Search found 103 matches
- Thu Mar 12, 2020 8:24 pm
- Forum: *Identifying Primary, Secondary, Tertiary, Quaternary Carbons, Hydrogens, Nitrogens
- Topic: Will the Final for 14B have Identification?
- Replies: 9
- Views: 5981
Re: Will the Final for 14B have Identification?
this topic has an asterisk, which means it is no longer covered in the curriculum/not needed to know for the tests
- Thu Mar 12, 2020 8:21 pm
- Forum: General Science Questions
- Topic: Who makes the Final
- Replies: 23
- Views: 1444
Re: Who makes the Final
My TA said that she has no idea what is going on the final, beyond what we are told as students.
- Thu Mar 12, 2020 8:20 pm
- Forum: Administrative Questions and Class Announcements
- Topic: take home FINAL DEADLINE
- Replies: 15
- Views: 1125
Re: take home FINAL DEADLINE
I'm very interested to know this as well. I scheduled my work as if the final were to be on Sunday, so I'd really like to know as well, especially as the weekend approaches.
- Thu Mar 12, 2020 8:18 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Final Topics
- Replies: 10
- Views: 846
Re: Final Topics
It's cumulative so everything! Though it is take home so you can refer to past notes.
- Tue Mar 10, 2020 12:21 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: intermediate species
- Replies: 4
- Views: 375
Re: intermediate species
I don't think that you can easily guess what an intermediate species is for a reaction, it has to be given to you if you were going to try to solve a problem using it.
- Fri Mar 06, 2020 1:50 am
- Forum: Balancing Redox Reactions
- Topic: Half rxns
- Replies: 27
- Views: 1477
Re: Half rxns
with acidic conditions you're balancing H2O and H+, and with basic conditions you're balancing H2O and OH-
- Fri Mar 06, 2020 1:43 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Electromotive force (emf)
- Replies: 9
- Views: 649
Re: Electromotive force (emf)
it's the voltage of the cell
- Fri Mar 06, 2020 1:41 am
- Forum: General Rate Laws
- Topic: Determining Order
- Replies: 6
- Views: 477
Re: Determining Order
Tai Metzger 3K wrote:let's say: rate = [A]^m * [B]^n
the order of reaction = m + n
I hope this helps!
thank you, I was confused as well!!
- Thu Mar 05, 2020 12:27 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Reasonable values for K
- Replies: 7
- Views: 555
Re: Reasonable values for K
I understand that values above 10^3 are large and below 10^-3 are small. I'm asking what values are reasonable, as in what are the minimum and maximum k values that have been found experimentally.
- Wed Mar 04, 2020 2:16 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Reasonable values for K
- Replies: 7
- Views: 555
Reasonable values for K
does anyone know what the possible ranges for K are? Like what is the the minimum/maximum value you would find for K in the real world?
- Sun Mar 01, 2020 4:52 pm
- Forum: Balancing Redox Reactions
- Topic: H+ or H2O
- Replies: 9
- Views: 632
Re: H+ or H2O
TimVintsDis4L wrote:One of the rules our TA taught us regarding this was that it's based off what type of reaction you have.
If it's acidic, balance O with water, then balance H with protons
If it's basic, balance O with OH-, and H with H20
this is a way simpler way to think about it than the worksheet, thanks!
- Sun Feb 23, 2020 11:20 pm
- Forum: Balancing Redox Reactions
- Topic: 6K.3D balancing problem?
- Replies: 4
- Views: 336
Re: 6K.3D balancing problem?
so fixing the balancing error I did get the right answer.
also, we can do Cl2 -> 2 Cl- because there was a typo in the book and the equation was supposed to be
Cl2 -> HClO + Cl- where we had to balance the extra H+ and waters.
also, we can do Cl2 -> 2 Cl- because there was a typo in the book and the equation was supposed to be
Cl2 -> HClO + Cl- where we had to balance the extra H+ and waters.
- Sun Feb 23, 2020 10:29 pm
- Forum: Interesting Applications: Rechargeable Batteries (Cell Phones, Notebooks, Cars), Fuel Cells (Space Shuttle), Photovoltaic Cells (Solar Panels), Electrolysis, Rust
- Topic: Respiration and Fermentation
- Replies: 1
- Views: 235
Respiration and Fermentation
Maybe this isn't included in this particular subcategory because it's more related to organic chem, but reduction and oxidation are the key ways in which cells get energy (fully reduced having the most available energy and fully oxidized having none available to use). This can either be through aero...
- Sun Feb 23, 2020 10:19 pm
- Forum: Balancing Redox Reactions
- Topic: 6K.3D balancing problem?
- Replies: 4
- Views: 336
6K.3D balancing problem?
Ok so I know that there's a typo for 6K3.D where the one of the products is meant to be 2Cl - rather than Cl 2 . For the oxidation reaction I have: Cl 2 + H 2 O -> HclO + H + + e - For the reduction reaction I have: Cl 2 + 2e - -> 2Cl - so when balancing the electrons you multiply the oxidation reac...
- Sun Feb 23, 2020 9:56 pm
- Forum: Balancing Redox Reactions
- Topic: 6K.3 part d
- Replies: 3
- Views: 267
Re: 6K.3 part d
thanks i was absolutely losing my mind at how to do this problem... can't believe there was a typo
- Sat Feb 22, 2020 8:40 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Nerst Equation
- Replies: 10
- Views: 1055
Re: Nerst Equation
if you're ever concerned, just check out the constants and equations sheet on lavelle's site. It's been the same since the course started and contains all the equations that will be given to us
- Sat Feb 22, 2020 8:39 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Reaction quotient
- Replies: 3
- Views: 348
when you have a larger Q, you have more products and less reactants. this makes for a low potential difference mathematically because you're subtracting the log of Q. Thinking of it in terms of electrical potential, since your reaction is going from left to right, anode to cathode, it would make sen...
- Sat Feb 22, 2020 8:33 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Concepts [ENDORSED]
- Replies: 2
- Views: 515
Re: Concepts [ENDORSED]
hi, another point is that it is the max potential difference for a reason. The potential difference is created by having a difference in charge, and this difference in charge is where the electromotive force comes from. When the cathode and anode are first connected, that's when there's a maximum po...
- Sat Feb 22, 2020 8:29 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: UA's
- Replies: 3
- Views: 343
Re: UA's
honestly biggest thanks to the UA's. make the material something that you can actually work through
- Sat Feb 22, 2020 8:28 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Ecell
- Replies: 5
- Views: 689
Re: Ecell
805097738 wrote:what is the difference between Ecell and Eocell?
the E0 always refers to standard conditions, I believe at equilibrium as well.
- Thu Feb 13, 2020 3:12 pm
- Forum: Calculating Work of Expansion
- Topic: Last Question on the Midterm [ENDORSED]
- Replies: 5
- Views: 497
Re: Last Question on the Midterm [ENDORSED]
I'd like to know as well, so I'm commenting to easily navigate back.
- Wed Feb 12, 2020 4:47 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Pizza Rolls REVIEW Session DOWNLOAD HERE
- Replies: 67
- Views: 5736
Re: Pizza Rolls REVIEW Session DOWNLOAD HERE
AysiaB1I wrote:Does anyone know how we got the temperature for question 6 on pizza rolls? I have 304.55 from the review session but i'm not sure how we got it.
solve for it with PV=nRT, everything except temp is given in the problem
- Wed Feb 12, 2020 11:09 am
- Forum: Administrative Questions and Class Announcements
- Topic: Pizza Rolls REVIEW Session DOWNLOAD HERE
- Replies: 67
- Views: 5736
Re: Pizza Rolls REVIEW Session DOWNLOAD HERE
Can someone please explain why the v2/v1 for He is 4 and and 4/3 for Kr for problem 5? Yeah so V2 is the sum of the volume in both chambers, where one is 3 times the size of the other. So in one chamer you have one liter, and the other you have 3. So the total (V2) is 4. He starts out in the 1L con...
- Wed Feb 12, 2020 2:17 am
- Forum: Administrative Questions and Class Announcements
- Topic: Pizza Rolls REVIEW Session DOWNLOAD HERE
- Replies: 67
- Views: 5736
Re: Pizza Rolls REVIEW Session DOWNLOAD HERE
Can someone explain how to solve #11 on the practice questions? What steps do you need to take? 1) since you're dealing with an acid, you want to convert the pKb to Ka 2) given the concentration of the acid, you can set up an ice table to help you write the equilibrium expression 3) after setting u...
- Tue Feb 11, 2020 11:26 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Pizza Rolls REVIEW Session DOWNLOAD HERE
- Replies: 67
- Views: 5736
Re: Pizza Rolls REVIEW Session DOWNLOAD HERE
Luc Zelissen 1K wrote:For Question 5, I havefor both gasses, but when I add them up I dont get the right answer. Is there another equation to find the change of Entropy of the 2 gasses combined, if so what is it?
don't forget to account for the temperature change!
- Fri Feb 07, 2020 9:19 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: overall definitions
- Replies: 4
- Views: 223
Re: overall definitions
Here are some of the important ones - deltaT = change in temperature deltaS = change in entropy deltaH = change in enthalpy deltaU = change in internal energy deltaV = change in volume deltaP = change in pressure W = work The other W = degeneracy, which helps you find entropy Csp = specific heat ca...
- Fri Feb 07, 2020 9:13 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Adiabatic System
- Replies: 3
- Views: 250
Re: Adiabatic System
Leonardo Le Merle 1D wrote:Also adding onto this question if that’s okay, do adiabatic and isothermal mean the same thing?
they do not! adiabatic means there is no transfer of heat, so q=0.
isothermal means that the temperature remains constant, so deltaT=0.
- Fri Feb 07, 2020 9:05 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: question on lecture notes about closed system
- Replies: 5
- Views: 209
Re: question on lecture notes about closed system
the beaker not being insulated is important for its distinction from an isolated system, since as it is not insulated heat/energy can be exchanged.
- Fri Feb 07, 2020 9:03 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Significance of open, closed, isolated
- Replies: 22
- Views: 1156
Re: Significance of open, closed, isolated
For a closed system, heat can be exchanged but volume remains constant. An open system has constant pressure yet a changing volume. An isolated system has no energy exchange whatsoever (eg. bomb calorimeter). exactly this! This information can be used to help solve problems. For example, for open s...
- Fri Feb 07, 2020 8:57 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Isolated vs Closed [ENDORSED]
- Replies: 34
- Views: 4294
Re: Isolated vs Closed [ENDORSED]
though you can never truly have an isolated system, if a problem says that the system is insulated (especially if it mentions being well insulated) then it's probably best to consider it isolated
- Sun Feb 02, 2020 11:57 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Hess's Law vs Bond Enthalpies vs Standard Enthalpies of Formation
- Replies: 7
- Views: 299
Re: Hess's Law vs Bond Enthalpies vs Standard Enthalpies of Formation
You use Hess's Law when you are giving the enthalpy change of the total reaction. You use bond enthalpies when they give you the enthalpy change based on bonds, for example C-H 413KJ/mol. And you use standard enthalpies of formation when they give you the enthalpy of a compound. Hess's Law is for t...
- Sat Feb 01, 2020 10:48 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Specific Heat Capacity, Molar Heat capacity, and the Third?
- Replies: 3
- Views: 134
Re: Specific Heat Capacity, Molar Heat capacity, and the Third?
The three things Dr. Lavelle was talking about are heat capacity, molar heat capacity, and specific heat capacity (also called specific heat). Heat capacity is the heat required to raise the temperature of any object by 1 degree Celsius and is a more general term than the others. Molar heat capacit...
- Sat Feb 01, 2020 10:46 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: H and q
- Replies: 7
- Views: 236
Re: H and q
Connie Chen 1E wrote:Delta H refers to the total heat in a system whereas q is the heat that is being transferred. Delta H is equal to q when the pressure is constant, but if the pressure changes, then they are not the same.
this explanation makes a lot of sense, thanks!
- Sat Feb 01, 2020 10:44 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: accuracy of bond enthalpies
- Replies: 8
- Views: 406
Re: accuracy of bond enthalpies
the values are experimental and are an estimate based off of a ton of different possible bond pairings!
- Sat Feb 01, 2020 10:42 pm
- Forum: Phase Changes & Related Calculations
- Topic: Open vs Isolated System
- Replies: 15
- Views: 1319
Re: Open vs Isolated System
it's isolated if energy cannot be exchanged with the surroundings thus, if the system is insulated it is often isolated.
- Sat Feb 01, 2020 10:36 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Hess's Law vs Bond Enthalpies vs Standard Enthalpies of Formation
- Replies: 7
- Views: 299
Hess's Law vs Bond Enthalpies vs Standard Enthalpies of Formation
Hey guys, what should I look for to help me determine which way (Hess's Law, Bond Enthalpies, Standard Enthalpies of Formation) to solve a problem? I know this is old material, but I'm still pretty confused.
- Fri Jan 24, 2020 1:42 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: suggestions
- Replies: 16
- Views: 571
Re: suggestions
crash course is good for concepts, khanacademy is better for understanding the math imo
- Fri Jan 24, 2020 1:40 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Prep for Test 1
- Replies: 16
- Views: 677
Re: Prep for Test 1
no need to memorize, only convert between and analyze
- Fri Jan 24, 2020 1:39 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Temperature
- Replies: 6
- Views: 215
Re: Temperature
heat can be considered a product (in an exothermic reax) or a reactant (in an endothermic reax), and thus adding or removing heat can cause the reaction to shift
- Fri Jan 24, 2020 1:37 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Partial Pressure
- Replies: 5
- Views: 252
Re: Partial Pressure
changing the pressure will cause the reaction to shift to minimize the change, as per le chatlier's principle
- Fri Jan 24, 2020 1:36 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Changes in Pressure
- Replies: 9
- Views: 410
Re: Changes in Pressure
if the pressure is changed without a change in volume (i.e. by adding an inert gas), then the reaction does not shift. If it is changed due to a volume change, then the reaction will favor the side with the fewest overall moles of gas.
- Sun Jan 19, 2020 4:24 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: pH values of weak and strong acids
- Replies: 6
- Views: 319
Re: pH values of weak and strong acids
pH is a -log[H+], which simply means that a lower pH is actually associated with a higher number of H+ ions. So saying a weaker acid has a higher pH actually means it has a lower concentration of H+ ions.
- Sun Jan 19, 2020 4:22 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Concentrations
- Replies: 12
- Views: 412
Re: Concentrations
Changing the concentration or pressure can cause the equilibrium to "favor" the products or reactants, but in both cases the equilibrium constant K will stay the same. Only when temperature is changed is K changed because of a permanent change in the system's dynamics (depending on if it'...
- Sun Jan 19, 2020 4:18 pm
- Forum: Ideal Gases
- Topic: "omitting" the units
- Replies: 7
- Views: 653
Re: "omitting" the units
You're not really "omitting" units, K is unitless (since it is a ratio, the units "cancel")
- Sun Jan 19, 2020 4:15 pm
- Forum: Ideal Gases
- Topic: ICE Table
- Replies: 11
- Views: 840
Re: ICE Table
It might be super helpful to watch some videos, on youtube or khanacademy, that walk you through the ICE table; It's a bit tough to understand from just an explanation, and easier to learn by walking through one.
- Sun Jan 19, 2020 4:12 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Why is K unitless?
- Replies: 10
- Views: 628
Re: Why is K unitless?
K is y a ratio (between products and reactants) and so it does not have units- but so does this mean that all ratios we use in this class will be unitless? Is it safe to apply this rule widely? If it's simply a ratio then most likely, yes. Constants will come with their units but ratios should rema...
- Thu Jan 09, 2020 5:33 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Le Chatelier's and Endo/Exothermic
- Replies: 5
- Views: 245
Re: Le Chatelier's and Endo/Exothermic
for exothermic, heat is released which makes it a product.
for endothermic, heat is absorbed which makes it a reactant.
for endothermic, heat is absorbed which makes it a reactant.
- Thu Jan 09, 2020 5:25 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: determining shift in equilibrium
- Replies: 4
- Views: 240
Re: determining [censored] in equilibrium
Yes, you need to take the ΔH into account. Since ΔH < 0, the forward reaction is exothermic and the reverse reaction is endothermic. In other words, the forward reaction gives off heat and the reverse reaction uses up heat. Since heat is added, Le Chatelier's Principle states that the system will t...
- Thu Jan 09, 2020 5:22 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: confused about ice table
- Replies: 5
- Views: 145
Re: confused about ice table
An ICE table is a method to keep track of initial and final concentrations during an equilibrium reaction. You will write down all your reactants and products and mark their initial concentrations (the I in ICE). Then you will look for the change, which is denoted by x. Reactants will lose concentr...
- Thu Jan 09, 2020 5:20 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Why is K unitless?
- Replies: 10
- Views: 628
Re: Why is K unitless?
it's just a constant representing the ratio of the concentrations. Looking at the units, they would cancel each other out :)
- Thu Jan 09, 2020 5:18 pm
- Forum: Ideal Gases
- Topic: R constant in PV=nRT
- Replies: 9
- Views: 298
Re: R constant in PV=nRT
What exactly is the ideal gas constant in R? When I looked it up, I realized that there are two different ones to use. How do we determine which one to use based on what information is given to us in a problem? the way to determine which constant to use would be to look at the units and find which ...
- Fri Dec 06, 2019 1:14 am
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Strong vs. weak acids and bases
- Replies: 3
- Views: 329
Re: Strong vs. weak acids and bases
Can someone explain why the trend makes sense with details? ok so as I understand it, a strong acid and a strong base dissolve COMPLETELY in a solution. A compound is will dissolve more easily if the bond is easier to break. The bond strength goes down as you go down in a group because the ionic ra...
- Fri Dec 06, 2019 12:48 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Coordination #
- Replies: 6
- Views: 495
Re: Coordination #
Coordination number refers to the number of points to which ligands are attached to the central atom. For example, in [NiCl4]2-, the nickel is attached to 4 Cl- ligands, so the coordination number is 4. so if there's only one molecule attached, but it's attached at 2 sites (chelating and bidentate)...
- Fri Dec 06, 2019 12:34 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Good resources for learning about coordination compounds?
- Replies: 2
- Views: 218
Good resources for learning about coordination compounds?
I'm still having difficulties with coordination compounds and am looking for good material to help me learn. The slides are never posted, the textbook isn't always the best way to learn information, and I haven't found much on Khan Academy. Does anyone have any good online resources for learning abo...
- Thu Dec 05, 2019 4:47 pm
- Forum: Naming
- Topic: sodium bisoxalato(diaqua)ferrate(III) (homework 9C.3D)
- Replies: 2
- Views: 219
sodium bisoxalato(diaqua)ferrate(III) (homework 9C.3D)
Hello, I'm having some trouble with coordination compounds, and so I have a few questions. For problem 9C.3D from the text it's asking to write the formula for sodium bisoxalato(diaqua)ferrate(III). I've been mostly able to do the previous parts of this problem, but I have a few questions regarding ...
- Thu Dec 05, 2019 4:35 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: What is this?
- Replies: 5
- Views: 362
Re: What is this?
You should memorize a few of the common ligands for naming purposes (my TA recommended that we memorize I-, Cl-, Br-, OH-, CN-, C2O4-, H2O, NH3, CO, en). You should know which coordination compounds/ligands can be polydentate due to structure. For coordination number you just need to know how many ...
- Sat Nov 30, 2019 9:07 pm
- Forum: Administrative Questions and Class Announcements
- Topic: finals
- Replies: 5
- Views: 294
Re: finals
805306082 wrote:VLi_1L wrote:Will there be a curve on the final?
There is no curve on the final. At the end of the quarter, the points we have acquired throughout the quarter will be given a grade. Lavelle takes the average points earned and assigns them grades.
ok so there is a curve class wide?
- Sat Nov 30, 2019 9:04 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Difficulty
- Replies: 14
- Views: 919
Re: Difficulty
this class has been one of the hardest for me this semester, but really it all depends on how much work you put in and how efficiently you are using your time.
- Sat Nov 30, 2019 9:03 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Points
- Replies: 11
- Views: 761
Re: Points
In terms of the grading, I don't know if we are getting graded on a week to week basis, or at the end of the quarter. Some people say that if we have 50 by the end of the quarter we get all the points, but others say that we need to do 5 a week or else we do not get the points. If anyone knows the ...
- Sat Nov 30, 2019 9:03 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Test 2 Grades
- Replies: 10
- Views: 672
Re: Test 2 Grades
the test grade is up now for me, so I would assume that it would just depend on when your TA enters the points
- Sat Nov 30, 2019 9:02 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Final Exam Content
- Replies: 14
- Views: 796
Re: Final Exam Content
one of the UAs said that it was cumulative
- Fri Nov 22, 2019 2:08 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Molecular Shape and Polarity
- Replies: 5
- Views: 334
Re: Molecular Shape and Polarity
the shape affects the polarity (due to the possibility of dipole moments cancelling), but as far as i know it doesn't work the other way around
- Fri Nov 22, 2019 2:03 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Test 2
- Replies: 5
- Views: 313
Re: Test 2
just 2E-2F!
- Fri Nov 22, 2019 2:00 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Test 2
- Replies: 14
- Views: 771
Re: Test 2
Does anyone have any advice for remembering molecular shape? Hi Cassandra, I think it's good to start by remembering molecular geometry, where lone pairs are counted as regions of electron density. If there are 2 areas of electron density, the shape is linear and the angle 180 degrees. If there are...
- Fri Nov 22, 2019 2:00 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Test 2
- Replies: 3
- Views: 202
Re: Test 2
the UA from the step-up i went to today said it would be IMF, VSEPR, and sigma/pi bonds :) good luck
- Thu Nov 21, 2019 10:00 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: dipole-dipole vs induced dipole
- Replies: 9
- Views: 589
Re: dipole-dipole vs induced dipole
induced dipole= van der waal's forces= london forces, right?
- Thu Nov 14, 2019 3:37 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: 3F.13
- Replies: 2
- Views: 160
3F.13
How are we supposed to approach this problem? the only thing I can think of is that if the bond lengths are shorter then the attraction is greater.
- Thu Nov 14, 2019 3:24 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: How to remember strength of different intermolecular forces
- Replies: 5
- Views: 807
Re: How to remember strength of different intermolecular forces
You can make a visual model of the intermolecular attractions by drawing the Lewis structures. Based on how close the electrons or nucleus of one molecule/atom can get to another nucleus or electron pair, you can distinguish what will be stronger. For instance, H forms Hydrogen bonds with lone pair...
- Thu Nov 14, 2019 3:22 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: How to remember strength of different intermolecular forces
- Replies: 5
- Views: 807
Re: How to remember strength of different intermolecular forces
Another way to remember the order of intermolecular force strength is to think about the strength of the charges on ions, dipoles, and induced dipoles respectively. Ions are charged atoms of elements, and thus, already have a full charge associated with them (+1, +3, -2 etc.) Thus, the interactions...
- Thu Nov 14, 2019 3:18 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3591836
Re: Post All Chemistry Jokes Here
Ayush Ray 1I wrote:My midterm grade
this is like the SI derived unit for frequency.
it hertz
- Thu Nov 14, 2019 2:29 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: How to remember strength of different intermolecular forces
- Replies: 5
- Views: 807
How to remember strength of different intermolecular forces
If I remember correctly, the types of intermolecular forces directly impact the strength of the bonds and thus influence properties such as melting points. Is there any way to remember which are stronger and which are weaker, other than just memorizing? If any part of my question is incorrect, pleas...
- Thu Nov 07, 2019 7:13 pm
- Forum: Lewis Structures
- Topic: Expanded Octet
- Replies: 10
- Views: 525
Re: Expanded Octet
how can we know if they're likely to use the expanded octect though?
- Thu Nov 07, 2019 7:11 pm
- Forum: Administrative Questions and Class Announcements
- Topic: What homework to turn in per week
- Replies: 7
- Views: 391
Re: What homework to turn in per week
Jessica Tran_3K wrote:I also agree ^^ I think as long as the concept is still relevant, it should be fine.
So we're still good to do stuff from chemical bonds this week? I'm never sure about what we can do.
- Wed Nov 06, 2019 4:38 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Polarizing Power (Trends)
- Replies: 3
- Views: 340
Re: Polarizing Power (Trends)
As anions get larger and larger, their polarizability increases. This is due to the decreasing effective nuclear charge on their valence electrons. As charge increases on cations and as they get smaller, their polarizing power increases since they can get close to the nucleus of anions and exert a ...
- Mon Nov 04, 2019 6:38 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: initial and final variables
- Replies: 4
- Views: 287
Re: initial and final variables
Which ones are initial and which ones are final don't really matter, what matters is that the correct molarity is paired with the correct volume :)
- Mon Nov 04, 2019 6:37 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: Textbook question
- Replies: 3
- Views: 300
Re: Textbook question
Chem_Mod wrote:I'm assuming c is concentration (ie Molarity), M is also molarity, n= number of moles, and v= volume. Can you elaborate on what "m" refers to?
I would assume that m is mass from the equation, since mass * molarity would result in n, the number of moles
- Thu Oct 31, 2019 9:27 pm
- Forum: Resonance Structures
- Topic: Homework 2C 1
- Replies: 3
- Views: 188
Re: Homework 2C 1
a radical is if a compound has an unpaired lone pair electron. radicals tend to be very reactive (due to them being unstable, often one electron away from a full valence shell)
- Thu Oct 31, 2019 9:23 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Formal Charges on Atoms Summed in Ions?
- Replies: 6
- Views: 205
Re: Formal Charges on Atoms Summed in Ions?
Kelvin Chung 1C wrote:In an ion, generally the formal charges should add up to the charge of the ion. In a molecule, I believe the formal charges should add up to 0.
exactly. for example Na has a formal charge of +1 and Cl has a formal charge of -1. Their combined compound NaCl has a formal charge of zero.
- Thu Oct 31, 2019 7:06 pm
- Forum: Octet Exceptions
- Topic: Radicals: Homework Problem #2C1
- Replies: 8
- Views: 462
Re: Radicals: Homework Problem #2C1
Jorge Ramirez_4H wrote:Are radicals that important for the midterm?
I'd like to know this as well. Will we be expected to select what compounds are radicals or not? And does this knowledge have some other application?
- Thu Oct 31, 2019 7:03 pm
- Forum: Ionic & Covalent Bonds
- Topic: Chemical Formula by expected charges
- Replies: 2
- Views: 121
Re: Chemical Formula by expected charges
Megan Ngai- 3B wrote:If you look up periodic table charges on Google images, it shows the charges per group (it's easy to remember). So Mg has a charge of +2 and As has a charge of -3. Therefore it would be Mg3As2.
Oh, this is super useful! Do you know where the formal charge comes from?
- Thu Oct 31, 2019 6:36 pm
- Forum: Ionic & Covalent Bonds
- Topic: Chemical Formula by expected charges
- Replies: 2
- Views: 121
Chemical Formula by expected charges
for 2A.23, it asks to give the chemical formula based on the charges of the elements involved in the compounds. How do you know what the charge is in order to calculate this? In the problem, it asks to give the formula for magnesium arsenide, so if that can be used as an example, it'd be helpful.
- Wed Oct 23, 2019 2:27 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Self-test 1E.2B
- Replies: 2
- Views: 130
Re: Self-test 1E.2B
[Ar]3d104s24p3, remembering to write 3d10 before 4s even though it comes after, because 3d10 is a lower energy level.
- Wed Oct 23, 2019 2:22 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: 4s and 3d
- Replies: 5
- Views: 183
Re: 4s and 3d
Emma Popescu 3D wrote:The 4s is filled first because it is at a lower energy than 3d. However, Dr. Lavelle wants us to put them from lowest to highest energy level (3d and then 4s) because 4s is ionized first.
ok so, 4s is filled first, and 3d is written first? thanks.
- Wed Oct 23, 2019 1:21 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Orbitals 1D.19
- Replies: 3
- Views: 286
Re: Orbitals 1D.19
First of all, s-subshell is l=0, p-subshell is l=1, d-subshell is l=2, f-subshell is l=3 For (a), it is 4p-subshell, so l= 1. If l=1, ml can be -1, 0, 1. Therefore, 4p-subshell can have 3 orbitals. For (b), it is 3d-subshell, so l=2. If l=2, ml can be -2, -1, 0, 1, 2. Therefore, 3d-subshell can hav...
- Wed Oct 23, 2019 12:59 am
- Forum: Trends in The Periodic Table
- Topic: Ionization Energy Across a Period
- Replies: 3
- Views: 129
Re: Ionization Energy Across a Period
it's easier to think about whether an elememt wants to gain or lose an electron to become stable. elements in group 1 and 2 want to lose their electrons to become stable so the ionization energy is low. elements in groups further along the period are closer to becoming stable (filling their electron...
- Wed Oct 23, 2019 12:43 am
- Forum: Quantum Numbers and The H-Atom
- Topic: 1D.1
- Replies: 5
- Views: 261
Re: 1D.1
Mariah wrote:Im kind of confused about this, what would cause an electron to transition into a higher energy level in the first place? Do they do this on their own or because of some outside force?
they can get excited by light, and the energy from the photon allows them to make the transition.
- Thu Oct 17, 2019 10:51 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Homework Problem 1B.27
- Replies: 6
- Views: 310
Re: Homework Problem 1B.27
When doing your calculation, the Δv you should use is 10m/s, which is your maximum uncertainty. Why do we have to use the maximum uncertainty velocity in the Heisenberg Indeterminacy equation? because the maximum uncertainty is the entire range that you are uncertain. when you say plus or minus fiv...
- Thu Oct 17, 2019 9:57 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Atomic Radius
- Replies: 4
- Views: 193
Re: Atomic Radius
To add on, atomic radius increases down a group with each new period because the outermost electrons occupy shells with increasing principal quantum number and therefore lie farther from the nucleus. Atomic radius decreases across a period because new electrons are in the same shell of the atom and...
- Wed Oct 16, 2019 2:44 am
- Forum: Properties of Electrons
- Topic: Question about diffraction patterns
- Replies: 3
- Views: 287
Re: Question about diffraction patterns
I would recommend checking out the crash course video "Light Is Waves: Crash Course Physics #39" . The entire video is very helpful.
https://www.youtube.com/watch?v=IRBfpBPELmE&t=
https://www.youtube.com/watch?v=IRBfpBPELmE&t=
- Wed Oct 16, 2019 2:39 am
- Forum: Properties of Electrons
- Topic: Equations and confused of when to use what
- Replies: 3
- Views: 238
Re: Equations and confused of when to use what
So primarily talking about the E= hv, c= λv, r = h/n^2, Ek = 1/2mv^2, λ = h/mv So in essence, ryberg equation, energy of a photon equation, speed of light equation, de broglies, etc. When do we use such equations? Which equations should I primarily memorize or understand the most? What are the spec...
- Wed Oct 16, 2019 2:19 am
- Forum: Properties of Light
- Topic: Defraction patters
- Replies: 3
- Views: 292
Re: Defraction patters
I am a bit confused about diffraction patters and wavelike properties. Do both light and electrons have wave like properties? If so, does that mean they both have Difraction patterns. Also, what are diffraction patters? ok so basically, light/electrons acting as waves means that there will be some ...
- Fri Oct 11, 2019 12:10 am
- Forum: DeBroglie Equation
- Topic: How to know which equation to use
- Replies: 9
- Views: 609
Re: How to know which equation to use
505106414 wrote:What is a real-world application of this equation?
the v for frequency is actually nu (fun fact), and this is used to calculate wavelength, where c=lambda*v.
- Fri Oct 11, 2019 12:02 am
- Forum: Properties of Light
- Topic: HW 1.A #11
- Replies: 4
- Views: 175
Re: HW 1.A #11
Doris Cho 1D wrote:Not sure what the question is even asking... I understand what the answer is saying but I don't understand the question (?) if anyone knows what I mean...
it's asking how the lines are related, in this case it's asking you to make a connection the the energy levels
- Thu Oct 10, 2019 11:50 pm
- Forum: Properties of Light
- Topic: HW 1.A #9
- Replies: 3
- Views: 179
Re: HW 1.A #9
I matched up the right radiation to each event but I only figured out the 3.3 x 10^-19 J to microwaves because of process of elimination... does it need to be converted to Hz or nm for me to see that it goes with microwaves? I've seen charts with Hz or m but never any with Joules, so that might be ...
- Thu Oct 10, 2019 5:05 pm
- Forum: Properties of Light
- Topic: "Work Function" (from Post-Assessment Module)
- Replies: 4
- Views: 198
Re: "Work Function" (from Post-Assessment Module)
Chem_Mod answered your question already but if you want the equation it's used in, here you go
The work function is the Φ in the equation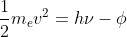 that relates the kinetic energy of the ejected electron to the energy supplied by a photon and the work function.
that relates the kinetic energy of the ejected electron to the energy supplied by a photon and the work function.
The work function is the Φ in the equation
- Thu Oct 10, 2019 12:06 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Rydberg Equation
- Replies: 3
- Views: 764
Re: Rydberg Equation
Rydberg Equation: 1/λ=R(1/n(1)^2−1/n(2)^2) You can change n(1) and n(2), which refer to different energy levels in the Hydrogen atom. For example, you can use n(1)= 1 and n(2)=2 to find the wavelength of light emitted when an electron drops from the n=2 to the n=1 energy levIel (or the wavelength o...
- Fri Oct 04, 2019 9:49 am
- Forum: Limiting Reactant Calculations
- Topic: Problem From Limiting Reactant Module
- Replies: 2
- Views: 187
Re: Problem From Limiting Reactant Module
Hi, to solve this problem, first the equation needs to be fully balanced. There's already a stoichiometric coefficient in front of AgNO3, however there also needs to be a 3 in front of AgCl. So, the resulting equation should be: C6H9Cl3 + 3AgNO3 ---> 3AgCl + C6H9(NO3)3 Then, find the molar masses o...
- Thu Oct 03, 2019 3:18 pm
- Forum: Limiting Reactant Calculations
- Topic: Problem From Limiting Reactant Module
- Replies: 2
- Views: 187
Problem From Limiting Reactant Module
Hey all, I was hoping for help on one of the problems from the module that I simply wasn't able to get even a remotely close answer for. Any explanation would be very helpful. The problem is as follows: 22. According to the following equation, 0.750 g of C 6 H 9 Cl 3 is mixed with 1.000 kg of AgNO 3...
- Tue Oct 01, 2019 12:40 am
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Accuracy vs Precision
- Replies: 10
- Views: 393
Re: Accuracy vs Precision
Jesse H 3 wrote:precision is consistent results while accuracy is how close to the true value we can get.
this response is succinct and how I'm going to think of it from now on. thanks
- Tue Oct 01, 2019 12:36 am
- Forum: SI Units, Unit Conversions
- Topic: Significant 0’s [ENDORSED]
- Replies: 8
- Views: 1385
Re: Significant 0’s [ENDORSED]
if it helps, think about how it would be written in scientific notation. for example .085 would be 8.5*10 -2 (meaning the 0 after the decimal is not significant) while .850 would be written as 8.50*10 -1 (so the zero is significant :). for a number like 100 or 200 the zeros aren't considered signifc...

