Does anyone know which of the ligands on the ligand naming sheet Lavelle gave us are polydentate and require the "bis, tris, tetrakis.." naming?
So far I have: EDTA, ethylene diamine, diethylene triamine, oxalato and carbonato. Are any of these errors/ should I add any?
Search found 58 matches
- Sat Dec 07, 2019 4:44 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: polydentate naming
- Replies: 1
- Views: 162
- Sat Dec 07, 2019 11:52 am
- Forum: Lewis Acids & Bases
- Topic: strength of bases
- Replies: 1
- Views: 215
strength of bases
I know that group 1 and 2 metal hydroxides are strong bases, but what about metal oxides? I know it's been said that Li2O is a strong base, but is this an exception to the list of strong bases?
- Fri Dec 06, 2019 12:45 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: speed of light
- Replies: 2
- Views: 270
speed of light
Today in Lavelle's review, he said that we know the electron cannot be inside the nucleus of an atom because the calculated uncertainty in the velocity was greater than the speed of light. Why are we comparing it to the speed of light? Also, if it were smaller than 3x10^8, could it then be found ins...
- Wed Dec 04, 2019 8:23 pm
- Forum: Einstein Equation
- Topic: units for energy
- Replies: 2
- Views: 366
units for energy
What are the units for energy in the E=hv equation? Is it Joules or Joules/photon?
- Wed Dec 04, 2019 12:54 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: atomic orbital probability
- Replies: 2
- Views: 393
atomic orbital probability
What does it mean to "describe the interpretation of atomic orbitals in terms of probability?" (this is an objective on the quantum outline and I don't understand what it means)
- Wed Dec 04, 2019 12:48 pm
- Forum: Conjugate Acids & Bases
- Topic: adding H to a molecule
- Replies: 1
- Views: 234
adding H to a molecule
When something asks for the conjugate acid of a molecule like CH3NH2, how do you know where to add the hydrogen to (can you explain general terms)? Also, if you are adding to a molecule like C6H5O-, how would you know to add the H to the O and not to the existing H's? (this makes C6H5OH and NOT C6H6O)
- Wed Dec 04, 2019 12:44 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: intermolecular energy equation
- Replies: 1
- Views: 560
intermolecular energy equation
On the chemical bonds outline at the very bottom, it says we should know the parameters that contribute to the intermolecular energy (E is proportional to 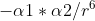 ). Can someone explain what this means and when we would use it?
). Can someone explain what this means and when we would use it?
- Mon Dec 02, 2019 5:32 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: increasing pH and decreasing pH
- Replies: 4
- Views: 330
increasing pH and decreasing pH
More protons means lower pH which means a stronger acid? Correct?
- Mon Dec 02, 2019 5:27 pm
- Forum: Amphoteric Compounds
- Topic: water
- Replies: 3
- Views: 196
water
Is water an amphoteric compound?
- Mon Dec 02, 2019 5:26 pm
- Forum: Amphoteric Compounds
- Topic: water
- Replies: 2
- Views: 215
water
Is water an amphoteric compound?
- Mon Dec 02, 2019 5:25 pm
- Forum: Bronsted Acids & Bases
- Topic: Defining brosted and lewis
- Replies: 5
- Views: 450
Defining brosted and lewis
So all Lewis acids are Bronsted acids but not all Bronsted acids are Lewis acids?
- Tue Nov 26, 2019 12:07 pm
- Forum: Lewis Acids & Bases
- Topic: identification of acid/base
- Replies: 2
- Views: 189
identification of acid/base
When asked which atom was the lewis acid or base, should you include the charge if it has one? Or is just saying copper (for example) sufficient?
- Tue Nov 26, 2019 12:05 pm
- Forum: Hybridization
- Topic: when to use
- Replies: 1
- Views: 117
when to use
If asked to show the electron configuration for an atom, would you have to be asked specifically for hybridization or is there certain cases where you should know to use hybridization?
- Tue Nov 26, 2019 12:02 pm
- Forum: Hybridization
- Topic: extra p orbital
- Replies: 1
- Views: 148
extra p orbital
Can someone explain why adding an electron to the atomic p orbital requires less energy than adding it to the hybridized orbital?
- Tue Nov 26, 2019 11:56 am
- Forum: Hybridization
- Topic: sigma and pi bonds
- Replies: 3
- Views: 224
sigma and pi bonds
How can you figure out the hybridization of sigma and pi bonds? How do the hybridization's differ if something is double bonded and has sigma and pi bonds?
- Tue Nov 26, 2019 11:53 am
- Forum: Coordinate Covalent Bonds
- Topic: identification
- Replies: 1
- Views: 230
identification
What's the best way to identify if something is a coordination compound?
- Thu Nov 21, 2019 1:07 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: test 2
- Replies: 3
- Views: 293
test 2
Will we not get our test 2 grades back in discussion next week since some people don't have discussion for the thanksgiving holiday?
- Wed Nov 20, 2019 9:33 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: cancelling dipoles
- Replies: 2
- Views: 196
cancelling dipoles
Dipoles only cancel for a tetrahedral if all the atoms bonded to the central atom are the same right? Is it possible for different atoms to cancel each other out?
- Tue Nov 19, 2019 1:39 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: h bonding and dipole dipole
- Replies: 4
- Views: 290
h bonding and dipole dipole
If a molecule has hydrogen bonding, does that automatically mean it has dipole dipole too?
- Tue Nov 19, 2019 10:32 am
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: kJ/mol
- Replies: 1
- Views: 134
kJ/mol
Should we know the value of the kJ/mol for each kind of bond? Like do we need to know that hydrogen bonding is -20kJ/mol or just know that h-bonding is fairly strong?
- Tue Nov 19, 2019 10:31 am
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: how to determine ion-ion
- Replies: 4
- Views: 275
how to determine ion-ion
Is it only ion-ion if the two atoms involved show a charge? Basically, is NaCl ion-ion or would it need to be Na+ and Cl- to be ion ion?
- Mon Nov 18, 2019 9:21 pm
- Forum: Ionic & Covalent Bonds
- Topic: HS vs HO
- Replies: 4
- Views: 859
HS vs HO
Is HS more tightly bound or is HO? why?
- Wed Nov 13, 2019 10:08 am
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Similar terms
- Replies: 3
- Views: 276
Similar terms
So is induced dipole only equivalent with London/dispersion if two induced dipoles are attached (induced dipole-induced dipole)?
- Wed Nov 13, 2019 10:05 am
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: hydrogen bonding
- Replies: 3
- Views: 180
hydrogen bonding
For hydrogen bonding to occur, can hydrogen only be bonded to O,N, or F? Or can it be bonded to those elements and another element in the same molecule?
- Wed Nov 13, 2019 10:03 am
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: ion-dipole
- Replies: 2
- Views: 225
ion-dipole
What is an example of an ion dipole? I am confused how this IM force is possible
- Wed Nov 13, 2019 10:02 am
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Ion-Ion
- Replies: 2
- Views: 102
Ion-Ion
Is ion-ion the same thing as ionic bonding? If not, what is the difference?
- Wed Nov 13, 2019 10:01 am
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Covalent bonding
- Replies: 5
- Views: 389
Covalent bonding
Are covalent bonds an intermolecular force? Why or why not?
- Thu Nov 07, 2019 6:51 pm
- Forum: Ionic & Covalent Bonds
- Topic: covalent relationship to polarizability
- Replies: 4
- Views: 171
covalent relationship to polarizability
How does high polarizability and high polarizing power lead to a more covalent bond? What is the relationship between these?
- Thu Nov 07, 2019 6:49 pm
- Forum: Bond Lengths & Energies
- Topic: Lone pairs
- Replies: 1
- Views: 89
Lone pairs
If lone pairs cause bonds to be weaker, does having more sets of lone pairs make a bond more and more weak? Or is it just the presence of lone pairs in general?
- Thu Nov 07, 2019 6:48 pm
- Forum: Ionic & Covalent Bonds
- Topic: polarizing power
- Replies: 7
- Views: 441
polarizing power
If polarizability increases down and left, does polarizing power increase up and right?
- Thu Nov 07, 2019 6:47 pm
- Forum: Bond Lengths & Energies
- Topic: dissociation energy
- Replies: 5
- Views: 372
dissociation energy
What is the trend for dissociation energy? Is there a trend at all?
- Thu Nov 07, 2019 6:45 pm
- Forum: Ionic & Covalent Bonds
- Topic: Polarizability
- Replies: 6
- Views: 457
Polarizability
Does polarizability follow the same trend as atomic size? Are there any exceptions? Why do they follow the same trend?
- Tue Nov 05, 2019 4:01 pm
- Forum: Bond Lengths & Energies
- Topic: Coordination compounds [ENDORSED]
- Replies: 1
- Views: 749
Coordination compounds [ENDORSED]
Do we need to know coordination compounds for the midterm? If so, to what extent?
- Fri Nov 01, 2019 4:46 pm
- Forum: Octet Exceptions
- Topic: radical
- Replies: 5
- Views: 445
radical
Can someone explain radicals and how they fit into octet exceptions?
- Fri Nov 01, 2019 4:44 pm
- Forum: Bond Lengths & Energies
- Topic: dissociation energy
- Replies: 7
- Views: 302
dissociation energy
Why is disassociation energy always positive?
- Fri Nov 01, 2019 4:43 pm
- Forum: Dipole Moments
- Topic: identification
- Replies: 3
- Views: 232
identification
How do you know when a dipole moment occurs between two atoms?
- Fri Nov 01, 2019 4:41 pm
- Forum: Dipole Moments
- Topic: induced dipole
- Replies: 4
- Views: 180
induced dipole
What is an induced dipole and how do you identify it?
- Fri Nov 01, 2019 4:39 pm
- Forum: Dipole Moments
- Topic: direction of arrow
- Replies: 2
- Views: 172
direction of arrow
In a dipole moment, does the arrow always point from the positively charged atom to the negatively charged atom?
- Thu Oct 24, 2019 11:35 am
- Forum: Ionic & Covalent Bonds
- Topic: valence
- Replies: 3
- Views: 242
valence
Are valence electrons the same thing as valence of an atom? How does all this relate to covalent bonds?
- Thu Oct 24, 2019 11:34 am
- Forum: Ionic & Covalent Bonds
- Topic: strength of bonds
- Replies: 14
- Views: 1020
strength of bonds
What determines bond strength? Is this a value you can calculate?
- Thu Oct 24, 2019 11:32 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: 2nd electron
- Replies: 4
- Views: 170
2nd electron
Can someone explain why removing the 2nd electron is harder?
- Thu Oct 24, 2019 11:31 am
- Forum: Trends in The Periodic Table
- Topic: Trends
- Replies: 5
- Views: 189
Trends
As someone who in unfamiliar with chemistry, what periodic trends are the most important for this class?
- Thu Oct 24, 2019 11:28 am
- Forum: Ionic & Covalent Bonds
- Topic: Cation
- Replies: 23
- Views: 1829
Cation
What is are cations and anions and why are they important?
- Tue Oct 15, 2019 11:47 am
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Light's effects
- Replies: 8
- Views: 281
Light's effects
Can someone explain how light is able to affect an electron's momentum and position but not a baseball? Does it relate to size or mass or both?
- Tue Oct 15, 2019 11:46 am
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Time of Use
- Replies: 5
- Views: 116
Time of Use
When will we know to use the Heisenberg equation? Will the question say something about indeterminacy or should we use it when asked for momentum and position at all?
- Tue Oct 15, 2019 11:43 am
- Forum: Properties of Light
- Topic: Relationships
- Replies: 3
- Views: 173
Relationships
I'm confused how light, energy, and photons are all related? Can you have a photon of light and a photon of energy? Is light a source of energy?
- Tue Oct 15, 2019 11:42 am
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Momentum
- Replies: 7
- Views: 336
Momentum
I'm not familiar with momentum. In the Heisenberg equation will we ever have to solve for momentum or will it be given?
- Mon Oct 14, 2019 8:16 am
- Forum: Properties of Electrons
- Topic: Two Kinds of Properties
- Replies: 3
- Views: 161
Two Kinds of Properties
What does it mean for an electron to have wavelike and particle like properties?
- Wed Oct 09, 2019 8:23 am
- Forum: Photoelectric Effect
- Topic: h and c
- Replies: 2
- Views: 144
h and c
What do 'h' and 'c' represent in the worked example from last lecture? Where do these numbers come from and how do we solve for them?
- Wed Oct 09, 2019 8:21 am
- Forum: Properties of Electrons
- Topic: electron energy
- Replies: 4
- Views: 199
electron energy
What is meant by 'energy needed to remove an electron'? Does this value vary and how would we know how much energy is needed?
- Wed Oct 09, 2019 8:20 am
- Forum: Photoelectric Effect
- Topic: Photon [ENDORSED]
- Replies: 6
- Views: 312
Photon [ENDORSED]
What exactly is a photon and how does it relate to electrons in the photoelectric effect?
- Wed Oct 09, 2019 8:18 am
- Forum: Photoelectric Effect
- Topic: Work Function
- Replies: 3
- Views: 285
Work Function
What is the work function and which part of the equation from class represents the work function?
- Wed Oct 09, 2019 8:16 am
- Forum: Photoelectric Effect
- Topic: Quantum
- Replies: 5
- Views: 305
Quantum
What does it mean for variables to be 'quantized' or 'discrete'?
- Wed Oct 02, 2019 6:26 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: Molarity
- Replies: 9
- Views: 379
Molarity
I know that the fraction of molarity is: moles of solution over volume of solution, but what does molarity actually represent and when would I need to calculate it?
- Wed Oct 02, 2019 6:23 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Molar Mass
- Replies: 10
- Views: 1125
Molar Mass
Is the molar mass calculated from the periodic table or does it have to be given? What is actually meant by molar mass?
- Wed Oct 02, 2019 6:22 pm
- Forum: Limiting Reactant Calculations
- Topic: Limiting reactant
- Replies: 4
- Views: 242
Limiting reactant
Is the limiting reactant the one with the smallest number of moles? I am confused on how to tell which one actually limits the reaction
- Wed Oct 02, 2019 6:21 pm
- Forum: Limiting Reactant Calculations
- Topic: Steps to calculate limiting reagant
- Replies: 4
- Views: 502
Steps to calculate limiting reagant
When solving for the limiting reactant, what step follows directly after calculating moles of each molecule? Also can you explain the next few steps and why they matter?
- Wed Oct 02, 2019 6:17 pm
- Forum: Significant Figures
- Topic: Powers of 10
- Replies: 10
- Views: 636
Powers of 10
Can someone explain when we know to write answers in powers of 10 and when it's not necessary?

