Search found 55 matches
- Sat Dec 07, 2019 8:26 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: different Rydberg
- Replies: 3
- Views: 371
Re: different Rydberg
Is it ok to use to use the second one? It's okay to use the alternative one as long as you can ensure that you can get the correct answer to any atomic spectra questions using it. From what I remember, Dr. Lavelle really pushed for us to use the equation that he put on his equation sheet. So, I wou...
Re: naming
Justin Vayakone 4H wrote:Wait there was a worksheet he emailed us? When did he email it? I don't see it.
https://lavelle.chem.ucla.edu/wp-conten ... pounds.pdf
It should be in this link here.
- Thu Dec 05, 2019 6:20 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Cancelling dipoles
- Replies: 2
- Views: 190
Re: Cancelling dipoles
You're on the right track. However, it's more important to visualize the molecule you're given based on its molecular geometry. Considering CH2Cl2, the dipoles wouldn't cancel even if you were to write the lewis structure by putting both Cl's on opposite sides and the same for the H's. This is becau...
- Thu Dec 05, 2019 6:07 pm
- Forum: Hybridization
- Topic: d hybridized orbital confusion
- Replies: 7
- Views: 373
d hybridized orbital confusion
Hello,
I have a question regarding notation of a hybridized orbital that includes the d subshell.
Should the d be written before the sp or after the sp?
Ex:
dsp^3 vs. sp^3d
I have a question regarding notation of a hybridized orbital that includes the d subshell.
Should the d be written before the sp or after the sp?
Ex:
dsp^3 vs. sp^3d
- Thu Dec 05, 2019 6:03 pm
- Forum: Significant Figures
- Topic: Significant Figures w/ Acid and Base Calculations
- Replies: 2
- Views: 290
Significant Figures w/ Acid and Base Calculations
Hello, I'm aware of the fact that when dealing with logarithms, your resulting answer will have a different amount of sig figs than normal. Ex: -log(0.00146) = 1.836 (There are three sig figs in this case since any value to the left of a decimal point from the solution to a logarithmic equation does...
- Thu Nov 28, 2019 7:14 pm
- Forum: Amphoteric Compounds
- Topic: Examples of amphoteric compounds
- Replies: 5
- Views: 342
Re: Examples of amphoteric compounds
I'd say that it wouldn't hurt to memorize them because they follow along a pattern in the periodic table. It's a slightly skewed diagonal line of elements. Since Dr. Lavelle showed us exactly which ones are in class, then there's a chance that we might have to recall them in some manner for the final.
- Thu Nov 28, 2019 7:05 pm
- Forum: Naming
- Topic: -ido vs -o
- Replies: 5
- Views: 345
Re: -ido vs -o
I did some digging, and I found this post back in chemistry community a few years ago: https://lavelle.chem.ucla.edu/forum/viewtopic.php?t=2351 Apparently, -ido is the modern way of representing names of these negative species. However, -o is still often used among chemists due to the changes in nam...
- Thu Nov 28, 2019 6:56 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Heisenberg Equation
- Replies: 4
- Views: 540
Re: Heisenberg Equation
As everyone above mentioned, the equation refers to how we can never know the exact position and velocity of an object at any given time. There's an inverse relationship in the certainty of knowing the position of an object and knowing the velocity of the same object. Heisenberg derived this equatio...
- Thu Nov 28, 2019 6:47 pm
- Forum: Lewis Acids & Bases
- Topic: Lewis acid and base definition
- Replies: 4
- Views: 357
Re: Lewis acid and base definition
When thinking about lewis acids and bases, think about what molecule/species donates or accepts an electron when combining to form a more complex molecule. The species that acts as an "electron donor" is the lewis base while the species that acts as an "electron acceptor" is a le...
- Thu Nov 28, 2019 6:33 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Final Practice
- Replies: 3
- Views: 314
Re: Final Practice
I'd recommend just going to all the UA workshop sessions, as those who lead them give out extra worksheets full of practice problems. Personally, I'm hoping that Lyndon will host another review session before the final.
- Sun Nov 24, 2019 6:47 pm
- Forum: Hybridization
- Topic: Lone pairs
- Replies: 9
- Views: 590
Re: Lone pairs
Hybrid orbitals still need to facilitate space for the presence of a central atom's lone pairs. As most above mentioned, lone pairs are still considered as electron groups and thus, need to be in an orbital.
- Sun Nov 24, 2019 6:44 pm
- Forum: Hybridization
- Topic: Describing a molecule using hybridization
- Replies: 4
- Views: 253
Re: Describing a molecule using hybridization
What does steric mean? It just refers to the amount of bonds and lone pairs that an atom has. For instance, consider H2O. Oxygen is the central atom with two single bonds connected to the two hydrogen atoms and two lone pairs. Because there are two lone pairs and two single bonds, the steric number...
- Sun Nov 24, 2019 6:40 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Why is CH2Cl2 polar?
- Replies: 12
- Views: 793
Re: Why is CH2Cl2 polar?
If you were to consider the molecule as a 3D shape as opposed to a 2D one, then it's possible to see that the chlorine atoms and the hydrogen atoms are not exactly opposite of each other, which a potential lewis structure would suggest. Because these atoms are not on opposite ends, their dipole mome...
- Sun Nov 24, 2019 6:27 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Hydrogen Bonding Rules
- Replies: 6
- Views: 381
Re: Hydrogen Bonding Rules
As everyone mentioned, yes, if nitrogen had two lone pairs, then those would be considered as two separate bonding sites for potential hydrogens to bind to. To answer your question, one lone pair is considered as a single hydrogen binding site.
- Sun Nov 24, 2019 6:19 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Extensive properties
- Replies: 1
- Views: 178
Re: Extensive properties
For intermolecular forces:
Ion-ion interaction.
Ion-dipole interaction.
Dipole-dipole interaction. (One example: hydrogen bonding, which is a unique case.)
Dipole-induced dipole interaction.
Induced-dipole-induced-dipole interaction/Van der waals/London forces.
Ion-ion interaction.
Ion-dipole interaction.
Dipole-dipole interaction. (One example: hydrogen bonding, which is a unique case.)
Dipole-induced dipole interaction.
Induced-dipole-induced-dipole interaction/Van der waals/London forces.
- Sun Nov 17, 2019 9:30 pm
- Forum: Bond Lengths & Energies
- Topic: Test 2 Topics
- Replies: 40
- Views: 2250
Re: Test 2 Topics
It should most likely include concepts pertaining to molecular shape and structure as well as those slides regarding dipole-dipole interactions, hydrogen bonds, covalent bonds, and ionic bonds that were covered by Dr. Lavelle on the last slide on the Friday before the midterm exam. I missed lecture...
- Sun Nov 17, 2019 9:25 pm
- Forum: Ionic & Covalent Bonds
- Topic: Character of Bonds
- Replies: 7
- Views: 525
Re: Character of Bonds
Will he give us the actual electronegativity values on the tests though? As everyone mentioned, we won't be given the actual electronegativity values on the tests. However, I feel that any question regarding comparing electronegativity values will be more intuitive to answer based on the electroneg...
- Sun Nov 17, 2019 9:20 pm
- Forum: Lewis Structures
- Topic: Why can Xenon break the octet rule?
- Replies: 8
- Views: 5529
Re: Why can Xenon break the octet rule?
Any element within the third period and below on the periodic table will break the octet rule and will be able to have more electrons than eight. In addition, the first four elements won't have an completed octet (H, He, Li, Be) while the Boron's column of elements also doesn't need to have a comple...
- Sun Nov 17, 2019 5:04 pm
- Forum: SI Units, Unit Conversions
- Topic: Memorizing Conversions
- Replies: 25
- Views: 1378
Re: Memorizing Conversions
Most if not all conversions that you'd need to perform on any assessment in this class appears to be on the equation sheet we're given. However, it would still be helpful to know the conversions so that you don't have to keep on looking back and forth during a test.
- Sun Nov 17, 2019 4:53 pm
- Forum: Hybridization
- Topic: Homework Outline for Test
- Replies: 5
- Views: 295
Re: Homework Outline for Test
If I remember correctly, the specific topics would be intermolecular forces, the VSEPR model, and pi and sigma bonds. I believe that hybridization won't be tested.
- Mon Nov 11, 2019 12:50 am
- Forum: Lewis Structures
- Topic: Lewis Dot Structures
- Replies: 7
- Views: 404
Re: Lewis Dot Structures
Compounds with an odd number of electrons are defined as radicals and are supposed to be very reactive in nature. I've honestly been trying to figure out which atom is supposed to get the extra electron for days leading up to the midterm exam. I found that either you comply to the necessary formal c...
- Mon Nov 11, 2019 12:47 am
- Forum: Lewis Structures
- Topic: Formal Charges
- Replies: 15
- Views: 984
Re: Formal Charges
In the case that it's impossible for the compound to not have nonzero values for its formal charges, then the negative charges must be delegated to the compound's most electronegative atoms while the least ones will typically have positive formal charges. For compounds with neutral charges, these fo...
- Mon Nov 11, 2019 12:43 am
- Forum: Bond Lengths & Energies
- Topic: Test 2 Topics
- Replies: 40
- Views: 2250
Re: Test 2 Topics
It should most likely include concepts pertaining to molecular shape and structure as well as those slides regarding dipole-dipole interactions, hydrogen bonds, covalent bonds, and ionic bonds that were covered by Dr. Lavelle on the last slide on the Friday before the midterm exam.
- Mon Nov 11, 2019 12:41 am
- Forum: Dipole Moments
- Topic: Dipole Moment
- Replies: 6
- Views: 336
Re: Dipole Moment
A dipole moment is essentially just the difference in charges between different atoms in a compound. This is typically found by comparing each atom's electronegativity to each other.
- Mon Nov 11, 2019 12:39 am
- Forum: Electronegativity
- Topic: Ionization energy of O vs N
- Replies: 6
- Views: 1809
Re: Ionization energy of O vs N
This is based on the electron configuration of both oxygen and nitrogen. Oxygen has two unpaired electrons in its 2p orbitals while Nitrogen has three unpaired electrons. Based on my experience with one of the UA's, the symmetry of the electrons in nitrogen indicated by having a half complete valenc...
- Mon Nov 11, 2019 12:37 am
- Forum: Electronegativity
- Topic: Atom size
- Replies: 22
- Views: 3294
Re: Atom size
Typically electronegativity has the "opposite" trend in comparison to that of atomic radius. This is because the trend in the periodic table for electronegativity is increasing from left to right and bottom to top. In contrast, atomic radius increases from right to left and from top to bot...
- Mon Nov 11, 2019 12:33 am
- Forum: Electronegativity
- Topic: Electronegativity between Oxygen and nitrogen
- Replies: 2
- Views: 494
Re: Electronegativity between Oxygen and nitrogen
As mentioned above, it's because of oxygen's number of protons. If you want a reason based on periodic trends, electronegativity increases from left to right and from bottom to top. Oxygen is on the right of nitrogen.
- Mon Nov 11, 2019 12:31 am
- Forum: Lewis Structures
- Topic: Polar and Non polar covalent bonds in lewis structures
- Replies: 5
- Views: 356
Re: Polar and Non polar covalent bonds in lewis structures
You don't necessarily have to define them as such, but understanding that some compounds are either polar or non-polar would help you understand the composition of a given compound. If they're polar, then they're represented with "dipole moments," as there's going to be a portion of the co...
- Mon Nov 11, 2019 12:28 am
- Forum: Properties of Electrons
- Topic: Electron affinity
- Replies: 3
- Views: 289
Re: Electron affinity
Electron affinity follows nearly the same trend in the periodic table as electronegativity. However, by definition, these traits differ. Electron affinity refers to the amount of energy that an atom releases when it takes an electron. In contrast, electronegativity refers to the general ability for ...
- Mon Nov 11, 2019 12:25 am
- Forum: Properties of Light
- Topic: How to find the longest wavelength?
- Replies: 6
- Views: 4685
Re: How to find the longest wavelength?
The longest wavelength, as everyone mentioned, comes from the work function. Using the value of energy from the work function, solve for wavelength using E = h(c)/(wavelength).
- Sun Nov 03, 2019 3:36 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Formal Charge
- Replies: 5
- Views: 344
Re: Formal Charge
Like everyone above mentioned, formal charge should be calculated always when drawing Lewis structures. However, when drawing the "best" structure or the one with the lowest energy, it's important to note that you should calculate formal charge only after: 1.) Making sure your structure co...
- Sun Nov 03, 2019 3:14 pm
- Forum: Trends in The Periodic Table
- Topic: Electronegativity vs. Electron Affinity
- Replies: 3
- Views: 240
Re: Electronegativity vs. Electron Affinity
Electronegativity refers to how well an atom can attract an electron to itself when that atom is within a molecule while electron affinity, as mentioned above, refers to how much energy is released when an atom takes in an electron (the more simple and easier to understand definition would be the at...
- Sun Nov 03, 2019 1:55 pm
- Forum: Trends in The Periodic Table
- Topic: Exceptions
- Replies: 4
- Views: 134
Re: Exceptions
Nitrogen has a higher ionization energy than oxygen. If you look at the electron configuration of both, Nitrogen has a half-filled 2p orbital while oxygen has two unpaired electrons in the 2p orbital. Due to the symmetry of the electrons, more energy is required to remove an electron from a nitrogen...
- Sat Nov 02, 2019 11:38 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: 1.E.1
- Replies: 2
- Views: 101
Re: 1.E.1
If I remember correctly from the UA review session from Friday, one of the UA's stated that the subshells, values of l, for the hydrogen ion are degenerate. This means that they will have the same energy (all e- in s,p,d, etc. because there's only 1 electron). Besides that, all other atoms should ha...
- Sat Nov 02, 2019 11:24 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Filling of Orbitals
- Replies: 2
- Views: 137
Re: Filling of Orbitals
Since we're on the topic, the commenter above has got the correct explanation. It's important to note that this scenario, that is, having the d-orbital filled, takes place for all elements within chromium's column and copper's column (silver is underneath copper in the periodic table). In the case o...
- Sun Oct 27, 2019 4:23 pm
- Forum: Properties of Electrons
- Topic: Subshell Exceptions
- Replies: 6
- Views: 505
Re: Subshell Exceptions
In one of the UA sessions that I went to today, the UA told us to write Cr as [Ar]3d 5 4s 1 instead of [Ar]4s 1 3d 5 . I do not know why he said that but can someone better explain this to me? As the 3d orbital gains more electrons, it ends up having less energy than the 4s orbital. Since we're sup...
- Sun Oct 27, 2019 4:07 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Block Confusion
- Replies: 4
- Views: 192
Re: Block Confusion
In short, 3d has more energy than 4s simply because it stole one of the electrons of 4s. This is because 3d has an incomplete set of electrons in its orbitals but the 4s orbital is complete and because 3d wants to be complete too, it takes an electron from 4s and gives its last orbital an electron....
- Sun Oct 27, 2019 3:48 pm
- Forum: *Shrodinger Equation
- Topic: Wave functions
- Replies: 9
- Views: 362
Re: Wave functions
Wave functions represent the mathematical likelihoods of finding an electron in an atom. The orbital shapes, like the s or p, can be described as "solutions" to those mathematical functions (wave functions).
- Sun Oct 27, 2019 3:34 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Quantum Number
- Replies: 3
- Views: 145
Re: Quantum Number
As Ariana mentioned, the spin projection quantum number can only be either +1/2 or -1/2 for any electron in a particular orbital. In an electron configuration, +1/2 is indicated by an arrow pointing upwards while -1/2 is indicated by an arrow pointing downwards.
- Sun Oct 27, 2019 3:30 pm
- Forum: Properties of Light
- Topic: 1.31
- Replies: 4
- Views: 234
Re: 1.31
Recall this equation:
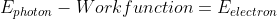
You're supposed to determine which laser to use. Take the appropriate laser's wavelength and use u = C/ lamda to solve for the energy of the photon.
Subtract the energy by the work function to find the energy emitted by the electron.
You're supposed to determine which laser to use. Take the appropriate laser's wavelength and use u = C/ lamda to solve for the energy of the photon.
Subtract the energy by the work function to find the energy emitted by the electron.
- Sun Oct 20, 2019 2:44 pm
- Forum: Photoelectric Effect
- Topic: if KE is 0 how can the electron be ejected?
- Replies: 4
- Views: 248
Re: if KE is 0 how can the electron be ejected?
If I remember correctly, Dr. Lavelle explained that the detector used to measure the kinetic energy of the ejected electron has a slight positive charge. This means that even if the electron is hit with an energy equal to the threshold needed (the work function), the electron will move slightly away...
- Sun Oct 20, 2019 2:39 pm
- Forum: DeBroglie Equation
- Topic: Wavelike properties of protons
- Replies: 2
- Views: 155
Re: Wavelike properties of protons
I agree with the post above, typically really small objects with momentum have perceivable wave-like properties. One exception to this is a photon. Despite it not having a mass, it can still have momentum. The reason as to why this is goes beyond the scope of this class, but because a photon can hav...
- Sun Oct 20, 2019 2:04 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Indeterminacy
- Replies: 1
- Views: 131
Re: Indeterminacy
You're on the right track, the value you'd use for uncertainty will need to utilize the +/- value.
Example: 5m/s with +/- 1.
5+1 = 6
5-1 = 4
6-4=2.
You'd use the value of 2 in the uncertainty equation for velocity.
Example: 5m/s with +/- 1.
5+1 = 6
5-1 = 4
6-4=2.
You'd use the value of 2 in the uncertainty equation for velocity.
- Sun Oct 20, 2019 2:00 pm
- Forum: Properties of Light
- Topic: Unit for Wavelength
- Replies: 34
- Views: 2494
Re: Unit for Wavelength
As mentioned by people in this thread, it looks like wavelength is going to measured in nm (nanometers). If you remember the equation: speed of light = frequency*wavelength You should notice that the speed of light has the units (meters/second). Since frequency is cycles per second or (per second), ...
- Mon Oct 14, 2019 8:35 pm
- Forum: Properties of Electrons
- Topic: Question from Heisenberg's Uncertainty Principle post test
- Replies: 2
- Views: 293
Re: Question from Heisenberg's Uncertainty Principle post test
1% of 0.05 nm is 0.01 x 0.05nm= 5e-4m This guy is essentially performing the first step of the problem. The uncertainty in position is equal to 1% of the radius of the hydrogen atom. Next, you'd need to get this value in meters. Finally, all you're doing is solving for velocity by dividing (h/4π) b...
- Sun Oct 13, 2019 2:31 pm
- Forum: DeBroglie Equation
- Topic: Problem 1B.7
- Replies: 3
- Views: 138
Re: Problem 1B.7
The units makes this question pretty tricky. Just remember that we're dealing with atoms of sodium. And in part 1, you solved for the energy emitted by a single sodium electron. So, in order to answer this question, we need to figure out how many atoms of sodium are present within 5.00mg of sodium. ...
- Sun Oct 13, 2019 2:21 pm
- Forum: Properties of Light
- Topic: 1A.3
- Replies: 3
- Views: 195
Re: 1A.3
I'm also confused about the relationship between the frequency of electromagnetic radiation and the extent of change in the electrical field. I second DHavo_3H's post. The extent of change in an electrical field at a given point has a positive correlation to the frequency of electromagnetic radiati...
- Sun Oct 13, 2019 12:44 am
- Forum: Photoelectric Effect
- Topic: How much energy to remove one electron?
- Replies: 8
- Views: 263
Re: How much energy to remove one electron?
Jk what i did was on the right track. Since the work function is the same as the threshold, I converted 150.6 kJ/mol to 1.506x10^5 J/mol. However, since the units are J/mol, I need to divide by Avogadro's constant, (6.022x10^23 electrons/mol) which would cancel out the moles and leave me with just ...
- Sun Oct 13, 2019 12:20 am
- Forum: Photoelectric Effect
- Topic: threshold energy
- Replies: 6
- Views: 323
Re: threshold energy
In the case of the photoelectric effect, the energy of a photon of light must be greater than or equal to the threshold energy required for an electron to be shot out or removed. If you remember the experiment, scientists were trying to figure out how intense light must be in order to remove electro...
- Sun Oct 13, 2019 12:14 am
- Forum: Einstein Equation
- Topic: Numbers to memorize [ENDORSED]
- Replies: 37
- Views: 4183
Re: Numbers to memorize [ENDORSED]
It would be convenient to memorize these numbers, but he does indeed offer a cheat sheet of equations to utilize for every test that we take. The particular cheat sheet is actually on his website: https://lavelle.chem.ucla.edu/wp-content/supporting-files/Chem14A/constants_equations.pdf (If I remembe...
- Thu Oct 03, 2019 9:13 pm
- Forum: Significant Figures
- Topic: Sig figs in intermediate steps??
- Replies: 5
- Views: 1002
Re: Sig figs in intermediate steps??
As mentioned before, decimal precision in immediate steps is the way to go. Just keep as many decimal points in your calculator as possible as you go through any problem and then your final answer should abide to the amount of sig figs you need. (Note: when showing work for intermediate steps, decim...
- Thu Oct 03, 2019 9:08 pm
- Forum: SI Units, Unit Conversions
- Topic: Kelvin, Celsius, & Fahrenheit Conversions
- Replies: 3
- Views: 119
Re: Kelvin, Celsius, & Fahrenheit Conversions
Kelvin (K) = 273.15 + ºC
Celsius (ºC) = K - 273.15
OR
(ºF)(5/9) - 32
Fahrenheit (ºF) = (ºC)(1.8) + 32
Celsius (ºC) = K - 273.15
OR
(ºF)(5/9) - 32
Fahrenheit (ºF) = (ºC)(1.8) + 32
- Thu Oct 03, 2019 4:53 pm
- Forum: SI Units, Unit Conversions
- Topic: E.1 7th edition
- Replies: 4
- Views: 291
Re: E.1 7th edition
This problem appears to be asking for the length of an entire line of Ag atoms stringed together. We're given the amount of atoms in moles (which again is a unit of measuring many atoms). If we want the total number of atoms in actual atoms and not moles, then we'd multiply 1 mol of Ag by avogadro's...
- Thu Oct 03, 2019 4:33 pm
- Forum: SI Units, Unit Conversions
- Topic: Sig Figs: follow the book or follow Sig Fig rules?
- Replies: 3
- Views: 200
Re: Sig Figs: follow the book or follow Sig Fig rules?
That's strange, can you state some examples of problems that display this? I'm looking around the solutions manual myself and it seems like sig figs are correct for the most part. Regardless of whether or not the solutions manual doesn't abide to sig figs, I'd say go for displaying your answers in s...
- Thu Oct 03, 2019 2:49 pm
- Forum: Properties of Light
- Topic: Quantum homework
- Replies: 4
- Views: 315
Re: Quantum homework
I just wanted to clarify: because the chemistry review section was "recently learned," we can theoretically submit five questions from that section for credit as opposed to submitting questions from the quantum world section?

