= 0
Search found 51 matches
- Fri Mar 13, 2020 10:11 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Concentration Cells
- Replies: 2
- Views: 252
Re: Concentration Cells
There’s a lot of things so I’ll let others answer, but one thing I make sure to remember is that 
= 0
= 0
- Fri Mar 13, 2020 10:07 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6M.1
- Replies: 1
- Views: 179
Re: 6M.1
Hi, what I came up with is because Ecell is negative, the Ecell = Er - El is reversed. This particular cell isn’t spontaneous.
So, to get negative (-) Ecell, the equation would be -Ecell = El - Er. In that case, -0.689V = El - 0.34 V. From there, you can add 0.34V to both sides to get El = -0.349V
So, to get negative (-) Ecell, the equation would be -Ecell = El - Er. In that case, -0.689V = El - 0.34 V. From there, you can add 0.34V to both sides to get El = -0.349V
- Thu Mar 12, 2020 11:45 am
- Forum: General Rate Laws
- Topic: Units of time
- Replies: 5
- Views: 418
Re: Units of time
I'd imagine you could use other units of time. Personally, I would stay uniform to the units used in the problem or whatever's specified.
- Thu Mar 12, 2020 11:42 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: How do you know a cell can do work?
- Replies: 7
- Views: 518
Re: How do you know a cell can do work?
Mariah wrote:Rebekah Alfred 1J wrote:When E cell is equal to zero, the cell cannot do work.
So can the cell do work whether it is negative or positive?
The cell can do work spontaneously if E cell is positive. E cell can do work non-spontaneously in an electrolytic cell in which an external energy source supplies power.
- Thu Mar 12, 2020 11:35 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Battery
- Replies: 19
- Views: 2406
Re: Battery
Yes, conceptually, there is no difference in electric potential between two cells so no electrons are being transferred between them. If the difference in electric potential is zero, no work can be done.
- Wed Mar 11, 2020 10:29 pm
- Forum: Second Order Reactions
- Topic: 7B.13
- Replies: 2
- Views: 348
Re: 7B.13
What I did was find k using the second order half-life equation. Following that, I divided the original original concentration by 16, 4, and 5 for a), b), and c). With both initial and final concentrations and k, I plugged those values into the second order integrated rate law equation to find t.
- Mon Mar 09, 2020 8:48 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Reversible vs Irreversible
- Replies: 3
- Views: 281
Re: Reversible vs Irreversible
For isothermal reversible expansion, the change is occurring over infinitesimally small steps. On the other hand, irreversible expansion occurs instantly over a constant pressure.
- Mon Mar 09, 2020 5:01 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Applying La Chateliers
- Replies: 4
- Views: 326
Re: Applying La Chateliers
Yes, Ecell is the voltage created by the difference in cell potentials. For instance, in a concentration cell, the higher the difference in concentrations between the cathode and anode, the more work can be done by the voltage that is created across the electrodes.
- Mon Mar 09, 2020 4:51 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Concentration
- Replies: 9
- Views: 569
Re: Concentration
Another helpful tip to know is that the cathode is usually of higher concentration and is considered a reactant. Similarly, the anode is of lower concentration is the product for Q.
- Mon Mar 09, 2020 4:49 pm
- Forum: Balancing Redox Reactions
- Topic: 6K.3 part D
- Replies: 3
- Views: 256
Re: 6K.3 part D
2Cl and Cl2- are different. 2Cl is neutral with an oxidation number of 0, whereas Cl2- is negatively charged with an oxidation number of -1.
- Mon Mar 09, 2020 4:41 pm
- Forum: Balancing Redox Reactions
- Topic: Balancing redox with h2o
- Replies: 9
- Views: 651
Re: Balancing redox with h2o
In acidic solution, you’d use H2O and H+ to balance your redox reaction, because H+ ions are present in acidic solutions. In basic solutions, you’d use H2O and -OH to balance because there’s -OH ions in basic solution. The main idea of balancing redox reactions is to use a combination of H2O and H+/...
- Mon Mar 09, 2020 4:37 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Concentration Cells
- Replies: 3
- Views: 282
Re: Concentration Cells
In a concentration cell, the chemical species involved have the same element but different concentrations. When electrons from a lower concentration solution travel across the electrode, a voltage is generated. When the electrons arrive at the cathode of higher concentration, it attracts ions of the...
- Thu Feb 27, 2020 12:35 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 6N3A
- Replies: 4
- Views: 398
Re: 6N3A
Why is 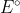 = 0? In what scenarios is
= 0? In what scenarios is 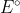 = 0?
= 0?
- Thu Feb 27, 2020 12:16 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 6N.1
- Replies: 4
- Views: 419
Re: 6N.1
From Appendix 2B,
In3+ + e- -> In2+ is -0.49V
U4+ + e- -> U3+ is -0.61V
In3+ + e- -> In2+ is -0.49V
U4+ + e- -> U3+ is -0.61V
- Tue Feb 25, 2020 9:28 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Hydrogen electrodes
- Replies: 1
- Views: 143
Re: Hydrogen electrodes
Hydrogen can either be H+(aq) and reduced to H2(g) or H2(g) and oxidized to H+(aq). In this reaction, there isn’t a solid to serve as an electrode so Platinum would help with that.
- Tue Feb 25, 2020 9:22 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Platinum electrode
- Replies: 4
- Views: 332
Re: Platinum electrode
Betania Hernandez 2E wrote: In some cases, there may be a solid on one side already but it needs to be conducting, if not then you would include Platinum solid.
I’m a little confused by the last part of your reply. What’s an example of a solid that isn’t conducting?
- Mon Feb 24, 2020 5:24 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Concentration and Cell Potential
- Replies: 5
- Views: 3128
Re: Concentration and Cell Potential
When either reactants increase or products decrease, cell potential increases. When either reactants decrease or products increase, cell potential decreases.
- Mon Feb 24, 2020 5:13 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Maximum Potential and Voltage
- Replies: 6
- Views: 452
Re: Maximum Potential and Voltage
Voltage is also the amount of work that can be done per Coulomb. So between two electrodes, when an electric current is flowing between them, it can do work.
- Mon Feb 24, 2020 5:06 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Electrolytic Cells
- Replies: 3
- Views: 310
Re: Electrolytic Cells
By using an external power source, you can drive a non-spontaneous redox reaction forward.
- Mon Feb 24, 2020 12:36 am
- Forum: Balancing Redox Reactions
- Topic: Balancing Redox Reactions
- Replies: 7
- Views: 463
Re: Balancing Redox Reactions
When balancing redox reactions under basic conditions.
- Mon Feb 24, 2020 12:34 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Nernst Equation usage
- Replies: 6
- Views: 399
Re: Nernst Equation usage
Matt Sanruk 2H wrote:What is the Nernst equation exactly?
The Nernst equation calculates cell potential based on concentration. It is E
- Mon Feb 24, 2020 12:29 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Platinum
- Replies: 5
- Views: 393
Re: Platinum
Platinum is the most common electrode because it is an inert conductor. It will not react with and affect the redox reaction.
- Mon Feb 24, 2020 12:27 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: cell potential
- Replies: 3
- Views: 262
Re: cell potential
To add to this answer, the units for volts are 1 Joule per coulomb, which specifies a certain amount in one coulomb.
- Wed Feb 12, 2020 1:36 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Cp and Cv
- Replies: 2
- Views: 206
Re: Cp and Cv
To add on to that, for diatomic gases under constant volume, you’d use 5/2R and for constant pressure, you’d use 7/2R. These equations are used to find the heat capacity of ideal gases.
- Wed Feb 12, 2020 1:33 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Calculating Enthalpy with Atoms, Linear Molecules, and Nonlinear Molecules.
- Replies: 2
- Views: 181
Re: Calculating Enthalpy with Atoms, Linear Molecules, and Nonlinear Molecules.
The monatomic gases where we use (-)R are for when calculating the heat capacity of ideal gases so everything on the right side of the periodic table. You’d use 3/2R for monatomic ideal gases at constant volume. You’d use 5/2R for diatomic ideal gases at constant volume.
- Wed Feb 12, 2020 1:27 am
- Forum: Calculating Work of Expansion
- Topic: Positive or negative work
- Replies: 15
- Views: 2180
Re: Positive or negative work
I like to think of a bicycle pump. When you push the pump down, you are exerting a positive force in work on the pump. However, if the gas were to push back against you, it is counteracting your work, so it’s negative work done by the pump (system).
- Mon Feb 10, 2020 1:21 am
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: irreversible vs reversible
- Replies: 3
- Views: 190
Re: irreversible vs reversible
A reversible reaction proceeds in infinitesimally small steps and can go in either direction. An irreversible reaction occurs instantaneously and cannot be reversed.
- Mon Feb 10, 2020 1:05 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: extensive and intensive
- Replies: 1
- Views: 73
Re: extensive and intensive
Heat capacity depends on the amount of substance present. Specific heat capacity describes the amount of energy needed to raise 1 gram of a substance by 1 degrees Celsius. Because heat capacity depends on the a non-specified amount of substance, it is extensive.
- Mon Feb 10, 2020 1:01 am
- Forum: Phase Changes & Related Calculations
- Topic: delta T
- Replies: 1
- Views: 183
Re: delta T
DeltaT would be Tfinal - Tinitial. Because it drops in temperature, let’s say to 80 degrees, Tfinal - Tinitial would be 80 - 100 = -20, indicating a drop in temperature with the negative sign.
- Mon Feb 10, 2020 12:58 am
- Forum: Phase Changes & Related Calculations
- Topic: equation for phase changes
- Replies: 3
- Views: 137
Re: equation for phase changes
Depending on the question, the deltaH fusion or vaporization is typically provided in J/mol or J/gram. With one or the other, you can convert between them with the molar mass.
- Mon Feb 10, 2020 12:54 am
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: identifying systems
- Replies: 3
- Views: 650
Re: identifying systems
It might be useful to visualize some examples. An example of an open system would be a pot on a stove where matter and energy is exchanged in the forms of ingredients and heat. A closed system would be like a rice cooker where heat is can be transferred to cook the rice, but not matter. An isolated ...
- Mon Feb 03, 2020 12:06 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: 4C.9A
- Replies: 2
- Views: 100
Re: 4C.9A
You are trying to heat the water to 100 degrees C and the copper is, in a sense, in the way. Both water and copper have different heat capacities so adding an amount of heat to one material isn't necessarily going to achieve the same temperature as adding the same amount of heat to another material....
- Sun Feb 02, 2020 11:52 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: method two
- Replies: 3
- Views: 119
Re: method two
Drawing out the Lewis structure is nice because you can visually see the number of bonds a certain molecule has, which will help you determine if you need to or how much to multiply the bond enthalpy by.
- Sun Feb 02, 2020 11:47 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: state functions
- Replies: 10
- Views: 380
Re: state functions
A state function is a property whose value is independent of the path taken to obtain the value. In other words, it doesn't matter how the particular value was achieved, but only as it exists in its current condition.
- Sun Feb 02, 2020 11:42 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Energy of a system
- Replies: 5
- Views: 380
Re: Energy of a system
When delta H is positive, the system absorbed heat. When delta H is negative, heat is removed. Delta H is the change in enthalpy of a system
- Sun Feb 02, 2020 11:35 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Delta H and U
- Replies: 3
- Views: 120
Re: Delta H and U
Delta H is the change in enthalpy. Delta U is the change in the internal energy of the system.
- Sun Jan 26, 2020 11:58 pm
- Forum: Phase Changes & Related Calculations
- Topic: State function
- Replies: 4
- Views: 142
Re: State function
I always conceptualized heat as a Thing that is passed around, while enthalpy is the net change in it. How accurate/inaccurate would that assessment be for the purpose of this class? I believe that's fair. For me, I think there must be a process to calculate heat, like a path, reaction, or change o...
- Sun Jan 26, 2020 11:53 pm
- Forum: Phase Changes & Related Calculations
- Topic: phase changes
- Replies: 5
- Views: 160
Re: phase changes
You won't have to calculate it. We will most likely have to refer to a table with enthalpy values.
- Sun Jan 26, 2020 11:51 pm
- Forum: Phase Changes & Related Calculations
- Topic: State Property
- Replies: 6
- Views: 206
Re: State Property
Of course! a state property is a value that isn't affected by how the system arrived at that state. At equilibrium, enthalpy describe the state of the system without considering how it got there. In contrast, heat doesn't describe a system but is instead a value that shows how a system changes. It d...
- Sun Jan 26, 2020 11:42 pm
- Forum: Phase Changes & Related Calculations
- Topic: State Function
- Replies: 3
- Views: 102
Re: State Function
I interpreted it as enthalpy is a state function and it therefore can be additive. Because it is a property of a system, meaning it is not affected by path taken to get the value, you can add values of enthalpy together.
- Sun Jan 26, 2020 11:36 pm
- Forum: Phase Changes & Related Calculations
- Topic: Temp. of sample
- Replies: 4
- Views: 118
Re: Temp. of sample
Yeah, if you refer to (or look up) the phase change graph, where the slopes are 0, heat is being added, yet temperature remains the same.
- Sun Jan 26, 2020 11:33 pm
- Forum: Phase Changes & Related Calculations
- Topic: ∆H
- Replies: 17
- Views: 681
Re: ∆H
Delta H is a numerical value that is a good indicator of the reaction being endothermic or exothermic. Another way can be to look at the bonds of a reaction. Overall, breaking a bond is endothermic and forming a bond is exothermic.
- Sun Jan 26, 2020 11:24 pm
- Forum: Phase Changes & Related Calculations
- Topic: Differentiating (q) and (w)
- Replies: 4
- Views: 196
Re: Differentiating (q) and (w)
Heat pertains to thermal energy, whereas work pertains to mechanical energy. Work is a means of transferring energy.
- Sun Jan 26, 2020 11:19 pm
- Forum: Phase Changes & Related Calculations
- Topic: delta H
- Replies: 3
- Views: 103
Re: delta H
Standard reaction enthalpy is the change in the total heat of the system during a chemical reaction. The standard enthalpy of formation is the change in the total heat of the system from forming a compound from elements.
- Sun Jan 26, 2020 11:10 pm
- Forum: Phase Changes & Related Calculations
- Topic: Phase changes
- Replies: 8
- Views: 234
Re: Phase changes
Eesha Sohail 1D wrote:On the heating curve, one axis is temperature, but can someone explain what exactly temperature means on a molecular level vs heat?
Heat deals with thermal energy, whereas temperature is the molecular kinetic energy (the molecules moving around in the ice/water/vapor).
- Sun Jan 26, 2020 11:04 pm
- Forum: Phase Changes & Related Calculations
- Topic: Enthalpy of Vaporization and Fusion
- Replies: 5
- Views: 166
Re: Enthalpy of Vaporization and Fusion
It may help to visualize the molecules that make up a solid, liquid and gas. When turning a solid to a gas, the molecules are more freely moving, but still packed together. However, when you turn a liquid into a gas, you are energizing the molecules so that they are quickly bouncing around completel...
- Sun Jan 19, 2020 10:59 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Approximations
- Replies: 2
- Views: 81
Re: Approximations
I don’t think you would need to include the (-x) for the final solution. A good way to check your approximation is the 5% rule.
- Sun Jan 19, 2020 10:55 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: pKa and pKb
- Replies: 17
- Views: 931
Re: pKa and pKb
PKa is calculated by the -log(kA) and pKb is calculated by the -log(kB). If you add pKa and pKb together, it will equal 14.
- Sun Jan 19, 2020 10:44 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Acid Base Equilibria
- Replies: 5
- Views: 194
Re: Acid Base Equilibria
I don't particularly understand the distinction between the K calculated through pressure of a gas and K from concentration. How are they different, and can they be converted into each other in any case? In response to Eesha, it’s the same process to calculate K, but pressure and concentration use ...
- Sun Jan 19, 2020 10:35 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Q < K
- Replies: 16
- Views: 850
Re: Q < K
I like to think about K on a number line. If Q is less than K, it is to the left of K and is trying to go right (towards products). If Q is greater than K, it is to the right of K and trying to go left (towards products). I hope this helps!
- Sun Jan 19, 2020 10:22 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5% rule
- Replies: 8
- Views: 279
Re: 5% rule
Do you only check with the 5% rule if you make the assumption about ignoring the change in concentration of product? In other words, if you solve chemical equilibrium problems with the quadratic formula, do you check with the 5% rule?

