Search found 50 matches
- Mon Mar 16, 2020 2:31 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Approximating X
- Replies: 13
- Views: 856
Re: Approximating X
If your K value is less than 1.0*10^-3, then you can assume that the x value will not greatly effect the values in your equation. So if the K value is that small, you can assume that the x value in the denominator is 0.
- Mon Mar 16, 2020 2:28 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kw and other constants
- Replies: 9
- Views: 729
Re: Kw and other constants
The Kw is the equilibrium constant for water (the autoprotolysis of water). So the w in Kw is water.
- Mon Mar 16, 2020 2:25 pm
- Forum: Phase Changes & Related Calculations
- Topic: state functions
- Replies: 13
- Views: 1408
Re: state functions
State functions only depend on the initial and final products. Examples are entropy, enthalpy, and gibbs free energy.
- Mon Mar 16, 2020 2:22 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: H2O
- Replies: 44
- Views: 2092
Re: H2O
You don't include water into your ice table because it's a liquid and when writing the equilibrium constants out, you disregard the liquids.
- Thu Mar 05, 2020 7:23 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6.M.13 part b
- Replies: 2
- Views: 241
Re: 6.M.13 part b
Cooper Baddley 1F wrote:The Mn is actually being reduced because it is going from a charge of +7 to +2 so that would be your cathode.
Oh that makes more sense! Thank you!!
- Thu Mar 05, 2020 4:24 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagram Order
- Replies: 8
- Views: 627
Re: Cell Diagram Order
As long as you have the anode on the left side and the cathode on the right side, it should be fine. Usually, you can determine what is losing the electron or gaining the electron when you're writing the half reactions if you know which are the anode and the cathode.
- Thu Mar 05, 2020 2:34 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Favoring reactions
- Replies: 7
- Views: 607
Favoring reactions
If the anode has a larger value than the cathode, does the reaction favor the products? Does it have to be the cathode that is a larger value for the reaction to favor the products? And is this something we should know?
- Thu Mar 05, 2020 2:05 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6.M.13 part b
- Replies: 2
- Views: 241
6.M.13 part b
SO the problem is asking: Identify the reactions with K > 1 in the following list and, for each such reaction, identify the oxidizing agent and calculate the standard cell potential. MnO4−(aq) + 8 H+(aq) + 5 Ce3+(aq)→5 Ce4+(aq) + Mn2+(aq) + 4 H2O(l) Both MnO4- and Ce3+ are losing electrons, does any...
- Tue Mar 03, 2020 5:37 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagrams
- Replies: 3
- Views: 258
Re: Cell Diagrams
Alicia Lin 2F wrote:The cathode is always written on the right side and the anode is always written on the left.
And does the flow of electrons go from the anode to the cathode?
- Tue Mar 03, 2020 5:23 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagrams
- Replies: 3
- Views: 258
Cell Diagrams
When we're given a cell diagram, how do we distinguish which side is the anode and which side is the cathode? I'm trying to find examples where we would figure out which one is which. Like would we be given elements and we just figure it out from that? (There might be an obvious answer to this and I...
- Sun Mar 01, 2020 8:57 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagrams
- Replies: 6
- Views: 487
Re: Cell Diagrams
I don't think it matters in what order you write them as long as you place a comma in between because of the similar phases.
- Sun Mar 01, 2020 8:54 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagram Comma vs Line
- Replies: 5
- Views: 355
Re: Cell Diagram Comma vs Line
A line is used to separate elements of different phases and a comma is used for elements of the same phase. So if you had Cu(s) and Cu2+(aq), there would be a line between them.
- Sun Mar 01, 2020 8:31 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Concentration Cell Examples
- Replies: 3
- Views: 286
Re: Concentration Cell Examples
So a concentration cell is just the same element with different concentrations? With the Ag and Ag+ example will the elements always have one that has a positive charge?
- Sun Mar 01, 2020 8:16 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Electrochemical Series
- Replies: 5
- Views: 452
Electrochemical Series
On Outline 5, one of the bulletins says to "understand what is meant by the electrochemical series". Did we go over this in class? I'm not sure what it is.
- Sun Mar 01, 2020 8:14 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Nernst Equation
- Replies: 3
- Views: 312
Nernst Equation
How do you derive the Nernst equation? Where do you start?
- Tue Feb 25, 2020 10:30 am
- Forum: Balancing Redox Reactions
- Topic: reducing agent
- Replies: 5
- Views: 359
reducing agent
Is the reducing agent what is losing electrons? What exactly is the reducing agent? I never really grasped the concept of the reducing and oxidation agents.
- Wed Feb 19, 2020 4:50 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Isothermal reversible and irreversible
- Replies: 2
- Views: 372
Isothermal reversible and irreversible
Are there different equations regarding isothermal reversible and irreversible free expansions?
- Wed Feb 19, 2020 4:47 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Delta G and Delta H
- Replies: 3
- Views: 434
Delta G and Delta H
On Q6B, how do you determine which process will have delta H and delta g be similar?
- Wed Feb 19, 2020 4:43 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: midterm question// Concentration ratio [ENDORSED]
- Replies: 9
- Views: 724
Re: midterm question// Concentration ratio [ENDORSED]
You have to think about how Ka=[A][H+]/[CB] (CB being conjugate base). By rearranging the equation, you can get Ka/[H+] = [A]/[CB], which is a ratio. Were we given this equation anywhere? I mean like the original, not manipulated, equation? And I don't recall using it before the midterm but I may b...
- Wed Feb 19, 2020 1:33 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Net charge given a pH
- Replies: 2
- Views: 287
Net charge given a pH
On Q3D on the midterm, it asked for the net charge given the pH and pKa and I did not know how to find the charge. Does anyone know how to find the charge given the pH? Do we use the pH to calculate it or the pH?
- Sun Feb 16, 2020 1:34 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Standard Enthalpies of Formation
- Replies: 10
- Views: 587
Re: Standard Enthalpies of Formation
Someone correct me if I'm wrong but I think all ideal gasses have an enthalpy of 0. I think the elements have to be in their standard state.
- Sun Feb 16, 2020 1:31 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Hess Law
- Replies: 6
- Views: 421
Re: Hess Law
Usually, you'll be given a couple or more equations that are related to the main one given and then you'll want to algebraically solve to get the main equation through the other ones. Whatever changes made on those equations you make onto the delta H's. Then you add them up to get the net change in ...
- Sun Feb 16, 2020 1:15 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: R constants
- Replies: 40
- Views: 2262
Re: R constants
You just have to make sure that you have the right units with the R value. For the most part in thermochemistry we used 8.314 but like in an instance of using PV=nRT, we would use the other R values.
- Sun Feb 16, 2020 1:10 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Why are exothermic reactions generally spontaneous?
- Replies: 16
- Views: 1239
Re: Why are exothermic reactions generally spontaneous?
Reactions will usually want to go towards a state that they are, in a sense, "comfortable" in, so they want to go towards favorable reactions. Usually, these reactions release heat. And I think an easy way to think about spontaneous reactions is that they can happen randomly (spontaneously...
- Mon Feb 10, 2020 7:50 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Pizza Rolls REVIEW Session DOWNLOAD HERE
- Replies: 67
- Views: 5797
Re: Pizza Rolls REVIEW Session DOWNLOAD HERE
Can someone help explain why and how the equation for 3b includes (1/2)(m)(delta H of fusion)? I get what you would isolate to get the answer but I'm not sure how they got that part of the equation.
- Sun Feb 09, 2020 3:26 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Hess's Law
- Replies: 10
- Views: 294
Re: Hess's Law
We're able to use Hess' Law because enthalpy is a state function. Since it's a state function, we're able to just add the enthalpies together.
- Sat Feb 08, 2020 9:56 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Isolated vs Closed [ENDORSED]
- Replies: 34
- Views: 4313
Re: Isolated vs Closed [ENDORSED]
For an isolated system, nothing can come in or out of the system. In a closed system, heat can come in and out of the system. Both can't have matter come in or out but the main difference between isolated and closed is that nothing can come inside an isolated system. It's like an insulated bottle, h...
- Sat Feb 08, 2020 9:52 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Reason for decrease in entropy
- Replies: 5
- Views: 306
Re: Reason for decrease in entropy
It can decrease in entropy when the system decreases in disorder, which could be thought of as molecules. When the temperature decreases, the disorder decreases because the movement of molecules slows down. Someone correct me if I'm wrong though.
- Sat Feb 08, 2020 9:48 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: q rev
- Replies: 9
- Views: 342
q rev
In the equation delta S equals q rev over temperature, what exactly is q rev?
- Sat Feb 08, 2020 9:47 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Endothermic vs Exothermic
- Replies: 10
- Views: 586
Re: Endothermic vs Exothermic
Yes, a negative is exothermic so then a positive would be endothermic. A way to remember it would be that exothermic is like "exit" and if something is exiting then it is leaving or losing something.
- Sun Feb 02, 2020 4:34 pm
- Forum: Phase Changes & Related Calculations
- Topic: phase change from liquid to vapor
- Replies: 8
- Views: 368
Re: phase change from liquid to vapor
It makes more energy to change from liquid water to water vapor. And as Matthew said, there is a longer flat line from liquid to vapor than from solid to liquid, which basically means that more energy is required to get to that vapor temperature.
- Sun Feb 02, 2020 4:15 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: ICE
- Replies: 20
- Views: 932
Re: ICE
It's mainly used to find the unknown value of a concentration. It's also based on what values you're given but you'll mainly be given the concentrations of the reactant and want to solve for the products.
- Sun Feb 02, 2020 4:13 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Calorimeter vs. Bomb Calorimeter
- Replies: 4
- Views: 324
Calorimeter vs. Bomb Calorimeter
What is the difference between a calorimeter and a bomb calorimeter? Are there different uses for both of them?
- Sun Feb 02, 2020 2:55 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: universe is an isolated system
- Replies: 4
- Views: 170
Re: universe is an isolated system
The universe is a system that has no surroundings to which heat or matter for that fact can be exchanged. This exactly the properties that make a system isolated. So the universe is isolated because heat or matter can't be exchanged? SO an isolated system means that heat can't be transferred to the...
- Sun Feb 02, 2020 2:52 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Closed Systems
- Replies: 14
- Views: 941
Closed Systems
How can you change the energy in a closed system? And does it differ with isolated systems?
- Sun Jan 26, 2020 4:16 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: suggestions
- Replies: 16
- Views: 571
Re: suggestions
Organic Chemistry Tutor is a really good one! I used it in 14a and it helped a lot!!
- Sun Jan 26, 2020 4:14 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Kw Equations
- Replies: 10
- Views: 547
Re: Kw Equations
The equation is [H3O^+][OH^-] = Kw. Generally, it would be [1.0x10^-7][1.0x10^-7] = 1.0x10^-14. This is when Kw is at 25 C., otherwise I think the Kw value would be different at different temperatures.
- Sun Jan 26, 2020 4:05 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Negative Square Root solving an ICE box
- Replies: 13
- Views: 587
Re: Negative Square Root solving an ICE box
If you got a negative number inside the square root, I would re-check the calculations inside of the square root. You shouldn't be getting a negative because that would result in an imaginary number.
- Sun Jan 26, 2020 3:55 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Preferences between Methods
- Replies: 3
- Views: 125
Preferences between Methods
Did Professor Lavelle say if there was a method preferred over another? And how would we know which one to use?
- Sun Jan 26, 2020 3:52 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Enthalpies
- Replies: 3
- Views: 139
Enthalpies
Is there a relationship between the standard reaction enthalpy and the standard enthalpy of formation?
- Sun Jan 19, 2020 4:27 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: ICE table
- Replies: 11
- Views: 391
Re: ICE table
You can use ICE tables for acids and bases but you mainly use them when you have weak acids and weak bases.
- Sun Jan 19, 2020 4:13 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Kb vs Ka
- Replies: 5
- Views: 166
Re: Kb vs Ka
Kb is for bases while Ka is for acids.
- Sun Jan 19, 2020 4:02 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: 5% vs. K < 10^-3
- Replies: 3
- Views: 137
Re: 5% vs. K < 10^-3
They're the same. You can disregard the X in the denominator when K < 10^-3 which essentially allows you to solve for X without the quadratic formula. It also means that X is small enough to be excluded in the equation.
- Sun Jan 19, 2020 3:55 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Conjugate Seesaw
- Replies: 5
- Views: 221
Conjugate Seesaw
What is the conjugate seesaw?
- Sun Jan 19, 2020 3:43 pm
- Forum: Phase Changes & Related Calculations
- Topic: Autoprotolysis
- Replies: 15
- Views: 822
Re: Autoprotolysis
It is the transfer of a proton between the same type of molecule. An example of it would be 2H2O 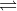 H3O
H3O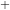 + OH
+ OH
- Sun Jan 12, 2020 5:18 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Direction
- Replies: 4
- Views: 150
Re: Direction
If the Q value is less than K, then there is more reactant than there is product in the reaction, and the direction which the reaction favors it forward. If the Q value is greater than K, then there is more product than there is reactant in the reaction, and the direction which the reaction favors i...
- Sun Jan 12, 2020 4:59 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Q
- Replies: 10
- Views: 489
Re: Q
Yes it can apply to both! They are both calculated the same way. Just the only difference between the two is that K is at equilibrium while Q can be calculated at anytime in the reaction.
- Sun Jan 12, 2020 4:57 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Reaction quotient
- Replies: 8
- Views: 422
Re: Reaction quotient
K can only be calculated when the reaction has reached equilibrium. Q can be calculated at any point in a reaction.
- Sun Jan 12, 2020 4:49 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Q
- Replies: 6
- Views: 331
Re: Q
It tells us whether there is more reactant or product during the reaction. And with that, it can determine whether a forward or reverse reaction is favored. So if Q is smaller than K, then there is more reactant than product, resulting in favoring a forward reaction. If K is smaller than Q, then the...
- Sun Jan 12, 2020 4:43 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: ICE tables
- Replies: 3
- Views: 107
Re: ICE tables
It's a way to help you track your steps but you don't have to use the table.

