Search found 51 matches
- Sun Dec 08, 2019 5:34 pm
- Forum: Dipole Moments
- Topic: dipole dipole
- Replies: 5
- Views: 419
Re: dipole dipole
Hydrogen bonds are stronger than dipole-dipole. The strength for intermolecular forces arranged from lowest to highest is London forces, dipole-dipole, H-bonding, and ion-dipole.
- Sun Dec 08, 2019 5:30 pm
- Forum: Naming
- Topic: Cation vs. Anion Transition Metal
- Replies: 3
- Views: 427
Re: Cation vs. Anion Transition Metal
The suffix -ate is added to the metal when the complex is anionic. So if the overall charge of the complex is negative like it was in Part C, the name of cobalt became cobaltate.
- Sun Dec 08, 2019 5:15 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: oxidation number
- Replies: 3
- Views: 404
Re: oxidation number
The elements outside of the bracket affect the oxidation number because in order for the coordination compound to be neutral, the charges of the elements within the bracket and outside of the bracket must cancel each other out.
- Sun Dec 08, 2019 5:07 pm
- Forum: Naming
- Topic: NH4[PtCl3(NH3)]
- Replies: 6
- Views: 863
Re: NH4[PtCl3(NH3)]
Whenever a complex is anionic, meaning that it has an overall negative charge, the ending of the metal will be -ate.
- Sun Dec 08, 2019 4:56 pm
- Forum: Naming
- Topic: bis- tris- tetrakis-
- Replies: 8
- Views: 628
Re: bis- tris- tetrakis-
The prefixes -bis, -tris, and -tetrakis are added when the ligand already has a Greek prefix or if the complex is polydentate.
- Sun Dec 01, 2019 10:56 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Ligands
- Replies: 4
- Views: 411
Re: Ligands
A chelating ligand is when a ligand has more than one bond with the metal atom.
- Sun Dec 01, 2019 10:41 pm
- Forum: Lewis Acids & Bases
- Topic: lewis vs. bronsted
- Replies: 10
- Views: 543
Re: lewis vs. bronsted
Lewis acids are electron acceptors and Bronsted acids are proton donors. Lewis bases are electron donors and Bronsted bases are proton acceptors.
- Sun Dec 01, 2019 10:30 pm
- Forum: Lewis Acids & Bases
- Topic: 6A.13
- Replies: 1
- Views: 220
Re: 6A.13
BF3 is a Lewis acid because Boron has an empty p-orbital. This means that it can accept an electron pair, which by definition is a Lewis acid. The new bond would be formed with the Boron.
- Sun Dec 01, 2019 10:16 pm
- Forum: Bronsted Acids & Bases
- Topic: Final
- Replies: 13
- Views: 854
Re: Final
As far as I know, the Final will be cumulative. However, there will be more of an emphasis on the topics after the midterm. Just make sure you know how to do everything mentioned on the outlines.
- Sun Dec 01, 2019 7:22 pm
- Forum: Naming
- Topic: Roman Numeral
- Replies: 13
- Views: 915
Re: Roman Numeral
Debora Fernandez Clemente_ 4H wrote:How do you calculate the oxidation number of the metal in a complex?
To calculate the oxidation number of the metal in a complex, you would use the equation:
(# metal atoms)(oxidation number of the metal)+
- Mon Nov 25, 2019 7:04 am
- Forum: Ionic & Covalent Bonds
- Topic: ligands
- Replies: 7
- Views: 1684
Re: ligands
Ligands are Lewise bases (electron donors) that are attracted to the central atom since it is a Lewis acid and transition metal. So the ligand can be either an ion or compound.
- Mon Nov 25, 2019 6:56 am
- Forum: Dipole Moments
- Topic: Melting points
- Replies: 15
- Views: 1344
Re: Melting points
Melting points are determined based on their intermolecular forces. The more intermolecular forces it has, the more energy it would take to break bonds, resulting in a higher boiling and melting point.
- Mon Nov 25, 2019 6:53 am
- Forum: Sigma & Pi Bonds
- Topic: Molecular Shape
- Replies: 11
- Views: 730
Re: Molecular Shape
Technically, sigma and pi bonds do affect bond length and bond angles. However, the change is minimal so it therefore doesn't have much influence on the molecular shape. Furthermore, the VSEPR model counts single, double, and triple bonds as one region.
- Mon Nov 25, 2019 6:45 am
- Forum: Sigma & Pi Bonds
- Topic: sigma and pi bonds
- Replies: 27
- Views: 1676
Re: sigma and pi bonds
Single bonds are all sigma bonds.
Double bonds are all sigma and pi bonds.
Triple bonds are all sigma and 2 pi bonds.
Double bonds are all sigma and pi bonds.
Triple bonds are all sigma and 2 pi bonds.
- Mon Nov 25, 2019 6:40 am
- Forum: Electronegativity
- Topic: Electronegativity
- Replies: 16
- Views: 1062
Re: Electronegativity
Electronegativity is basically how much an atom attracts electrons. So if there is a high electronegativity difference, it means that there is a strong bond and that the bond length will be shorter.
- Sun Nov 17, 2019 10:18 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: bond angles
- Replies: 8
- Views: 456
Re: bond angles
Bond angles get smaller with more lone pairs at the central atom due to lone pair repulsion. Lone pairs want to be far away from the other elements so it will repel the other bonds away from it, causing a decrease in bond angle.
- Sun Nov 17, 2019 10:04 pm
- Forum: Electronegativity
- Topic: 2D.3
- Replies: 3
- Views: 407
Re: 2D.3
The periodic trend for electronegativity is that electronegativity decreases as you go down the periodic table and electronegativity increases as you go from left to right of the periodic table (excluding noble gases). With that being said, although Ba and Be are the same distance from Br in terms o...
- Sun Nov 17, 2019 9:52 pm
- Forum: Octet Exceptions
- Topic: Octet expansion
- Replies: 6
- Views: 550
Re: Octet expansion
An expanded octet can occur if the atomic number of the central atom is 11 or more. This is because elements of the third principal energy level and above have a d-oribital that it can fill.
- Sun Nov 17, 2019 9:37 pm
- Forum: Lewis Structures
- Topic: Balanced Lewis Structures
- Replies: 6
- Views: 447
Re: Balanced Lewis Structures
You would use formal charges to determine which way to write a Lewis structure that is most stable. However, Lewis structures are 2-D and only portray which atoms are bonded together rather than in which position (such as horizontally or vertically). In order to determine it's molecular shape, you w...
- Sun Nov 17, 2019 9:26 pm
- Forum: Bronsted Acids & Bases
- Topic: Test2
- Replies: 7
- Views: 425
Re: Test2
My TA said that Test 2 would consist of all the material we covered after Topic 2D. In regards of memorizing, I would highly recommend memorizing the different molecular shapes and bond angles since I highly doubt that they would provide us with that kind of information.
- Sun Nov 10, 2019 8:32 pm
- Forum: Electronegativity
- Topic: Electron Affinity and Electronegativity
- Replies: 7
- Views: 437
Re: Electron Affinity and Electronegativity
Electron affinity refers to the amount of energy released when an electron is added to an atom. Electronegativity, on the other hand, refers to how much an atom attracts electrons. So an easier way to think of it is electron affinity is how much they want electrons whereas electronegativity is how m...
- Sun Nov 10, 2019 8:25 pm
- Forum: Electronegativity
- Topic: definitions
- Replies: 8
- Views: 494
Re: definitions
Ionization energy refers to the minimum amount of energy needed to remove an electron whereas electron affinity is the amount of energy released when an electron is added.
- Sun Nov 10, 2019 8:05 pm
- Forum: Lewis Structures
- Topic: Lewis acids and bases?
- Replies: 7
- Views: 410
Re: Lewis acids and bases?
Are the cations usually Lewis acids and the anions usually Lewis bases? Yes! So Lewis acids are electron pair acceptors and Lewis bases are electron pair donors. Ions with a positive charge signifiy that it can accept a pair of nonbonding electrons. Ions with a negative charge signify that it can d...
- Sun Nov 10, 2019 7:51 pm
- Forum: Lewis Structures
- Topic: Midterm grades
- Replies: 26
- Views: 1439
Re: Midterm grades
My TA said that we would be getting our midterm grades by Wednesday on 11/13.
- Sun Nov 10, 2019 7:47 pm
- Forum: Ionic & Covalent Bonds
- Topic: Both types of bonds
- Replies: 6
- Views: 382
Re: Both types of bonds
It is possible, however the differentiating factor between the two types of bonds comes down to their physical and chemical properties. For example, covalent bonds are typically between two nonmetals whereas ionic bonds are typically between a metal and a nonmetal. You could also observe their diffe...
- Sun Nov 03, 2019 9:32 pm
- Forum: Ionic & Covalent Bonds
- Topic: Ionic vs Covalent
- Replies: 14
- Views: 887
Re: Ionic vs Covalent
Ionic bonds are usually between a metal and a nonmetal. Covalent bonds, on the otherhand, are typically between two nonmetals. Another way to differentiate is by looking at the electronegativity difference. If the electronegativity difference is greater than 2, then it is an ionic bond. If the elect...
- Sun Nov 03, 2019 9:20 pm
- Forum: Properties of Light
- Topic: Focus 1.3
- Replies: 2
- Views: 178
Re: Focus 1.3
In order to find the amount of power produced in watts, you would use the equation: E = \frac{hc}{\lambda } This equation is used to help you find the energy per photon. From here, you would plug in Planck's constant (6.62608 x 10-34 J.s), the speed of light (2.998 x 108 m/s), and the given waveleng...
- Sun Nov 03, 2019 9:08 pm
- Forum: Properties of Light
- Topic: HW #1.7
- Replies: 5
- Views: 1393
Re: HW #1.7
Part a: So in order to convert the answer from meters to nanometers, you would try to cancel out the meters in order to get nm by itself. For example: (4.22 * 10^{-7} m) * (\frac{1 nm}{10^{-9} m}) This would leave the result as 422 nm. Part b: This follows the same concept as Part a,...
- Sun Nov 03, 2019 8:45 pm
- Forum: Properties of Light
- Topic: 1A.15
- Replies: 3
- Views: 305
Re: 1A.15
Yes! Great start so far. From here, you would try to get \frac{1}{n_{2}^{2}} by itself by moving all other values to the other side to get: \frac{-2.922 * 10^{15}}{3.29 * 10^{15}} + 1 = \frac{1}{n_{2}^{2}} From here, you would solve the left side which is approximately: 0.11185 = \frac{1}{n_{2}^{2}}...
- Sun Nov 03, 2019 8:34 pm
- Forum: *Black Body Radiation
- Topic: Info for Midterm
- Replies: 13
- Views: 1117
Re: Info for Midterm
I don't believe this concept will be on the test. Just focus on the learning objectives that Lavelle listed in each outline. After looking at the last test, I'm pretty sure he would test us on problems that are similar to the homework problems.
- Sun Oct 27, 2019 9:28 pm
- Forum: Ionic & Covalent Bonds
- Topic: Ionic VS. Covalent Bond
- Replies: 8
- Views: 467
Re: Ionic VS. Covalent Bond
Ionic bonds consist of ions in a ratio that result in electrical neutrality. They consist of a metal (cation) and a nonmetal (anion). Metals are cations since they give electrons, whereas nonmetals are anions because they gain electrons. On the other hand, covalent bonds consist of electrically neut...
- Sun Oct 27, 2019 9:06 pm
- Forum: Einstein Equation
- Topic: Total Energy of Light
- Replies: 2
- Views: 238
Re: Total Energy of Light
To calculate the energy of a photon, you would use E = hv. However, some problems might require you to use E = hc/lambda instead.
- Sun Oct 27, 2019 8:48 pm
- Forum: DeBroglie Equation
- Topic: 1.3
- Replies: 2
- Views: 219
Re: 1.3
Since the problem is asking you to find the amount of power produced in watts, you would use the equation: E = \frac{hc}{\lambda } This would help you find the energy per photon. So then, you plug in Planck's constant (6.62608 x 10 -34 J.s), the speed of light (2.998 x 10 8 m/s) and the given wavele...
- Sun Oct 27, 2019 8:13 pm
- Forum: Ionic & Covalent Bonds
- Topic: Double bond placement
- Replies: 15
- Views: 874
Re: Double bond placement
You can use the octet rule, but I personally like to look at bonding preferences, such as how hydrogen has one bond, oxygen has two bonds, nitrogen has three bonds, and carbon has four bonds.
- Sun Oct 27, 2019 8:07 pm
- Forum: Ionic & Covalent Bonds
- Topic: Covalent Bonds
- Replies: 11
- Views: 562
Re: Covalent Bonds
Covalent bonds can only be formed by nonmetals, and metals are more likely to form ionic bonds. Remember that metals often times become cations because it is easier for them to give up electrons. Non metals have high ionization energy. Nonmetals can also form ionic bonds, right? An ionic bond is ty...
- Sun Oct 20, 2019 8:31 pm
- Forum: Properties of Light
- Topic: 1A.9 Energy of Photon
- Replies: 12
- Views: 493
Re: 1A.9 Energy of Photon
This problem in particular requires the use of two equations: c= (wavelength)(frequency) E = h v E represents the energy of a photon h represents Planck's constant (6.626 x 10[/sup]-34[/sup] J * s) v represents the frequency of radiation Since the first and third parts of the problem provide you wit...
- Sun Oct 20, 2019 7:56 pm
- Forum: Properties of Light
- Topic: What are the units of hertz
- Replies: 41
- Views: 2118
Re: What are the units of hertz
Victoria Otuya 4F wrote:Is hz the same as s-1?
Hz is one cycle per second (1/s) and it is the same as s-1. It just becomes s-1 since it is read in the numerator rather than the denominator.
- Sun Oct 20, 2019 7:49 pm
- Forum: Properties of Light
- Topic: Question 1A.3
- Replies: 3
- Views: 168
Re: Question 1A.3
I think the best way to approach this problem is to reason through each choice to see why each one does or doesn't happen. So for example: (a) The speed of radiation doesn't decrease as the frequency of electromagnetic radiation decreases since it is a constant (c= 2.998 x 10 8 m/s). (b) The wavelen...
- Sun Oct 20, 2019 7:17 pm
- Forum: Properties of Light
- Topic: Question 1A.15
- Replies: 5
- Views: 270
Re: Question 1A.15
The equation we will be using to solve this problem is: \nu = R (\frac{1}{n_{1}^{2}}- \frac{1}{n_{2}^{2}}) So the problem states that the spectral line 102.6 nm is in the ultraviolet spectrum of atomic hydrogen. We already know that the Lyman series is the set of lines in the ultraviolet reg...
- Sun Oct 20, 2019 2:33 pm
- Forum: Properties of Light
- Topic: Unit for Wavelength
- Replies: 34
- Views: 2467
Re: Unit for Wavelength
c = \lambda \nu The standard units are: c= Speed of Light in meters per second (m/s) \lambda = wavelength in meters (m) \nu = frequency in Hertz (Hz or 1/s) However, the wavelength can be in other length units if the problem states otherwise. In that case, you would have to use dimensional analysis.
- Sun Oct 13, 2019 4:55 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: E 17
- Replies: 6
- Views: 811
Re: E 17
Parts (a) and (b) use the equation m= n * M.
m- mass of the sample
n- amount in moles
M- molar mass
Part (c) uses the equation n = N/NA
n- amount in moles
N- number of objects
NA- Avogadro's constant
m- mass of the sample
n- amount in moles
M- molar mass
Part (c) uses the equation n = N/NA
n- amount in moles
N- number of objects
NA- Avogadro's constant
- Sun Oct 13, 2019 3:35 pm
- Forum: Balancing Chemical Reactions
- Topic: Combustion
- Replies: 17
- Views: 1049
Re: Combustion
Combustion refers to a chemical reaction that includes burning in air. The most basic set-up is usually:
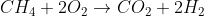 O
O
The main point is that the products will always include CO2 and H2O. It's just a matter of balancing the equation based on what is given to you.
The main point is that the products will always include CO2 and H2O. It's just a matter of balancing the equation based on what is given to you.
- Sun Oct 13, 2019 3:10 pm
- Forum: Balancing Chemical Reactions
- Topic: When are atoms lost or created?
- Replies: 14
- Views: 1794
Re: When are atoms lost or created?
The law of conservation of mass states that atoms are neither created or destroyed. Therefore, if a chemical reaction occurs, then the chemical formula will result in the reactants and/or products being multiplied by factors that result in the same number of atoms.
- Sun Oct 13, 2019 2:56 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Theoretical vs. Actual Yield
- Replies: 38
- Views: 14058
Re: Theoretical vs. Actual Yield
The actual yield will most of the time be less than the theoretical yield because of competing reactions, measurement errors, and/or limiting reactants. The theoretical yield is the maximum quantity of product that can be obtained from a chemical reaction.
- Sun Oct 13, 2019 9:19 am
- Forum: SI Units, Unit Conversions
- Topic: Practice Problems?
- Replies: 11
- Views: 610
Re: Practice Problems?
If you want more practice problems aside from the ones Lavelle assigned, I would highly recommend participating in the Step-Up Program and Workshops. Some clubs also provide their own Peer Learning Groups for certain subjects. Khan Academy is also a great resource to use.
- Sat Oct 05, 2019 10:49 am
- Forum: Empirical & Molecular Formulas
- Topic: Empirical Formula Purpose
- Replies: 13
- Views: 3966
Re: Empirical Formula Purpose
Empirical formulas show the relative number of atoms a molecule has. The ratio can then be used to determine the molecular formula, which is the actual number of atoms in a molecule. However, there are some cases in which the empirical formula is also the molecular formula.
- Sat Oct 05, 2019 10:27 am
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Avogadro's number
- Replies: 9
- Views: 541
Re: Avogadro's number
Hey, I was just curious. What does Avogadro's number actually represent? Thank you guys. :) Avogadro’s number is referring to the pure number “6.0221 x 10^23,” which is unitless. On the other hand, Avogadro’s constant is a constant with units, 6.0221 x 10^23 mol^-1, which represents the number of o...
- Wed Oct 02, 2019 3:28 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Balancing Equations [ENDORSED]
- Replies: 25
- Views: 2059
Re: Balancing Equations [ENDORSED]
When balancing equations how do you determine what to balance first? My TA recommended that we start with balancing the elements that appear the least in a chemical equation. For example: C_{4}H_{10} + O_{2} \rightarrow CO_{2} + H_{2}O You would start with either the Carbons or Hydrogens since they...
- Tue Oct 01, 2019 7:14 pm
- Forum: Significant Figures
- Topic: Rounding the elements
- Replies: 12
- Views: 863
Re: Rounding the elements
I would recommend using the exact values provided by the periodic table and then use the significant figure rules at the end of the calculation to round off properly.
- Tue Oct 01, 2019 7:06 pm
- Forum: Empirical & Molecular Formulas
- Topic: Rounding [ENDORSED]
- Replies: 12
- Views: 822
Re: Rounding [ENDORSED]
I just looked over Dr. Lavelle’s worksheet regarding significant figures. According to Lavelle, one of the rules for rounding off is to wait until the end of a calculation. For calculations using multiplication and division, your answer should have the same number of significant figures as the value...
- Tue Oct 01, 2019 6:11 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Advice from a Medical Student - Part II [ENDORSED]
- Replies: 298
- Views: 260394
Re: Advice from a Medical Student - Part II [ENDORSED]
Thank you so much for sharing your experience as a medical student! It is truly inspiring to hear about all the things you have accomplished over the past three years. I am also pursuing a career in medicine. However, I am a first-generation student and I have no family members or friends who are in...

