We use the pre-equilibrium approach which involves replacing the intermediates in the rate law of the slow step with reactants of the fast step.
Here's a picture of my notes for further detail
https://ibb.co/7zYNtJ3
Search found 50 matches
- Mon Mar 16, 2020 12:41 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: method
- Replies: 4
- Views: 459
- Mon Mar 16, 2020 12:35 pm
- Forum: General Rate Laws
- Topic: Rate Constants
- Replies: 3
- Views: 387
Re: Rate Constants
To be pseudo-first-order, the concentration of one of the reactants in the rate law must be increased to be much larger than the concentration of the other reactant. For example, look at #15 from ENDGAME. The concentration of A has been increased so much that even when A is used up by the reaction, ...
- Mon Mar 16, 2020 12:28 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Final Exam #15
- Replies: 3
- Views: 547
Final Exam #15
On the final, #15 asked us to find \Delta H ionization for H20 --> OH- + H+. How were we supposed to find this? I googled the \Delta H formation of H2O and OH- and used \sum \Delta H products - \sum \Delta H reactants . Was this the right way to go about it or did we have to use the pH or \Delta H f...
- Sat Mar 14, 2020 8:50 pm
- Forum: First Order Reactions
- Topic: Rate of reaction and temperature
- Replies: 2
- Views: 242
Re: Rate of reaction and temperature
temperature only affects k, so if the temperature is constant for your reaction, then we don't factor it into our calculations
- Sat Mar 14, 2020 8:47 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Lyndon Review: 1D
- Replies: 5
- Views: 529
Re: Lyndon Review: 1D
Mass of the anode will not affect Ecell because sometimes they are inert in the reaction (such as when we use platinum electrodes) and because they are solids and therefore will not affect the concentration of the solutions they are in and don't necessarily participate in the reaction;
- Sat Mar 14, 2020 8:43 pm
- Forum: Balancing Redox Reactions
- Topic: Endgame 3B
- Replies: 3
- Views: 375
Re: Endgame 3B
Is anyone having a hard time figuring out how to do Endgame 3B?? I can balance the oxidation reaction, but I can't figure out how to do the reduction reaction with Br2. Hi y'all, it's me again with a mental breakdown over this problem. Make sure to balance your half reactions correctly with the rig...
- Sat Mar 14, 2020 8:41 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Adding water [ENDORSED]
- Replies: 4
- Views: 406
Re: Adding water [ENDORSED]
I don't think that's correct. According to class notes, when concentration decreases (ie when pure water is added), cell potential will decrease. Not sure about the reasoning, so if anyone knows, please comment!
- Sat Mar 14, 2020 8:20 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: S = 0
- Replies: 21
- Views: 1220
Re: S = 0
Mariah wrote:rabiasumar2E wrote:It's zero in an isothermal reversible reaction.
Can you explain how we know whether or not it is isothermal?
The problem usually say whether or not a reaction is isothermal
- Sat Mar 14, 2020 6:42 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Endgame Q15
- Replies: 2
- Views: 303
Re: Endgame Q15
Yes, this is done to determine the order of B. This is done because A is in such a large amount that even when it is used in the reaction, the effect to the concentration of A is relatively small and therefore the concentration of A doesn't really change. So, we ignore the concentration of A when wr...
- Sat Mar 14, 2020 6:40 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: h20 in our rate law
- Replies: 3
- Views: 321
Re: h20 in our rate law
Generally when water is in excess, like in the dilute solution you're talking about, we wouldn't include water because even when water is used up by the reaction, the change to the concentration water is pretty small. It's like when Lavelle says a billionaire gives away $10, they still have a billio...
- Sat Mar 14, 2020 6:13 pm
- Forum: Student Social/Study Group
- Topic: Tips for Staying Focused
- Replies: 64
- Views: 4656
Re: Tips for Staying Focused
I've found that when I'm in a space where other people are also working, like the library or a coffee shop, I can stay focused for longer periods. Sometimes...peer pressure is good..
Also, NEVER STUDY ON YOUR BED OR IN YOUR BEDROOM IN GENERAL!
Also, NEVER STUDY ON YOUR BED OR IN YOUR BEDROOM IN GENERAL!
- Sat Mar 14, 2020 6:11 pm
- Forum: Student Social/Study Group
- Topic: Thoughtful Poetry Time
- Replies: 3
- Views: 545
Re: Thoughtful Poetry Time
chemistcry
- Sat Mar 14, 2020 6:06 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Chem Community Posts Due Date
- Replies: 13
- Views: 1172
Re: Chem Community Posts Due Date
In an email Lavelle said, "Chemistry Community will also be available up to the day of the final exam." which I would assume is today at midnight
- Sat Mar 14, 2020 3:17 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Steady State
- Replies: 2
- Views: 256
Re: Steady State
I believe that we were told by Dr. Lavelle to not use the steady state method and instead use the pre-equilibrium method that he taught us in class. The pre equil method involves looking at the rate laws of the intermediate reactions and seeing whether or not those rate laws match up with the given...
- Sat Mar 14, 2020 3:16 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Endgame 5d
- Replies: 3
- Views: 490
Re: Endgame 5d
Not sure about why n is 1 (I had the same question) but for temperature, unless stated otherwise, assume that the temperature is 25 C or 298 K
- Sat Mar 14, 2020 3:10 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Endgame #5 [ENDORSED]
- Replies: 2
- Views: 386
Endgame #5 [ENDORSED]
In the Endgame review packet, #5 says: "Calculate the value of Ka for HF using the standard cell potentials: F2 + 2 H + (aq) + 2e- --> 2 HF (aq) E o = +3.03 V F2 + 2 e- --> 2 F- (aq) E o = +2.87 V " I understand why E o is -0.16 V but I don't understand why n (the moles of electrons transf...
- Sat Mar 14, 2020 2:03 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: E vs Eo
- Replies: 6
- Views: 541
Re: E vs Eo
Nicholas Chin 1G wrote:In some cases, standard E will be zero, so this equation can be used to find Ecell in concentration cells.
Is Eo only ever 0 in a concentration cell or is there another situation in which Eo = 0 ?
- Sat Mar 14, 2020 11:29 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: E vs Eo
- Replies: 6
- Views: 541
Re: E vs Eo
Rory Simpson 2F wrote:Eº is the standard cell potential which in this case refers to a cell under standard conditions (25ºC, 1atm, 1mol/L). E doesn't have these conditions, so you'd be calculating the cell potential for any differences in temperature or concentration, as the equation suggests.
Ah thank you sm!
- Sat Mar 14, 2020 10:15 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: E vs Eo
- Replies: 6
- Views: 541
E vs Eo
In the equation
Ecell = Eo -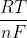 ln Q
ln Q
what is the difference between Ecell and Eo?
Ecell = Eo -
what is the difference between Ecell and Eo?
- Sat Mar 14, 2020 9:55 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Steady State
- Replies: 2
- Views: 256
Steady State
How exactly do we use the steady-state method to determine the reaction mechanism? My notes are a bit confusing so I don't understand how to use this and why we would use this method.
- Sat Mar 14, 2020 9:48 am
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: rate constant
- Replies: 5
- Views: 447
Re: rate constant
I believe they're trying to differentiate this k from the equilibrium constant K.
In this case, kr is the k we mean in the rate of reaction (rate = k[Reactant]^n)
In this case, kr is the k we mean in the rate of reaction (rate = k[Reactant]^n)
- Fri Mar 13, 2020 11:58 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Endgame 5 and 7
- Replies: 2
- Views: 282
Re: Endgame 5 and 7
MingdaH 3B wrote:You can add them in 5 because they're part of a cathode anode system, but standard Ecell is not a state function in either case.
Thank you!! This was confusing me as well
- Fri Mar 13, 2020 11:55 pm
- Forum: Second Order Reactions
- Topic: Stoichiometric coefficients
- Replies: 2
- Views: 366
Re: Stoichiometric coefficients
Here's a picture of my notes showing how a coefficient would affect the rate law and half-life equations. I think a general rule of thumb is that wherever the k goes, the coefficient goes with it.
https://ibb.co/wJSnv2H
(Sorry for the link, chemistry community won't let me just upload a picture)
https://ibb.co/wJSnv2H
(Sorry for the link, chemistry community won't let me just upload a picture)
- Fri Mar 13, 2020 11:44 pm
- Forum: First Order Reactions
- Topic: Units
- Replies: 2
- Views: 263
Re: Units
The units for a second-order reaction are actually L*mol^-1*s^-1. Why? When you think about what the rate is for second-order reactions: \frac{1}{[A]}=kt+\frac{1}{[A]0} the units of k need to work with all the other pieces in the equation. The units of 1/[A] are L*mol^-1 while the units of t are usu...
- Fri Mar 13, 2020 11:32 pm
- Forum: Van't Hoff Equation
- Topic: Test 2 #5
- Replies: 2
- Views: 306
Re: Test 2 #5
Thank you so much, this makes so much more sense!
- Fri Mar 13, 2020 11:30 pm
- Forum: General Rate Laws
- Topic: Natural Log Rate Order
- Replies: 4
- Views: 369
Re: Natural Log Rate Order
Here's a screenshot of my notes explaining why first-order rates are a function of natural log [A] and time

- Fri Mar 13, 2020 11:21 pm
- Forum: General Rate Laws
- Topic: Elementary reactions
- Replies: 4
- Views: 408
Re: Elementary reactions
I think you're confusing an elementary reaction for some kind of a different reaction. An elementary reaction is just the term we use for the smaller steps that when added together, result in the complete reaction. So if a reaction is ever broken down into several steps, each of those steps is an el...
- Fri Mar 13, 2020 11:12 pm
- Forum: General Rate Laws
- Topic: ENDGAME 14f
- Replies: 2
- Views: 271
Re: ENDGAME 14f
First, we find that the overall reaction is A + B + E --> F + G + B. We got this by adding together the two steps and canceling out the intermediates C and D. We cannot cancel out B because it's a catalyst (enters the initial reaction and exits from the final reaction). Then the condition that must ...
- Fri Mar 13, 2020 9:24 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: 14F
- Replies: 1
- Views: 145
Re: 14F
First, we find that the overall reaction is A + B + E --> F + G + B. We got this by adding together the two steps and canceling out the intermediates C and D. We cannot cancel out B because it's a catalyst (enters the initial reaction and exits from the final reaction). Then the condition that must ...
- Fri Mar 13, 2020 9:03 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: 7.9
- Replies: 1
- Views: 128
Re: 7.9
So the two possible mechanisms are one where the rate law = k[C12H22O11] and another where the rate law = k[H2O][C12H22O11]. (I got these by looking at what the slow step is). The only difference between these two rate laws is that one is water-dependent and the other runs independently of water. Th...
- Fri Mar 13, 2020 8:53 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Molecularity
- Replies: 1
- Views: 137
Re: Molecularity
Molecularity refers to the number of molecules that collide is an elementary step. Molecularity is helpful in identifying the rate of an elementary step. For example, when we say that an elementary step where molecule A reacts to form product P is bimolecular, we then know that 2 molecules of A must...
- Fri Mar 13, 2020 8:43 pm
- Forum: Properties of Electrons
- Topic: z effective
- Replies: 2
- Views: 561
Re: z effective
You can think of Z effective as the relative force (or 'effect') protons have/exert on each electron. If there are a lot of electrons surrounding the nucleus (such as Radon for example), the effect protons have on any one electron is pretty low therefore the Z effective is low. But if there are only...
- Fri Mar 13, 2020 8:35 pm
- Forum: Van't Hoff Equation
- Topic: Test 2 #5
- Replies: 2
- Views: 306
Test 2 #5
On the most recent test there was the question: Given that \Delta H rxn o = -42 kJ/mol and K = 1.63106 for a reaction at 25 C, calculate K at 110 C. Assume that \Delta H rxn o and \Delta S rxn o remain constant over this temperature range. The equation sheet had the Van't Hoff Equation written as ln...
- Fri Mar 13, 2020 8:23 pm
- Forum: Ionic & Covalent Bonds
- Topic: Ionic bonds in solutions
- Replies: 9
- Views: 849
Re: Ionic bonds in solutions
Ionic bonds between the ions are broken but they are replaced by hydrogen bonds with the oppositely charged atom in a water molecule. I.e. when NaCl is dissolved in water, Na+ forms a hydrogen bond with negatively charged oxygen.
- Fri Mar 13, 2020 8:20 pm
- Forum: Ionic & Covalent Bonds
- Topic: Bond Forming
- Replies: 8
- Views: 604
Re: Bond Forming
Molecules must also collide with enough energy to overcome the activation energy needed to form a bond.
- Tue Mar 10, 2020 9:10 pm
- Forum: Administrative Questions and Class Announcements
- Topic: What is the plan for the final?
- Replies: 16
- Views: 1071
What is the plan for the final?
Has Lavelle addressed what he's going to do for the final? I've heard from all my other professors and need to know soon
- Tue Mar 03, 2020 10:52 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6N.13
- Replies: 1
- Views: 223
6N.13
I'm a bit confused on how to understand non-typical cell reactions like the one from 6N.13 part b: Calculate the reaction quotient, Q, for the following cell reactions, given the measured values of the cell potential. Balance the chemical equations by using the smallest whole-number coefficients: b)...
- Tue Feb 25, 2020 11:16 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 6N3c
- Replies: 2
- Views: 245
Re: 6N3c
The first step is to break up this cell into the two half-reactions which are: 2 H + + 2e - --> H 2 and 2 Cl - --> Cl 2 + 2 e - We can find the E o for each half-reaction by using the Standard Electric Potential chart, and we find that E o for the 1st reaction is 0.00V and +1.36 V. And since the fin...
- Tue Feb 18, 2020 8:46 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Salt tunnel in Galvanic cells
- Replies: 1
- Views: 91
Re: Salt tunnel in Galvanic cells
The salts used in these reactions are specifically chosen so they do not react with any species used in the cells. Their only purpose like you indicated is to balance charges but they won't change equilibrium concentrations of main reaction's species.
- Tue Feb 18, 2020 8:40 pm
- Forum: Balancing Redox Reactions
- Topic: Electrochemistry Outline
- Replies: 1
- Views: 145
Re: Electrochemistry Outline
I think this picture summarizes the relationship between Gibbs Free Energy, K and cell potential
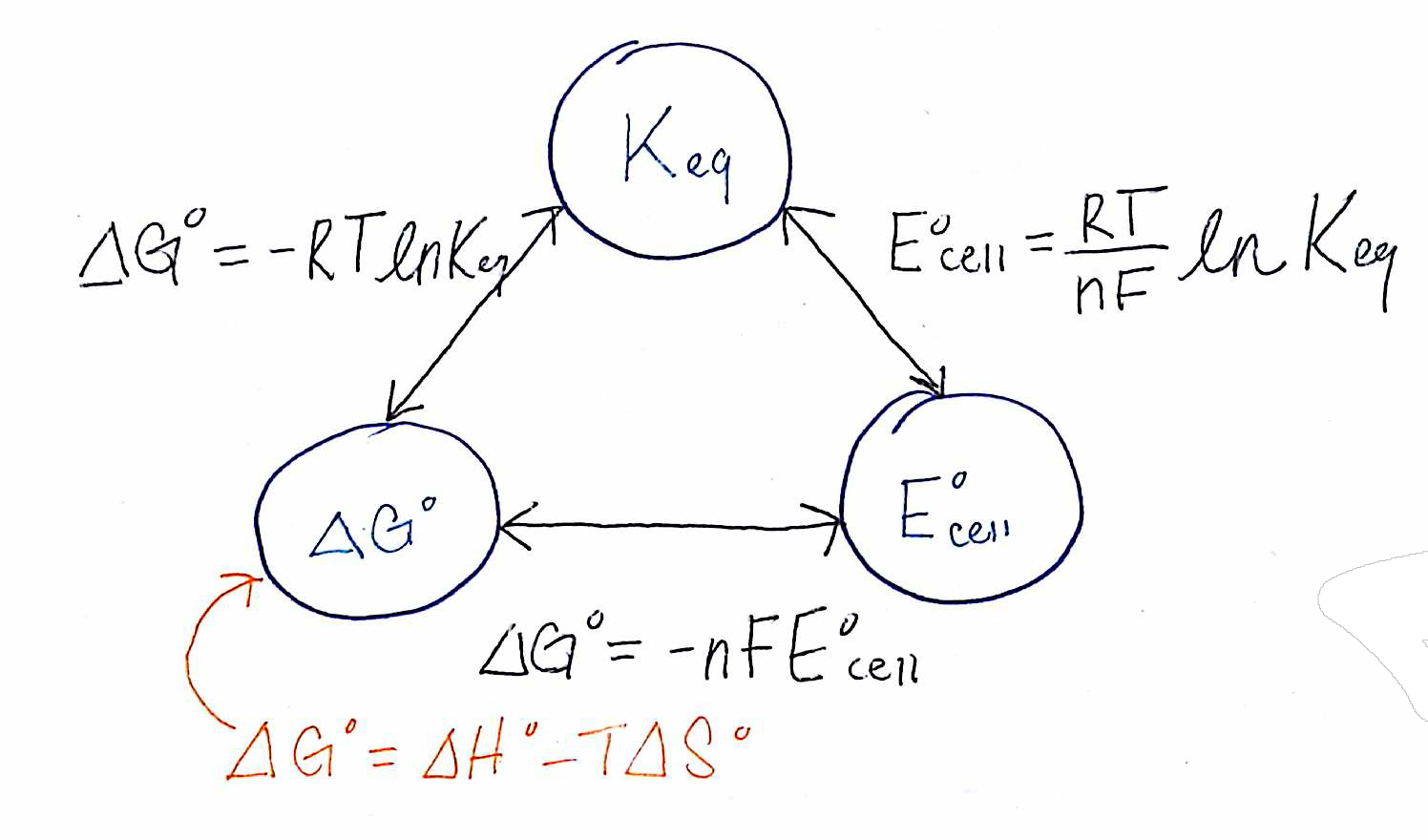

- Tue Feb 18, 2020 8:23 pm
- Forum: Balancing Redox Reactions
- Topic: 6K.5
- Replies: 1
- Views: 435
Re: 6K.5
I really like this one from organic chemistry tutor: https://www.youtube.com/watch?v=fdbrhQAM9Gw
(all of his videos are really good btw, he's super helpful)
(all of his videos are really good btw, he's super helpful)
- Sat Feb 08, 2020 10:35 am
- Forum: Administrative Questions and Class Announcements
- Topic: Pizza Rolls REVIEW Session DOWNLOAD HERE
- Replies: 67
- Views: 5965
Re: Pizza Rolls REVIEW Session
Thank you so much!! Will you be providing another packet or will you go over this packet on your Monday workshop?
- Tue Feb 04, 2020 2:16 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Reversible Isothermic Reaction
- Replies: 1
- Views: 107
Reversible Isothermic Reaction
On Monday's lecture (2/3), Lavelle was talking about a reversible isothermic reaction and then he mentioned that the system expands, therefore does work. But ΔU does not change because the system is isothermic. But how does that make sense? In the diagram, we see that the system takes in heat, so ho...
- Tue Feb 04, 2020 2:07 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Heat vs Temp
- Replies: 3
- Views: 225
Re: Heat vs Temp
Temperature is the average kinetic energy of all the molecules/atoms in a substance (ie. what is the average of how much is each molecule/atom vibrating?) Heat, on the other hand, is the total amount of energy a thing has since heat is a form of energy. For example, heat can be transferred from one ...
- Wed Jan 29, 2020 10:46 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Enthalphy of Combustion
- Replies: 2
- Views: 108
Re: Enthalphy of Combustion
ΔH c o is the energy released or absorbed by 1 mol of substance when burned in oxygen. We can use these values to solve for the overall ΔH rxn by doing ∑ΔH c o products - ∑ΔH c o reactants Looking at 4D.15, we calcuate the reaction enthalpy by doing the following calculation: -1560 kJ - (-1300 kJ + ...
- Tue Jan 28, 2020 6:44 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: How to do 4A.13
- Replies: 1
- Views: 170
Re: How to do 4A.13
we have to use the equation q cal =CΔT. so for the first reaction, we can solve for C by plugging in q cal (-3.50 kJ) and ΔT (7.32°C). We then get that C = -0.478 kJ/°C. then for the second reaction, we use this C value to solve for q cal . Plug in the C value we solved above (-0.478 kJ/°C) and ΔT o...
- Fri Jan 24, 2020 10:04 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Calculation methods
- Replies: 6
- Views: 297
Re: Calculation methods
Method 1: When two reactions are given and both their ΔH rxn are also given, you can add both reactions and their ΔH rxn to get the new reaction's ΔH rxn . (See the example he gave in class) Method 2: Use bond enthalpies. Bond enthalpies are always given on a chart. You can try to figure out what ar...
- Thu Jan 23, 2020 10:15 pm
- Forum: Phase Changes & Related Calculations
- Topic: Work, reversible path
- Replies: 2
- Views: 272
Re: Work, reversible path
More work is done when a process is very slow because there is a lot of energy that goes into getting the task done. For example, it take a lot of energy to climb a mountain than a small bump in the round -- one takes a lot longer than the other. How does this apply? In a reversible reaction, there ...
- Thu Jan 16, 2020 11:18 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Stress
- Replies: 4
- Views: 139
Re: Stress
Types of stressors include: change in temperature, change in volume/pressure, and adding or removing a reactant or product
- Thu Jan 09, 2020 4:55 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Exercise 5I.15
- Replies: 3
- Views: 184
Exercise 5I.15
I'm a bit confused on how to solve this question: When solid NH 4 HS and 0.400 mol NH 3 (g) were placed in a vessel of volume 2.0L at 24 C, the equilibrium NH 4 HS (s) ⇄ NH3 (g) + H2S (g), for which K c = 1.5 x 10^-4, was reached. What are the equilibrium concentrations for NH3 and H2S? I understand...

