Search found 102 matches
- Wed Mar 11, 2020 1:06 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Saying Thank You to Dr. Lavelle
- Replies: 490
- Views: 547566
Re: Saying Thank You to Dr. Lavelle
Dear Dr. Lavelle, Prior to attending UCLA I had very little chemistry experience and was afraid of what I had signed up for. I am very grateful I have had the opportunity to learn from you for the past 2 quarters and be well introduced to one of my now favorite branches of science. I really apprecia...
- Wed Mar 11, 2020 12:41 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Concentration
- Replies: 9
- Views: 563
Re: Concentration
E=E^0-\frac{RT}{nf}ln([P]/[R]) E and T were given and R is a constant (8.314 J/Kmol). E^0= 0 since this is a concentration cell. The question also provided that the cathode reaction had some concentration which for concentration cells is the [R] in Q. Considering that the redox reaction was...
- Wed Mar 11, 2020 12:33 pm
- Forum: Second Order Reactions
- Topic: linear graph
- Replies: 7
- Views: 550
Re: linear graph
A linear plot is in the form y=mx+b and for second order 1/[A] = kt + 1/[A]0 so the plot is 1/[A] versus time.
- Wed Mar 11, 2020 12:21 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Catalysts vs. Intermediates
- Replies: 8
- Views: 891
Re: Catalysts vs. Intermediates
A catalyst is something that lowers the activation energy of a reaction and leaves K(equilibrium) the same, a catalyst should appear on both sides of the total net reaction, while an intermediate is an actual product between steps of the reaction that will not appear in the final net reaction.
- Wed Mar 11, 2020 12:17 pm
- Forum: Balancing Redox Reactions
- Topic: Balancing basic reactions
- Replies: 8
- Views: 564
Re: Balancing basic reactions
For balancing basic reactions, add H2O's to balance oxygens, after that add H+'s to balance the hydrogens and finally, since this is basic, for every H+ you have added add that same amount of OH- onto both sides and note that OH-+H+=H2O, so there will be some cancelling.
- Wed Mar 11, 2020 12:15 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Work and Delta G
- Replies: 4
- Views: 240
Re: Work and Delta G
G=Wmax always, but I think that Wmax=-nfE is only for standard conditions since that equation is from G^0=-nFE^0 which the knots imply standard conditions.
- Fri Mar 06, 2020 3:15 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Units G=-nFE
- Replies: 6
- Views: 857
Re: Units G=-nFE
Volts = Joules/Coulomb so in deltaG=-nFE the Coulombs cancel out from the multiplication between E (J/C) and F (C/mol), and n has no units so the final units will be J/mol.
- Fri Mar 06, 2020 3:12 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Calculating ln Q
- Replies: 20
- Views: 1590
Re: Calculating ln Q
Q is the reaction quotient, [Products]/[Reactants] for concentration cells the lower concentration is the products. Or the cathode concentration is the reactants and the anode concentration is the products.
- Fri Mar 06, 2020 3:10 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Inert electrode
- Replies: 9
- Views: 598
Re: Inert electrode
You add an inert electrode like Pt(s) when the reaction does not have a conducting solid already involved.
- Fri Mar 06, 2020 3:04 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Forward and reverse reaction rates
- Replies: 4
- Views: 360
Re: Forward and reverse reaction rates
k and k' have different numerical values, it is not necessarily a derivative or anything like that. Its the same as just arbitrarily writing k(forward) and k(reverse) they are both rate constants but they aren't the same number.
- Wed Mar 04, 2020 3:14 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 6.65
- Replies: 3
- Views: 355
Re: 6.65
The equation to use is E^0=(RT/nF)lnK and not E since pH's are always recorded at equilibrium, but that's about all I know. I'm also really unsure why K=[H+]/[OH-] either... the only reaction I can think of relating the two is H + OH -> 2H2O but that doesn't work here.
- Thu Feb 27, 2020 4:41 pm
- Forum: General Science Questions
- Topic: Test 2
- Replies: 16
- Views: 1005
Re: Test 2
Lavelle said in an email that the test will include the 2nd page of outline 4 and all of outline 5 (which can be found on his website)
- Thu Feb 27, 2020 4:36 pm
- Forum: Balancing Redox Reactions
- Topic: OH- in Basic Solutions
- Replies: 6
- Views: 443
Re: OH- in Basic Solutions
You add xOH-'s to both sides after adding xH+'s. Notice that OH-+H+= H2O so that will likely lead to some cancelling. The general steps for balancing a redox reaction in a basic solution is 1.balance everything EXCLUDING H&O 2. balance O's by adding H2O 3. balance H's by adding H+ 4. add as many...
- Thu Feb 27, 2020 4:30 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 6N3A
- Replies: 4
- Views: 398
Re: 6N3A
n is the moles of electrons within the redox reaction, so for this question n=2 since your reduction reaction and oxidation reaction both have 2e-. Concerning the separate Nernst equations either is fine to use, however the -.05916V one always assumes temperature is 298k (this one might also be more...
- Thu Feb 27, 2020 4:21 pm
- Forum: Balancing Redox Reactions
- Topic: 6k3d
- Replies: 3
- Views: 237
Re: 6k3d
I ran into this too, I believe your right, it is supposed to be Cl- instead of Cl2 on the products side.
- Thu Feb 27, 2020 4:12 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: HW 6O.1
- Replies: 1
- Views: 168
Re: HW 6O.1
Compare the reduction potentials (E^0) of Ni2+ + 2e- to the H2O reduction reaction provided at the top of the 6O exercises. The cathode will be determined by which of the two has the greatest reduction potential, the one with the greater reduction potential will be the cathode and the one with the l...
- Fri Feb 21, 2020 2:21 pm
- Forum: Balancing Redox Reactions
- Topic: balancing half reactions in a basic solution
- Replies: 7
- Views: 447
Re: balancing half reactions in a basic solution
Yes, add OH- to both sides and when you do also notice that OH- + H+ = H2O.
- Fri Feb 21, 2020 2:17 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Electrode
- Replies: 4
- Views: 312
Re: Electrode
Platinum is not very reactive, so it is just a means of getting electrons from one source to the next for the actual electron transfer to occur.
- Fri Feb 21, 2020 2:15 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Finding Gibbs free energy with K
- Replies: 3
- Views: 262
Re: Finding Gibbs free energy with K
R is a constant, so refer to the constants and equations sheet on Lavelle's website and choose whichever has the correct units you need. T is provided in the question.
- Fri Feb 21, 2020 2:11 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: E v E(standard)
- Replies: 4
- Views: 312
Re: E v E(standard)
E is the electromotive force of the system at any condition where 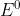 is the electromotive force of a system at standard conditions 1M, 1atm, 273K.
is the electromotive force of a system at standard conditions 1M, 1atm, 273K.
- Fri Feb 21, 2020 2:09 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: 5J.15
- Replies: 3
- Views: 288
Re: 5J.15
You have to use the H's and S's instead of G's because you have to calculate G for temperatures different than 25C. So you have to use 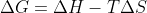 where T= the different temperature.
where T= the different temperature.
- Thu Feb 13, 2020 10:36 am
- Forum: Student Social/Study Group
- Topic: Midterm
- Replies: 8
- Views: 706
Re: Midterm
Off the top of my head I think something like 4I.9 and 4.43 were homework questions very similar to two on the midterm.
- Thu Feb 13, 2020 10:34 am
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Isobaric systems
- Replies: 16
- Views: 836
Re: Isobaric systems
When a system is isobaric that means that the pressure is constant, which implies that 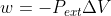 . I think this is all you can assume from this statement. Dr. Lavelle didn't really talk specifically about the terms isobaric and isochoric, so I wouldn't worry too much about it.
. I think this is all you can assume from this statement. Dr. Lavelle didn't really talk specifically about the terms isobaric and isochoric, so I wouldn't worry too much about it.
- Thu Feb 13, 2020 10:29 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Why are exothermic reactions generally spontaneous?
- Replies: 16
- Views: 1232
Re: Why are exothermic reactions generally spontaneous?
\Delta G=\Delta H - T\Delta S If delta G is negative then the reaction is spontaneous. So, using the formula above you can see that in most cases when \Delta H is negative and T\Delta S is positive or T\Delta S< \Delta H then delta G will be a negative value. There are generally more scenarios of d...
- Thu Feb 13, 2020 10:21 am
- Forum: Ideal Gases
- Topic: STP
- Replies: 12
- Views: 1606
Re: STP
STP is 0 Celsius, 273.15K and 1.00 atm. The equations should work the same except for when your dealing with things like \Delta G_{formation}^{0} where typically those constants are calculated from 298 K. Its probably best to make sure the constants you're dealing with explicitly say it was calculat...
- Thu Feb 13, 2020 10:15 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: S vs Stotal
- Replies: 7
- Views: 576
Re: S vs Stotal
Usually if its just
- Fri Feb 07, 2020 3:50 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Change in internal energy
- Replies: 3
- Views: 177
Re: Change in internal energy
\Delta U = 0 when it is isothermal, meaning temperature does not change so there is no net addition or loss of energy to the system. \Delta U = q + w, but since w can be written as w = - P_{ext}\Delta V in the cases where \Delta V = 0 (the volume is constant) then \Delta U = q + 0, thus \Delta U = ...
- Fri Feb 07, 2020 3:42 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: ∆S for summation
- Replies: 3
- Views: 186
Re: ∆S for summation
I'm not very sure, but my guess is that since its standard Gibbs free energy the only thing you need to do to calculate it is to do a Hess's approach only involving standard G's of formation.
- Thu Feb 06, 2020 12:27 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: 4F.17
- Replies: 1
- Views: 229
Re: 4F.17
Recall that S= Cln(T2/T1) so that's where heat capacities comes into play. Additionally since S= q/T then S= deltaHvap/T Those will be the formulas you will use for this question. Also, I think you have misread the question, you're reading 4F.18, 4F.17 uses water (however they both are the same proc...
- Thu Feb 06, 2020 12:13 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Isothermal Process Slow Expansion
- Replies: 2
- Views: 188
Re: Isothermal Process Slow Expansion
For an isothermal process there is no addition or loss of energy, so the change in internal energy is 0. Since delta U = q +w and the definition of isothermal is no addition or loss of energy from the surroundings 0 = q + w. Doing some algebra can yield q = -w (remember that usually work= -number, a...
- Wed Feb 05, 2020 9:27 am
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Change in entropy for a monatomic ideal gas vs diatomic molecules
- Replies: 3
- Views: 608
Change in entropy for a monatomic ideal gas vs diatomic molecules
If you were to compare the change in entropy of 1 mole of a monatomic ideal gas to 1 mole of atoms making up diatomic molecules by increasing both their temperatures, why would the 1 mole of monatomic ideal gas have a greater change in entropy? Problem 4H.9 in the book says something like this as an...
- Wed Jan 29, 2020 10:48 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Temperature
- Replies: 6
- Views: 396
Re: Temperature
Say A->B is exothermic, meaning that B is a lower energy state than A. B is therefore more stable to have, since it is lower energy meaning that the reaction will spontaneously go towards B (until it reaches equilibria) The less stable A requires more energy to be maintained. So if you add more heat...
- Wed Jan 29, 2020 10:41 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Bomb Calorimetry
- Replies: 2
- Views: 129
Re: Bomb Calorimetry
Bomb Calorimetry is the measure the amount of heat given off by a combustion reaction with constant volume.
- Wed Jan 29, 2020 10:39 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: approximation
- Replies: 4
- Views: 198
Re: approximation
When the K is less than 10^-3 you can assume that something like (.15-x) in the denominator will become just .15 since the x is so small it does practically nothing to that amount. But in the numerator you will still have an x present like x^2.
- Tue Jan 28, 2020 4:37 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: qp vs qv
- Replies: 6
- Views: 358
Re: qp vs qv
I think that the only difference between using the two is their corresponding heat capacities (in q=mCdeltaT). So for qp you'd use Cp and for qv you'd use Cv in your calculations.
- Tue Jan 28, 2020 4:34 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: delta H units
- Replies: 4
- Views: 527
Re: delta H units
For the most part delta H will be in kJ/mol, as it represents energy absorbed or released per mole. I think that the only time it would not include "/mol" is probably when units in an analysis cause moles to cancel out. To be safe, I would stick with kJ/mol as it is what I've seen for majo...
- Thu Jan 23, 2020 11:34 am
- Forum: Phase Changes & Related Calculations
- Topic: Adding and subtracting properties
- Replies: 6
- Views: 393
Re: Adding and subtracting properties
State properties only depend on the final and initial states. They only care about the destinations not the journey. So for deltas like enthalpy (which is state dependent), to find it you do: Final state value - Initial state value. State dependent properties are only involved with total changes fro...
- Thu Jan 23, 2020 11:29 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Le chatelier and Temperature
- Replies: 9
- Views: 309
Re: Le chatelier and Temperature
For Delta H= +, then the reaction is endothermic meaning it needs input For Delta H= -, then the reaction is exothermic and is spontaneous/needs no input. The way I like to think about these is that for endothermic reactions, they are going from generally a more stable form to a less stable form thu...
- Tue Jan 21, 2020 4:17 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: H2O in K Expressions
- Replies: 6
- Views: 326
Re: H2O in K Expressions
Only liquids and solids are supposed to be ignored in K expressions. While it is H2O we're talking about (which tends to be liquid and therefore ignored), if it is in the gas phase it should be used in the expression.
- Tue Jan 21, 2020 4:15 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6D.15
- Replies: 1
- Views: 110
Re: 6D.15
This one is a little tricky, you have to recall that Al^3+ is a central metal ion in chelation. So for AlCl3(aq)+H2O(l) the Cl3- ion and Al^3+ will depart and Cl3- doesn't affect the pH so it can be ignored. With the Al^3+ in water 6 H2O molecules will bond to make an octahedral Al(H2O)^3+. So the r...
- Tue Jan 21, 2020 4:05 pm
- Forum: Ideal Gases
- Topic: pressure
- Replies: 10
- Views: 405
Re: pressure
Partial pressure just means that it is a part/contributing to the total pressure of the system. Total pressure is all of the components of a reaction that would cause pressure combined (added), while partial pressure is just the pressure created by one aspect or compound.
- Wed Jan 15, 2020 10:11 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Real reason explaining Le Chatelier's principle
- Replies: 4
- Views: 152
Re: Real reason explaining Le Chatelier's principle
Le Chatelier's Principle says that a reaction will counteract a change in order to stay at equilibrium. If you increase pressure, P=(nRT)/V then you are essentially altering n/v (either making v smaller or n larger) also known as the concentration, and if the concentration of everything in the react...
- Wed Jan 15, 2020 10:01 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Q=K
- Replies: 14
- Views: 666
Re: Q=K
Q can be calculated at any time (including when it has already reached equilibrium) during the reaction and is compared to K to see the direction of the reaction, if Q equals K then that just means the reaction is at equilibrium.
- Wed Jan 15, 2020 9:58 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: 5I.11 units
- Replies: 5
- Views: 359
Re: 5I.11 units
From my ebook it says 1.20 milli-mol SO2, I think you may have misread it or there was a typo.
- Wed Jan 15, 2020 9:55 am
- Forum: Ideal Gases
- Topic: Partial Pressure
- Replies: 8
- Views: 322
Re: Partial Pressure
It is partial pressure because each compound contributes to the whole pressure, so the pressure caused by one compound would be called partial.
- Tue Jan 14, 2020 2:54 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6D.3
- Replies: 1
- Views: 130
Re: 6D.3
I think its cause .10M is the initial value of HClO2, I think the way that Ka and Kb work is similar to regular K in that the system must be at equilibrium. So while there was .10M of HClO2 at the start, .06 of it became the product(s) thus the final HClO2 you would put into the calculations would b...
- Thu Jan 09, 2020 3:31 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: When to assume x=0 for ICE box problems
- Replies: 1
- Views: 95
When to assume x=0 for ICE box problems
In question 5i.29 of the textbook, the solution has you omit x in the denominator (or treat it as 0) since the provided K is 3.2x10^-34. For what range of K's are you allowed to say is small enough to treat x as a 0? And what is the justification for treating the denominator's x like this?
- Wed Jan 08, 2020 5:12 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: How to find stability based off of equilibrium concentrations
- Replies: 2
- Views: 164
How to find stability based off of equilibrium concentrations
Problem 5i13 part c for reference, asks to determine which dissociation reaction is more thermodynamically stable, dichloride to chlorine vs difluorine to fluorine. I know that a reaction reaches its equilibrium constant so that its ratio of products to reactant is as stable as possible, but how can...
- Tue Jan 07, 2020 3:19 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: How to interpret reactions based on quotient in relation to equilibrium constant
- Replies: 5
- Views: 210
How to interpret reactions based on quotient in relation to equilibrium constant
How can you tell if more products or more reactants will be formed in a reaction based on the Q compared to K? Is there a rule? Like if Q<K it makes products?
- Tue Jan 07, 2020 11:14 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Heterogenous vs. Homogenous equations
- Replies: 6
- Views: 297
Re: Heterogenous vs. Homogenous equations
Heterogenous equations contain compounds with different (hetero) phases while homogenous equations contain the same (homo) phases. For example a chemical equation with all of the compounds in the gas phase is homogenous.
- Tue Jan 07, 2020 11:12 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Hw Problem G2
- Replies: 5
- Views: 382
Re: Hw Problem G2
I believe it is true, the reaction will reach equilibrium ending with satisfying the ratio of products to reactants (and/or reactants to products ratio) that makes up the equilibrium constants k and k^-1.
- Fri Dec 06, 2019 5:02 pm
- Forum: Electronegativity
- Topic: Electronegativity of Atoms We Should Know About
- Replies: 4
- Views: 364
Re: Electronegativity of Atoms We Should Know About
It is probably best to memorize the periodic trends that electronegativity has. As you go up the table EN increases, and as you go right from the table EN increases. I don't believe we will have to know the exact hierarchy/numbers for electronegativity, we just need to be able to work with the idea ...
- Fri Dec 06, 2019 4:50 pm
- Forum: Conjugate Acids & Bases
- Topic: oxoacids
- Replies: 3
- Views: 352
Re: oxoacids
Oxoacids are a category of acids that contain oxygen(s) which hold/bond to the soon-to-be-donated hydrogen/proton. HClO3 falls into the oxoacids category.
- Fri Dec 06, 2019 4:46 pm
- Forum: Hybridization
- Topic: 2F.15
- Replies: 1
- Views: 103
Re: 2F.15
S character of a bond refers to how much S is relatively in the hybridization, so for example sp3 has less S character than sp2 which will have less S character than sp (ie sp will have the most S character). Notice the corresponding molecular shapes that one can make with the central atom being sp3...
- Fri Dec 06, 2019 11:25 am
- Forum: Properties & Structures of Inorganic & Organic Bases
- Topic: Identifying electron donors vs electron withdrawers (6C 21)
- Replies: 1
- Views: 414
Identifying electron donors vs electron withdrawers (6C 21)
I understand that electron withdrawers would make a molecule more likely to lose a proton and electron donors less likely to lose a proton. Like in 6C 21 how acetic acid is a weaker acid than formic acid since the methyl group -CH3 on the acetic acid is a electron donor to the molecule making it sli...
- Fri Dec 06, 2019 11:09 am
- Forum: Properties & Structures of Inorganic & Organic Bases
- Topic: Determining relative strengths of bases (6C 17)
- Replies: 2
- Views: 404
Determining relative strengths of bases (6C 17)
How does one determine which base is stronger than the other. For example, in 6C 17 we are given BrO- and C17H19O3N (morphine). My answer would be that BrO- is more basic because it has a full negative charge to pull in protons compared to morphine's possible lone pairs (I haven't attempted to creat...
- Sun Dec 01, 2019 2:41 pm
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: What is kA value?
- Replies: 5
- Views: 567
Re: What is kA value?
kA is the ionization constant which lets you know how ionized/dissociated a solution is. The more ionized the stronger it is, the less ionized the weaker.
- Sun Dec 01, 2019 2:32 pm
- Forum: Sigma & Pi Bonds
- Topic: Ionic bond --> sigma and pi bonds
- Replies: 8
- Views: 1544
Re: Ionic bond --> sigma and pi bonds
Sigma and Pi bonds are overlapping of orbitals/ sharing electrons. Ionic bonds do not share electrons, so they do not include sigma or pi bonds.
- Sun Dec 01, 2019 2:29 pm
- Forum: Lewis Acids & Bases
- Topic: Lewis Acids
- Replies: 3
- Views: 250
Re: Lewis Acids
I remember it as a base will take electrons while an acid will receive electrons (I use bronsted definition).
- Sun Dec 01, 2019 2:27 pm
- Forum: Naming
- Topic: Names of ligands
- Replies: 4
- Views: 260
Re: Names of ligands
I believe it is primarily the naming rules for coordinate compounds, which involves memorization of ligands. Dr. Lavelle sent a email with a pdf for the naming rules and etc around 2 weeks ago. I would go off of that.
- Tue Nov 26, 2019 3:50 pm
- Forum: Student Social/Study Group
- Topic: -ate Nomenclature for final?
- Replies: 2
- Views: 138
-ate Nomenclature for final?
I'm currently doing the Acids and Bases HW and a handful of questions require knowledge of ate/ite nomenclature which I don't think we have covered thus far. For the final will it be necessary that we fully know "-ate" nomenclature? Thanks.
- Sat Nov 23, 2019 11:33 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: T-shaped v. Trigonal pyramid
- Replies: 9
- Views: 560
Re: T-shaped v. Trigonal pyramid
A= central atom, X= bonded atoms, E= lone pairs.
T shape: AX3E2
trigonal pyramid: AX3E
T shape: AX3E2
trigonal pyramid: AX3E
- Sat Nov 23, 2019 11:31 pm
- Forum: Biological Examples
- Topic: Biological Importance
- Replies: 6
- Views: 383
Re: Biological Importance
I'm not sure specifically. I believe the fact that pH impacts function in biological systems is probably the biggest takeaway. I'll just list what other parts i remember that where mentioned: Copper, Zinc, and Nickel contribute to enzyme function (which speed up reactions), Iron is important in elec...
- Sat Nov 23, 2019 11:20 pm
- Forum: Biological Examples
- Topic: Cisplatin
- Replies: 15
- Views: 712
Re: Cisplatin
I believe cisplatin bonds to two nucleotides preventing the DNA to unravel itself and therefore can't be replicated.
- Sat Nov 23, 2019 11:17 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSEPR and its relation to Hybridization
- Replies: 3
- Views: 229
Re: VSEPR and its relation to Hybridization
I think that the amount of electron densities a molecule has corresponds to the kind of hybridization shells may be present so VSEPR may be helpful in that if you know its shape you know the amount of electron densities. .
- Sat Nov 23, 2019 11:13 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: ligand
- Replies: 3
- Views: 273
Re: ligand
A ligand is a ion or molecule that creates a coordinate bond to a metal atom.
- Fri Nov 15, 2019 10:10 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSEPR Notation
- Replies: 5
- Views: 306
Re: VSEPR Notation
Usually A will not be accompanied by a number as it represents the central atom of the molecule. Whatever number follows X represents how many atoms are bonded to the central atom A. The number that comes with E represents how many lone pairs there are on the molecule's central atom.
- Fri Nov 15, 2019 10:08 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: regions of electron density
- Replies: 7
- Views: 560
Re: regions of electron density
I think of regions of electron density just as another way to say a general area of a group of electrons, so on a molecule wherever either lone pairs or bonds are is a general area of a group electrons therefore regions of electron density.
- Fri Nov 15, 2019 10:04 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bent or Angular
- Replies: 13
- Views: 1123
Re: Bent or Angular
Personally I refer to it as angular for the same reasons as the post before me. I think either is acceptable, but double check with your TA to be sure.
- Fri Nov 15, 2019 10:02 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: test 2
- Replies: 13
- Views: 734
Re: test 2
Unless on Monday Dr. Lavelle teaches hybridization, I don't think it will show up on test two. He said for test two it will be everything between the midterm up until Monday (which I believe he will teach sigma and pi bonds).
- Fri Nov 15, 2019 9:58 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Polarizing power vs polarizability
- Replies: 5
- Views: 1049
Re: Polarizing power vs polarizability
polarizing power is how powerful a specific ion can polarize another ion (pull/distort a electron cloud towards it)
polarizability is how much a ion can be polarized (how distorted it can become)
polarizability is how much a ion can be polarized (how distorted it can become)
- Thu Nov 07, 2019 2:04 pm
- Forum: Administrative Questions and Class Announcements
- Topic: What homework to turn in per week
- Replies: 7
- Views: 391
What homework to turn in per week
In general, what range of time should we use as a rule of thumb to determine what section of homework we should turn in for that week? I'm guessing 2 but I'm not sure if thats too long.
- Thu Nov 07, 2019 11:50 am
- Forum: Ionic & Covalent Bonds
- Topic: Mini Dino Nuggets 2b
- Replies: 6
- Views: 262
Re: Mini Dino Nuggets 2b
As you go up and right electronegativity increases. Using this rule Florine is more electronegative than Bromine so the differences are EN(F) - EN(C) versus EN(Br) - EN(C) since you know Florine's EN will be larger the difference between F and C is greater than C and Br. The more significant the ele...
- Thu Nov 07, 2019 11:36 am
- Forum: Ionic & Covalent Bonds
- Topic: Correcting Ionic Model
- Replies: 4
- Views: 291
Re: Correcting Ionic Model
Yes you are correct in both aspects. The way I think of it is a covalent bond is a sharing of electrons and ionic bond is an anion stealing an electron from a cation. Some ionic bonds show covalent character because as an ionic bond is polarized some electrons (or the electron cloud) "stretch&q...
- Thu Nov 07, 2019 11:29 am
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Bohr Frequency
- Replies: 6
- Views: 333
Re: Bohr Frequency
When energy is absorbed the system is gaining energy so that is a positive delta E going from a lower n to a higher n.
- Tue Nov 05, 2019 3:54 pm
- Forum: Administrative Questions and Class Announcements
- Topic: For the midterm will we need to know how to do electron configurations for the f shell? [ENDORSED]
- Replies: 2
- Views: 804
For the midterm will we need to know how to do electron configurations for the f shell? [ENDORSED]
For the midterm will we need to know how to do electron configurations for the f shell?
Also, do we have to memorize what general ranges the different types of electromagnetic radiation are in?
Also, do we have to memorize what general ranges the different types of electromagnetic radiation are in?
- Fri Nov 01, 2019 5:00 pm
- Forum: Bond Lengths & Energies
- Topic: dissociation energy
- Replies: 7
- Views: 300
Re: dissociation energy
I'm not 100% on this but I believe Dissociation energy is always positive because breaking a bond will always require an input of energy, if it were to release energy dissociation energy would be negative but that can't happen because breaking a bond is going from a lower potential energy to a highe...
- Fri Nov 01, 2019 11:30 am
- Forum: Ionic & Covalent Bonds
- Topic: How does one find a most likely charge for ions for a given element?
- Replies: 6
- Views: 518
How does one find a most likely charge for ions for a given element?
Currently working on Homework 2A #15, essentially it asks to find a most likely charge for a given element if it were to become an ion (like for S or Ga). I'm not sure how to approach this, do I focus on ionization energy and electron affinity? How does one determine how many electrons gained or los...
- Thu Oct 31, 2019 10:22 am
- Forum: Administrative Questions and Class Announcements
- Topic: Midterm
- Replies: 7
- Views: 453
Re: Midterm
If you go onto Dr. Lavelle's website under the class websites tab, scroll down slightly, on the right you should see a section with the title "Exam information." Click "midterm review sessions and rooms" and it should bring you to a pdf with all the info you need about the midter...
- Thu Oct 31, 2019 10:18 am
- Forum: Einstein Equation
- Topic: Joules units
- Replies: 6
- Views: 941
Re: Joules units
Planck's Constant's units are J * s (joules times seconds) not necessarily just joules, thats why the Planck's units are (kg m^2 s^-2) * (s) = kg m^2 s^-1.
- Thu Oct 31, 2019 10:14 am
- Forum: Properties of Electrons
- Topic: Formal Charge of an Atom
- Replies: 5
- Views: 321
Re: Formal Charge of an Atom
Formal Charge is assigned to an atom by following the formula: FC= V - (L+S/2) where V is the amount of valence electrons the atom has, L are the lone pairs it has (on a Lewis structure it would be the pairs of dots like ":"), and s being the bonds the atom has.
- Fri Oct 25, 2019 11:10 am
- Forum: Formal Charge and Oxidation Numbers
- Topic: charges and roman numerals
- Replies: 5
- Views: 944
Re: charges and roman numerals
Roman numerals next to atoms/metals just indicate the specific charge it has since metals can have a variety of different charges for the same thing. So this one the (III) indicates a +3 charge
- Fri Oct 25, 2019 11:05 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Atomic Radius
- Replies: 18
- Views: 654
Re: Atomic Radius
The atomic radius is half the distance between the centers of neighboring atoms, I don't think we will have to ever calculate a measurement for this (it would probably be given). I believe to answer this question you go based off of the periodic trends of atomic radii, as you go down and left of the...
- Thu Oct 24, 2019 11:20 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Electron Configurations
- Replies: 13
- Views: 2965
Re: Electron Configurations
For me, I don't memorize the order I just have memorized the exceptions like when you get to Cr the D shell can have 10 electrons and Cu's 4s shell can only hold 1 electron. The main thing I try to do when finding electron configuration is to build up from the shells (counting the electrons) and to ...
- Thu Oct 24, 2019 11:03 am
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Uncertainty value in equation
- Replies: 9
- Views: 383
Re: Uncertainty value in equation
Uncertainty accounts for both the positive and negative, so if your v was 103 +or- 3 then the uncertainty would be 3(2)= 6.
- Mon Oct 21, 2019 5:20 pm
- Forum: SI Units, Unit Conversions
- Topic: Unit conversion
- Replies: 15
- Views: 1562
Re: Unit conversion
For conversions involving angstroms use the ratio: 10^-10m/Angstrom. For example 100pm*(10^-12m/pm)*(1Angstrom/10^-10m)= 100*10^-2 Angstroms.
- Fri Oct 18, 2019 5:33 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: 3d104s2
- Replies: 1
- Views: 86
Re: 3d104s2
I'm not entirely sure on this either, but here is my two cents: 3d orbitals are lower energy than 4s orbitals because the n=3 on the 3d and n=4 on the 4s and we know n refers to energy and the electron configuration is written out in increasing order (so 4s2,3d10 is not correct because the order of ...
- Fri Oct 18, 2019 5:15 pm
- Forum: DeBroglie Equation
- Topic: Linear Momentum
- Replies: 6
- Views: 235
Re: Linear Momentum
Linear momentum is p, where its formula is composed of p=mv or Momentum = Mass (usually kg) * Velocity (usually m/s).
- Thu Oct 17, 2019 4:22 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Spin State
- Replies: 17
- Views: 422
Re: Spin State
+1/2 refers to spin up and -1/2 refers to spin down. I believe it is a reference to what would happen if you performed an experiment similar to the one Dr. Lavelle showed in lecture Oct 16th (the silver atoms one). Some electrons end up going upwards after going through a magnetic field while some o...
- Thu Oct 17, 2019 4:16 pm
- Forum: DeBroglie Equation
- Topic: Constant Question
- Replies: 7
- Views: 356
Re: Constant Question
Personally, I would use whatever number is more accurate. In test scenarios I would go for every digit given on the constants sheet. It shouldn't be too much of a big deal though to opt for 6.63 so long as it is clear that you're using that number and your answer is pretty much the same.
- Thu Oct 17, 2019 4:12 pm
- Forum: Photoelectric Effect
- Topic: Unit of measurements for E=hv
- Replies: 6
- Views: 252
Re: Unit of measurements for E=hv
For E=hv, h has the unit J*s and v has hz or s^-1 so the answer should come out to be in just joules J (s*s^-1=1), however for this question the v calculated is per photon so it is ok to make it J per photon.
- Thu Oct 10, 2019 3:55 pm
- Forum: Properties of Light
- Topic: Energy of light
- Replies: 4
- Views: 192
Re: Energy of light
Yes, it is always true that shorter wavelengths lead to higher energy because based off of c=wavelength *frequency if wavelength decreases the frequency has to increase to satisfy the equation and if frequency goes up energy goes up based off of the equation E= h * frequency.
- Thu Oct 10, 2019 10:47 am
- Forum: Administrative Questions and Class Announcements
- Topic: Can't find my old posts
- Replies: 1
- Views: 142
Can't find my old posts
After I've commented (or I look at one of my comments) when I click on the box on the right with my name I see that it says I have posted 10 times, I click on it and it shows me my posts, but I can only find 9 of the 10. I remember commenting on a photoelectric effect post this week but I can't find...
- Thu Oct 10, 2019 10:42 am
- Forum: Properties of Light
- Topic: Finding Wavelength and Energy of Photon
- Replies: 1
- Views: 86
Re: Finding Wavelength and Energy of Photon
To find the wavelength of Krypton-86 I would use the ratio that they gave you so: (1 meter/1,650,763.73 krypton wavelengths) * (1 Krypton wavelengths) to convert to krypton wavelength in meters (or nanometers). From there I would use the formula c= Wavelength * Frequency, solve for frequency, and pl...
- Thu Oct 10, 2019 10:32 am
- Forum: Properties of Light
- Topic: 1A 3 Chemical Principle 7th edition
- Replies: 2
- Views: 90
Re: 1A 3 Chemical Principle 7th edition
Decreasing frequency of light leads to less energy per photon, (E=hv). So I think that the extent of the change in the electrical field at a given point decreases because the energy of the light (electromagnetic radiation) has decreased. Or another way is that the electric field vector's height have...
- Tue Oct 08, 2019 4:45 pm
- Forum: Empirical & Molecular Formulas
- Topic: empirical to molecular formula [ENDORSED]
- Replies: 9
- Views: 609
Re: empirical to molecular formula [ENDORSED]
I believe it goes: (Molar Mass Given)/(Empirical molar mass)= Number you need to multiply the empirical formula to get the molecular formula.
- Thu Oct 03, 2019 8:45 pm
- Forum: SI Units, Unit Conversions
- Topic: Scientific Notation (general requirement for the course)
- Replies: 5
- Views: 346
Re: Scientific Notation (general requirement for the course)
I'm not entirely sure, I think that you're only required to write the correct amount of sig figs, but there can be cases were scientific notation would be more practical. I would just make sure that the reader can understand the sig figs in your answer.
- Wed Oct 02, 2019 6:08 pm
- Forum: Balancing Chemical Reactions
- Topic: H13 Is there a good step by step way to balance this?
- Replies: 4
- Views: 203
Re: H13 Is there a good step by step way to balance this?
So there is not really a step by step process for a reaction like this? Do you just kinda have to use your intuition to figure out problem like this one?
- Wed Oct 02, 2019 5:55 pm
- Forum: Balancing Chemical Reactions
- Topic: H13 Is there a good step by step way to balance this?
- Replies: 4
- Views: 203
H13 Is there a good step by step way to balance this?
Usually I have no issues balancing reactions, but the second part of problem H13 broke my usual solving steps. I usually balance from least frequent to most frequent but when I try to do that on NO + O2 = NO2 it just confuses me. I go from least frequent to most, so I first balance the N's which is ...
- Tue Oct 01, 2019 3:50 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Mole Units
- Replies: 2
- Views: 143
Re: Mole Units
Moles can be used for anything because it is like using the term dozen. If there are 12 eggs present you can say there are a dozen eggs, if there were 6 eggs you apply the the dozen ratio: (12 things = 1 Dozen things) and say there are .5 dozen eggs. In the chemistry world chemists like to use moles...

