-oxidation-numbers-607567.
- Fri Feb 21, 2020 9:04 am
- Forum: Balancing Redox Reactions
- Topic: Oxidation number of Ozone
- Replies: 10
- Views: 753
Re: Oxidation number of Ozone
Oxidation numbers of many elements are not fixed. Here is a site with more information about some rules that one must follow when assigning oxidation numbers: https://www.thoughtco.com/rules-for-assigning-oxidation-numbers-607567.
- Thu Feb 13, 2020 11:20 am
- Forum: Calculating Work of Expansion
- Topic: Work for an isothermal reversible expansion
- Replies: 3
- Views: 298
Re: Work for an isothermal reversible expansion
In the case of an isothermal reversible expansion, n = number of moles of gas = constant, R = gas constant, and T = constant. By the ideal gas law, since nRT is constant, PV is also constant. It does not matter whether you use the initial or final volume or pressure as their product will be constant.
- Thu Feb 13, 2020 11:16 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Delta U = n*Cv,m*deltaT
- Replies: 7
- Views: 2612
Re: Delta U = n*Cv,m*deltaT
For constant pressure the Cv would actually be switched to CP. Is this actually true? I thought that this equation for delta U only works with Cv. For example, in an isobaric process, q = nCp(delta T) and w = -P(delta V) = -nR(delta T). Thus delta U = q + w = n(Cp - R)(delta T). Since Cv = Cp - R, ...
- Thu Feb 13, 2020 9:44 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Entropy total =0 in reversible isothermal system
- Replies: 1
- Views: 201
Re: Entropy total =0 in reversible isothermal system
The definition of a reversible process requires that the entropy of the universe (total delta S) does not change. This is because the system undergoing this process is always in equilibrium with the surroundings. For an irreversible isothermal free expansion into a vacuum, the pressure is constant a...
- Thu Feb 13, 2020 9:24 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Why are exothermic reactions generally spontaneous?
- Replies: 16
- Views: 1254
Re: Why are exothermic reactions generally spontaneous?
Looking at the equation delta G = delta H - T*delta S, delta S must simply be greater than delta H/T for delta G to be less than 0 (spontaneous reaction). For spontaneous exothermic reactions (delta H and delta G < 0), this means that delta S can be negative as long as it is greater than delta H/T.
- Thu Feb 13, 2020 9:16 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: entropy and pressure
- Replies: 2
- Views: 202
Re: entropy and pressure
One equation for entropy is delta S = nR*ln(P1/P2). From this, it follows that increasing pressure decreases entropy and decreasing pressure increases entropy.
- Fri Feb 07, 2020 11:27 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: 4F.9
- Replies: 2
- Views: 71
Re: 4F.9
Because the process is isothermal, q = -w. Decreasing pressure must mean that volume is increasing, therefore work is negative. Then q is positive and delta S = q/T is also positive.
- Fri Feb 07, 2020 11:22 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Entropy and Equations (reversible vs irreversible)
- Replies: 2
- Views: 102
Re: Entropy and Equations (reversible vs irreversible)
While it does not matter whether or not a process is reversible, delta S = q/T cannot be used to calculate entropy for non-isothermal reactions. In those cases, an integral must be set up using dS = dq/T from T1 to T2. At constant pressure, dq = n(Cp)(dT). At constant volume, dq = n(Cv)(dT).
- Fri Feb 07, 2020 11:13 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: calculating entropy
- Replies: 4
- Views: 230
Re: calculating entropy
why is the change in entropy sometimes equal to (-deltaH/T)? in other words, what does the - imply? The change in entropy of the surroundings is equal to the negative change in the enthalpy of the system divided by temperature. This makes sense because when the system gives off heat (negative delta...
- Fri Feb 07, 2020 11:08 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Delta U equals zero
- Replies: 7
- Views: 351
Re: Delta U equals zero
As noted previously, all isothermal processes by definition have delta U = 0. This means that the work done does not necessarily have to be 0. In the case that work is not zero, q will simply be the negative of that so they cancel out.
- Fri Feb 07, 2020 10:50 am
- Forum: Calculating Work of Expansion
- Topic: Isothermal
- Replies: 17
- Views: 803
Re: Isothermal
Isothermal means that delta T = 0 and therefore delta U = nc(delta T) = 0. This does not mean that delta Q = 0.
- Fri Feb 07, 2020 10:44 am
- Forum: Calculating Work of Expansion
- Topic: Understanding equation for work at constant pressure
- Replies: 3
- Views: 143
Re: Understanding equation for work at constant pressure
405318478 wrote:Hey.
Could someone explain where the equation for work, W=nR(deltaT) comes from? This is for calculating work at constant pressure.
This comes from using the Ideal Gas Law to replace the P(delta V) in the original equation for work with nR(delta T).
- Wed Jan 29, 2020 9:26 am
- Forum: Calculating Work of Expansion
- Topic: 4A.3
- Replies: 9
- Views: 705
Re: 4A.3
BeylemZ-1B wrote:They aren’t the same in the answer key though.
That's strange. In my answer key, they are indeed the same (about 28 J).
- Wed Jan 29, 2020 9:07 am
- Forum: Calculating Work of Expansion
- Topic: Calculating Work
- Replies: 2
- Views: 148
Re: Calculating Work
Use the first equation for irreversible expansions/contractions occurring against constant external pressure. Use the second equation for reversible expansions where the external pressure is always equal to the internal pressure (pressure of the expanding/contracting gas).
- Tue Jan 28, 2020 6:59 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Best Method of the 3 Given?
- Replies: 7
- Views: 264
Re: Best Method of the 3 Given?
I don't know if there is a best method but I remember Dr. Lavelle specifically saying that using bond enthalpies was the least accurate method because these are calculated as averages across many different molecular configurations.
- Tue Jan 28, 2020 6:56 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Calculating Work
- Replies: 3
- Views: 108
Re: Calculating Work
More generally, W =-\int_{V_{i}}^{V_{f}}P\ dV . In the case of an irreversible process against a constant pressure, this gives you W = -P(V_{f} - V_{i}) = -P\Delta V . In the case of a reversible process, the P in the work equation is equal to the P of the gas -- the external pressure on the...
- Tue Jan 28, 2020 6:39 pm
- Forum: Phase Changes & Related Calculations
- Topic: 4A.9
- Replies: 4
- Views: 152
Re: 4A.9
Brittney Hun 2C wrote:How do you know the copper releases energy?
Heat always flows from objects of higher temperature to objects of lower temperature.
- Fri Jan 24, 2020 9:36 am
- Forum: Ideal Gases
- Topic: When does the Ideal Gas Law Fail?
- Replies: 7
- Views: 1402
Re: When does the Ideal Gas Law Fail?
The Van der Waals equation modifies the Ideal Gas Law slightly to better predict the behavior of real gases, taking intermolecular forces and volume taken up by gas molecules into account. It's really interesting to see how this was done but for the purposes of this course it should be safe to assum...
- Fri Jan 24, 2020 9:20 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Molar concentration of H3O
- Replies: 6
- Views: 576
Re: Molar concentration of H3O
Even if the temperature is not 25C, assuming that you are dealing with pure water, [H3O+] = [OH-] so 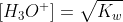 .
.
- Fri Jan 24, 2020 9:12 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Delta H
- Replies: 10
- Views: 777
Re: Delta H
To see if something is endothermic r exothermic we look at delta G. If delta G is positive, the reaction is endothermic, but if it is negative the reaction is exothermic. When determining whether a reaction is exothermic/endothermic, one should look at the sign of delta H (change in enthalpy). The ...
- Fri Jan 24, 2020 9:07 am
- Forum: Phase Changes & Related Calculations
- Topic: Phase Changes
- Replies: 3
- Views: 180
Re: Phase Changes
The names for the phase changes you listed are, in order, condensation, freezing, and deposition. These are indeed simply the reverse of vaporization, melting, and sublimation, respectively, and are therefore exothermic.
- Fri Jan 24, 2020 9:00 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: k<10^-3
- Replies: 9
- Views: 374
Re: k<10^-3
I don't believe that there is a strict cutoff of k = 10^-3 when determining whether or not a given compound is a weak acid. An acid is considered weak if it does not completely ionize in solution. That means that even acids with a Ka of 1 can technically be considered weak because this would imply t...
- Thu Jan 16, 2020 10:06 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Module 4 Q15
- Replies: 2
- Views: 127
Re: Module 4 Q15
To be completely honest I have no idea what's going on here either. I was under the assumption that Le Chatelier's Principle, like the equilibrium constant, does not take pure solids and liquids into account. Any further insight would be greatly appreciated! Edit: So I did some digging through sever...
- Thu Jan 16, 2020 9:59 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Endothermic/Exothermic Rxns and Delta H
- Replies: 3
- Views: 556
Re: Endothermic/Exothermic Rxns and Delta H
The net chemical equation for photosynthesis is deceptively simple and excludes many of the complex intermediate steps that must take place when converting the reactants to products. For this class, those details are not important. Just know that when you sum all the energy expended to break bonds a...
- Thu Jan 16, 2020 9:44 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Factors
- Replies: 7
- Views: 265
Re: Factors
The reaction quotient depends on the concentrations/pressures of the reactants and products. For reactions not at equilibrium, these values are constantly changing and thus Q is nonconstant. Even for reactions at equilibrium, changes in the concentrations/pressures (adding/removing more reactants/pr...
- Thu Jan 16, 2020 9:40 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Today's lecture
- Replies: 5
- Views: 187
Re: Today's lecture
The Henderson-Hasselbalch equation states that ) . Use an ICE table to determine the concentrations of the conjugate acid-base pair.
. Use an ICE table to determine the concentrations of the conjugate acid-base pair.
- Thu Jan 16, 2020 9:37 am
- Forum: Ideal Gases
- Topic: R constant
- Replies: 6
- Views: 282
Re: R constant
As previous posters have already noted, pay careful attention to the units of the given values in each problem when determining which R constant to use. For example, when converting from Keq to delta G, the correct R value would be about 8.314 J*K^{-1}*mol^{-1} , which makes sense seeing as your fin...
- Thu Jan 09, 2020 4:29 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kc and Kp
- Replies: 2
- Views: 124
Re: Kc and Kp
- Thu Jan 09, 2020 4:22 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Textbook 5H.1 Part 1
- Replies: 4
- Views: 160
Re: Textbook 5H.1 Part 1
I assume you're referring to part b and not part a as the answer to part a should be 1/41. First note that the given information tells you K = \frac{[N_{2}][H_{2}]^{3}}{[NH_{3}]^{2}} = 41 . Then note that the K value for part b is K = \frac{[N_{2}]^{\frac{1}{2}}[H_{2}]^{\frac{3}{2}}}{[NH_{3}]} = \sq...
- Thu Jan 09, 2020 4:17 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Equilibrium Constants with Ionic Compounds
- Replies: 2
- Views: 187
Re: Equilibrium Constants with Ionic Compounds
A net-ionic equation is one where the species (ions, solvents, etc.) that appear in both the reactants and the products are omitted, leaving only the ions that actually change in some way during the course of the reaction. An net-ionic equation for the dissociation of a solid salt in water would loo...
- Thu Jan 09, 2020 4:03 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kp v. Kc
- Replies: 5
- Views: 210
Re: Kp v. Kc
Kp is calculated using partial pressures and can therefore only be used for reactions that exclusively involve gaseous substances. Kc is calculated using molar concentrations and can be used for all reactions. In those calculations, the concentration of any given gas would simply be the number of mo...
- Thu Jan 09, 2020 3:55 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Units when Calculating Kp
- Replies: 1
- Views: 103
Units when Calculating Kp
Is the value of Kp supposed to change depending on the units used to measure pressure? For part c of 5G.5, the squared term in the numerator of the expression for Kp means that multiplying each pressure measurement by a constant (as one would do when converting between units) would result in a compl...
- Thu Dec 05, 2019 11:56 am
- Forum: Bronsted Acids & Bases
- Topic: Relative strength of base?
- Replies: 3
- Views: 249
Re: Relative strength of base?
Yes you would look at the same characteristics to determine the strength of a base. Essentially a stronger base, wants to accept a proton easier, and will give off more hydroxide ion. So how would that work though? Cause like for acid, you look at the strength between H and A (the bigger the A, the...
- Thu Dec 05, 2019 11:41 am
- Forum: Conjugate Acids & Bases
- Topic: conjugate base and pH
- Replies: 4
- Views: 2073
Re: conjugate base and pH
The seesaw relationship between the relative strengths of a conjugate acid/base pair states that the conjugate base of a strong acid will be a very weak base and the conjugate acid of a strong base will be a very weak acid. In these cases, the conjugates are so weak that they have basically no effec...
- Thu Dec 05, 2019 9:25 am
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: oxoacid strength - conceptual
- Replies: 1
- Views: 215
Re: oxoacid strength - conceptual
One of the factors that affect the strength of an acid is the net electron-withdrawing force acting on the H+ that the acid can donate. In the case of oxoacids, increasing the number of oxygens increases this electron-withdrawing force and further polarizes the compound by shifting the electron dens...
- Thu Dec 05, 2019 9:17 am
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: J.17
- Replies: 4
- Views: 392
Re: J.17
Unfortunately, memorizing a table of common ions may be your best bet here. As far as I know, there is no easy way to determine the charge of an ion given its molecular formula alone.
- Thu Dec 05, 2019 9:15 am
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Are polar/partially charged molecules always acidic?
- Replies: 1
- Views: 171
Re: Are polar/partially charged molecules always acidic?
I don't actually know the answer to your question but it did remind me of the fact that all amino acids with acidic side chains are negatively charged when deprotonated and all amino acids with basic side chains are positively charged when protonated. Whether or not an acid/base is protonated depend...
Re: 9C #9c/d
En is the abbreviation for ethylenediamine (C2H4(NH2)2) and edta is the abbreviation for ethylenediaminetetraacetic acid (C10H16N2O8).
- Mon Nov 25, 2019 12:38 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Chelateing Ligands
- Replies: 3
- Views: 315
Re: Chelateing Ligands
Chelating ligands are polydentate ligands that are able to form a ring-like structure of atoms that includes the central metal cation. As an example, each ethylenediamine (molecular formula: C2H4(NH2)2) ligand in the compound pictured below binds the central Cobalt cation to form two such rings. htt...
- Mon Nov 25, 2019 12:22 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: How to tell?
- Replies: 11
- Views: 958
Re: How to tell?
Sometimes it can be difficult to tell whether a given molecule is an acid or a base from its molecular formula alone. For example, NH3 (ammonia) is a weak base despite having several H atoms.
- Mon Nov 25, 2019 12:18 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Ligands
- Replies: 5
- Views: 353
Re: Ligands
Alison Trinh 1A wrote:Can someone explain what exactly a ligand is?
In this context, a ligand refers to an electron-pair donor that forms one or more coordinate covalent bonds with a central metal ion.
- Mon Nov 25, 2019 12:17 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Strong acids vs weak acids
- Replies: 8
- Views: 526
Re: Strong acids vs weak acids
Neutralization reactions involving weak acids and bases are a lot more complicated than those that only deal with strong acids and bases. This is because the incomplete ionization of the weak acids and bases results in an equilibrium that one would then need to create a so-called ICE table to solve ...
- Fri Nov 22, 2019 8:18 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Definition of a Ligand
- Replies: 4
- Views: 252
Re: Definition of a Ligand
In this context, a ligand is a Lewis base (electron pair donor) that can form coordinate covalent bonds with transition metal cations. To make things confusing, ligands take on an entirely different definition in the context of biochemistry (cell signaling, enzymatic reactions, etc.).
- Fri Nov 22, 2019 8:15 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Absorption of O2
- Replies: 1
- Views: 181
Re: Absorption of O2
We haven't yet covered equilibrium yet so you likely don't need to know the specifics, but logically speaking it should make sense that the heme group has a higher chance of binding oxygen when the oxygen is at a higher concentration (which is essentially what a higher partial pressure means). Conve...
- Fri Nov 22, 2019 8:13 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Acids
- Replies: 6
- Views: 477
Re: Acids
A strong acid is one that fully dissociates in water while a weak acid is one that only partially dissociates (equilibrium dictated by something called a Ka value).
- Fri Nov 22, 2019 8:10 pm
- Forum: Hybridization
- Topic: lone pairs in hybridization
- Replies: 8
- Views: 544
Re: lone pairs in hybridization
When determining the hybridization scheme of a certain atom, all electron domains should be counted, whether they be bonding or lone pairs. Bonding electrons will essentially singly occupy their respective hybridized orbitals (allowing sigma bonds to form via interactions with other orbitals only co...
- Fri Nov 22, 2019 8:07 pm
- Forum: Hybridization
- Topic: hybridization
- Replies: 11
- Views: 576
Re: hybridization
^^ What is a step by step method? I don't think there is any kind of step-by-step method. All you need to determine the hybridization scheme of a certain central atom is the number of regions of electron density around that atom (which one can very easily determine using the Lewis dot structure). 2...
- Thu Nov 14, 2019 9:27 am
- Forum: Lewis Structures
- Topic: 2.7
- Replies: 2
- Views: 168
Re: 2.7
Honestly, I think you just have to approach the problem like any other Lewis structure question and work it out by hand using the number of total valence electrons. Wikipedia says that this ion (the pentazenium cation) has six possible resonance structures given by: [N≡(N+)−(N−)−(N+)≡N]+ ↔ [(N−)=(N+...
- Thu Nov 14, 2019 9:22 am
- Forum: Lewis Structures
- Topic: Curiosity [ENDORSED]
- Replies: 6
- Views: 1214
Re: Curiosity [ENDORSED]
Not 100% sure if this is correct, but I drew it with the Se in the middle and the O's surrounding it. For 2 of the O's I drew double bonds and 2 lone pairs, and for the other 2 O's I attached H's and 2 lone pairs. Yeah, a quick Google search reveals that this is the correct Lewis structure. Now for...
- Thu Nov 14, 2019 9:18 am
- Forum: Ionic & Covalent Bonds
- Topic: Electronegativity
- Replies: 7
- Views: 472
Re: Electronegativity
Electronegativity is actually one of the more consistent periodic trends and generally increases across a period and decreases down a group. While a similar trend characterizes the ionization energies, do note (as mentioned above) that there is no decrease in electronegativity from N to O and from B...
- Thu Nov 14, 2019 9:08 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Difference between molecular shape
- Replies: 3
- Views: 305
Re: Difference between molecular shape
I found this simple table online that might help you visualize and remember all the various molecular geometries.
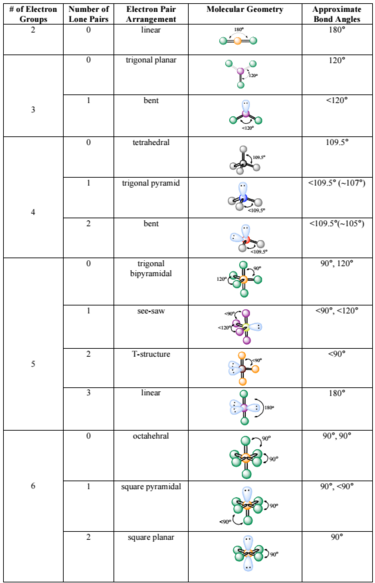

- Thu Nov 14, 2019 9:03 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Deciding Between Trigonal Planar vs Trigonal Pyramidal
- Replies: 4
- Views: 202
Re: Deciding Between Trigonal Planar vs Trigonal Pyramidal
You can count the number of regions of electron density and the number of lone pairs around a central to quickly determine its corresponding molecular geometry. Trigonal planar geometries occur when there are 3 regions of electron density of which none are lone pairs. Trigonal pyramidal geometries o...
- Fri Nov 08, 2019 12:13 pm
- Forum: Dipole Moments
- Topic: Drawing Unpaired Electrons
- Replies: 7
- Views: 353
Re: Drawing Unpaired Electrons
Always make sure that the total number of electrons in your final Lewis dot structure (whether they be in lone pairs, bonds, or single dots in the case of the unpaired and nonbonding electrons of radicals) is equal to the sum of the valence electrons of all the atoms in the structure.
- Fri Nov 08, 2019 12:06 pm
- Forum: Electronegativity
- Topic: Determining Electronegativity
- Replies: 4
- Views: 225
Re: Determining Electronegativity
Remember that electronegativity generally increases going across a period (left to right) and decreases going down a group. You can use this trend to figure out the relative magnitudes of the electronegativity differences.
- Thu Nov 07, 2019 12:15 pm
- Forum: Ionic & Covalent Bonds
- Topic: 2D.9
- Replies: 3
- Views: 219
Re: 2D.9
The way I like to remember this is that ions with larger charge/size ratios have higher polarizing power. Thus, as both smallest by ionic radii and one of only two in your list carrying a relatively large 2+ charge, Be2+ has the largest charge/size ratio and therefore also has the highest polarizing...
- Thu Nov 07, 2019 12:08 pm
- Forum: Ionic & Covalent Bonds
- Topic: coulomb potential energy and Madelung constant, as well as lattice energy
- Replies: 4
- Views: 507
Re: coulomb potential energy and Madelung constant, as well as lattice energy
Lattice energy represents the change in energy that occurs when separated gaseous ions are packed together to form the ionic solid, which is always negative due to the process being exothermic. This kinda gets into the realm of physics but essentially the negative derivative of the electrostatic for...
- Thu Nov 07, 2019 11:23 am
- Forum: Ionic & Covalent Bonds
- Topic: Ionic vs Covalent
- Replies: 14
- Views: 896
Re: Ionic vs Covalent
Additionally, an electronegativity difference less than 0.5 generally represents a nonpolar covalent bond while an electronegativity difference between 0.5 and around 1.5 represents a polar covalent bond.
- Mon Oct 28, 2019 1:32 pm
- Forum: Lewis Structures
- Topic: lewis structures
- Replies: 5
- Views: 241
Re: lewis structures
While it is true that the atoms with the lowest ionization energies generally go in the center, this is not always the case. For example, I challenge anyone who does not already know the structure of HNO2 (nitrous acid) to draw its Lewis structure from scratch and compare that with its real structur...
- Mon Oct 28, 2019 1:18 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Formal Charge equation?
- Replies: 5
- Views: 222
Re: Formal Charge equation?
Honestly, while memorizing the equation is fine and good, I like to tally up the number of electrons to subtract by simply counting each bond as one electron as each lone pair as two electrons. For example, if I saw a double bond, a single bond, and two lone pairs around a hypothetical atom, I could...
- Mon Oct 28, 2019 1:02 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Finding Most Stable Structure
- Replies: 9
- Views: 309
Re: Finding Most Stable Structure
How do you know which atom can hold more of the negative charge? To maximize the stability of your structure, any remaining negative formal charge should be preferably assigned to the more electronegative elements first. I'm sure Lavelle will not expect us to memorize the electronegativities for al...
- Mon Oct 28, 2019 12:55 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: In Class Example, Sulfate
- Replies: 3
- Views: 423
Re: In Class Example, Sulfate
After getting the formal charge on each atom as close to zero as possible, place the remaining negative formal charge(s), if present, on the more electronegative atom(s) and the positive formal charge(s), if present on the less electronegative atom(s) to get the most stable structure. The reason tha...
- Mon Oct 28, 2019 12:30 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Finding Most Stable Structure
- Replies: 9
- Views: 309
Re: Finding Most Stable Structure
In cases where it is impossible to entirely eliminate the formal charge on each atom, the most stable structure will have the negative formal charges on the more electronegative atoms and the positive formal charges on the less electronegative atoms.
- Wed Oct 23, 2019 4:09 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Electron Spin
- Replies: 8
- Views: 510
Re: Electron Spin
Does Hund's rule apply to the Pauli Exclusion Principle? This is a bit of a strange question to ask. Hund's Rule describes the order in which electrons fill a subshell and is composed of two main statements: every orbital in a subshell must be singly occupied before any are doubly occupied, and all...
- Mon Oct 21, 2019 10:39 am
- Forum: Quantum Numbers and The H-Atom
- Topic: Z
- Replies: 5
- Views: 319
Re: Quantum Number Z
Hi, To my understanding, Z is not one of the four types of quantum numbers [those are principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (ml), and the electron spin quantum number (ms)]. Z, in this context, is involved with the quantum number ...
- Mon Oct 21, 2019 10:32 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Difference between the electron shell and orbitals
- Replies: 4
- Views: 575
Re: Difference between the electron shell and orbitals
All the answers above me are close but just ever so slightly off. While it is true that each shell contains one or more orbitals, it is not entirely correct to say that each angular momentum quantum number l represents an orbital within a given shell with principal quantum number n. This becomes obv...
- Mon Oct 21, 2019 10:08 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Wave Function and Uncertainty
- Replies: 3
- Views: 119
Re: Wave Function and Uncertainty
If my understanding is correct, the squared absolute value (well, technically modulus but I won't get into that) of the wave function describes the probability density of observing an electron in the orbital described by the function, essentially how likely you are to find an electron at any given t...
- Mon Oct 21, 2019 9:53 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Orbitals and Energy Levels
- Replies: 2
- Views: 136
Re: Orbitals and Energy Levels
If it helps, you can think of the various quantum numbers for electrons as a hierarchy of sorts. Starting from the bottom going up, the spin quantum number ( m_{s} ) represents the spin of a given electron. The magnetic quantum number ( m_{l} ) represents the specific orbital that contains that elec...
- Mon Oct 21, 2019 9:35 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Why is 4s before 3d?
- Replies: 9
- Views: 997
Re: Why is 4s before 3d?
As mentioned by previous posters, the 4s orbital is filled before the 3d orbitals because it actually has lower energy than the 3d orbitals before they are populated. Note however that this reverses as soon as electrons begin to fill the 3d orbitals, at which point the electrons in the 4s orbital wi...
- Wed Oct 16, 2019 12:44 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: What are subshells
- Replies: 4
- Views: 261
Re: What are subshells
As posted above, the letters s, p, d, and f are refer to subshells, which, as their name implies, are subsets of shells. Each of these subshells contain one or more discrete orbitals that are represented by the various possible orbital angular momentum quantum numbers for a given shell and subshell....
- Wed Oct 16, 2019 12:30 pm
- Forum: DeBroglie Equation
- Topic: Given the energy, calculate the wavelength of y-rays
- Replies: 12
- Views: 3361
Re: Given the energy, calculate the wavelength of y-rays
also how do you convert the 10^-12 answer into nm As stated above, the answer to your first question is that c in this case simply represents the speed of light, which is a constant that is approximately equal to 3\times 10^{8} m/s. To convert from 10^{-12} m to nm, simply multiply by the conversio...
- Wed Oct 16, 2019 12:20 pm
- Forum: DeBroglie Equation
- Topic: Calculating Wavelength
- Replies: 5
- Views: 309
Re: Calculating Wavelength
Hi! I haven't done this problem before but I think what it means is that after you calculate the difference of the wavelengths (between protons and neutrons), you need to then calculate how much of the wavelength of neutron does this difference in wavelengths take up. Namely, diff divide by wavelen...
- Wed Oct 16, 2019 12:15 pm
- Forum: DeBroglie Equation
- Topic: Wavelength of Radiation
- Replies: 3
- Views: 171
Re: Wavelength of Radiation
I almost got tripped up by this question as well. After thinking it over, I believe that it is asking for the wavelength of the light that would eject an electron that with the given final velocity. In this case, you need to use the equation \frac{1}{2}mv^{2}=h\nu -\Phi , where m is the mass of an e...
- Wed Oct 16, 2019 12:02 pm
- Forum: DeBroglie Equation
- Topic: 1B.9 HW Question
- Replies: 8
- Views: 252
Re: 1B.9 HW Question
Drake Choi_1I wrote:When converting from photons to moles, would it be 6.022 x 10^23 photons to 1 mole?
Yes, that is correct. Whenever you are converting to moles you simply divide the amount of whatever you have by Avogadro's number.
- Sat Oct 12, 2019 12:52 pm
- Forum: Properties of Electrons
- Topic: Electron Energy Levels
- Replies: 8
- Views: 349
Re: Electron Energy Levels
This contradicts a lot of what's already been posted here, but I always thought that an electron could be excited to any electron level from 1 to a theoretical infinity (e.g. completely removed from the atom) depending on the energy of the photon that it interacts with. I don't think the energy leve...
- Sat Oct 12, 2019 12:33 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Rydberg equation [ENDORSED]
- Replies: 73
- Views: 9192
Re: Rydberg equation [ENDORSED]
Can someone please tell me which units cancel out when solving the Rydberg equation? The principal quantum numbers (the n's) have no units. The Rydberg constant has the units m^{-1} . Basically, everything on the right side will work out to some answer with the units m^{-1} . This matches the left ...
- Sat Oct 12, 2019 12:29 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Rydberg equation [ENDORSED]
- Replies: 73
- Views: 9192
Re: Rydberg equation [ENDORSED]
are the final units wirtten in Hz or Js? This depends on what the question is asking for. The traditional form of the Rydberg formula, as posted by MAC 1G above, allows you to plug in constants on the left side to get 1 over the wavelength of the photon that will be emitted/absorbed when an electro...
- Sat Oct 12, 2019 12:21 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Rydberg equation [ENDORSED]
- Replies: 73
- Views: 9192
Re: Rydberg equation [ENDORSED]
In class, Professor Lavelle wrote the Rydberg equation with a negative sign on the right to show that energy was being lost. Unfortunately when I used the equation that way, my negatives got all messed up and my answer was wrong...Should I just forget that negative? When using the Rydberg formula, ...
- Sat Oct 12, 2019 12:00 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Test 1 [ENDORSED]
- Replies: 107
- Views: 23381
Re: Test 1 [ENDORSED]
curry 1E wrote:Can someone explain what Angstroms are? I was confused on the test on this question.
Angstroms are a unit of length equivalent to 10^(-10) m.

