Search found 101 matches
- Mon Mar 09, 2020 1:24 pm
- Forum: General Rate Laws
- Topic: rate laws and graphs
- Replies: 4
- Views: 555
Re: rate laws and graphs
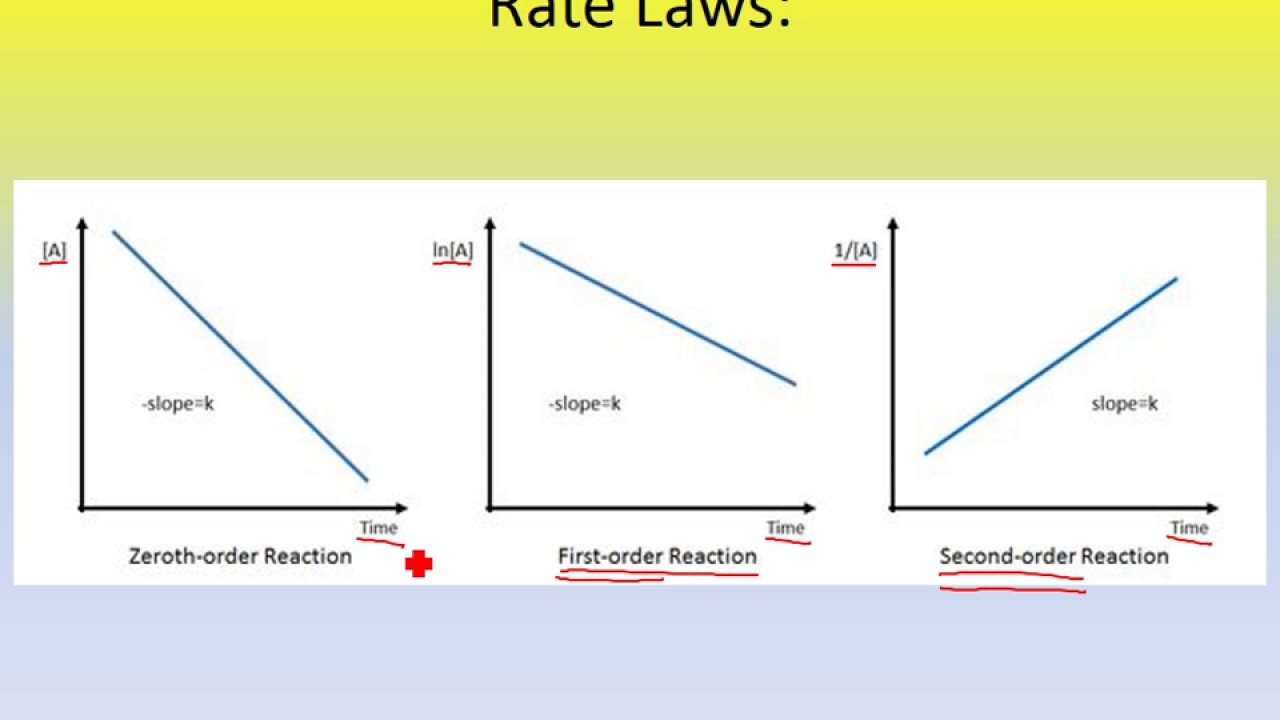
For your reference, here are the graphs showing the slope line trends and axes labels for each order of reaction:


- Mon Mar 09, 2020 1:18 pm
- Forum: General Rate Laws
- Topic: Rate constant k
- Replies: 3
- Views: 459
Re: Rate constant k
To add on, the rate constant, k, does have different units depending on what order the reaction is. The general rule is as follows for the units of k: 1/(Mn-1*t-1), where n is the order of the reaction.
- Mon Mar 09, 2020 1:13 pm
- Forum: General Rate Laws
- Topic: Rate Constant Units
- Replies: 3
- Views: 322
Re: Rate Constant Units
To add on, the general rule for rate constant units is:
1/(Mn-1*t-1), where n is the order of the reaction.
1/(Mn-1*t-1), where n is the order of the reaction.
- Mon Mar 09, 2020 1:08 pm
- Forum: General Rate Laws
- Topic: Difference between each order of reaction
- Replies: 3
- Views: 333
Re: Difference between each order of reaction
Here's a general diagram with the given slope trend lines and axes labels for each type of order:


- Mon Mar 09, 2020 1:06 pm
- Forum: General Rate Laws
- Topic: First vs Second vs Zero Order
- Replies: 7
- Views: 605
Re: First vs Second vs Zero Order
Here's a digram with the given slope line trends and axes labels for each reaction order, if it helps:


- Mon Mar 02, 2020 2:17 pm
- Forum: General Rate Laws
- Topic: Order
- Replies: 5
- Views: 533
Re: Order
Here is a diagram of the graphs mentioned above, in case you might find it helpful:


- Mon Mar 02, 2020 2:11 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Units for Gibbs
- Replies: 3
- Views: 266
Re: Units for Gibbs
As a general rule, anything with the ∆_ r is per mole. Regarding ∆G (according to the reply of a similar question), free energy can have the units of kJ or kJ/mol depending on whether the question refers to the molar free energy of a substance or the free energy of a specific amount of the substance...
- Mon Mar 02, 2020 1:43 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Free Energy and Work
- Replies: 5
- Views: 427
Re: Free Energy and Work
Free energy is the maximum amount of energy from a system that can be used to perform useful work, in other words, work not associated with the expansion of a system.
- Mon Mar 02, 2020 1:39 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: The sign of ∆Gº
- Replies: 2
- Views: 326
Re: The sign of ∆Gº
∆G˚ really tells you the thermodynamic favorability of a reaction using the ratio of the amount of product to the amount of reactant at equilibrium. Also, you are correct in the relationship shown by that equation; if ∆G˚is negative, then K>1 and it may be important to note that E˚ is positive.
- Mon Mar 02, 2020 1:22 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Outline of Thermodynamics
- Replies: 3
- Views: 334
Re: Outline of Thermodynamics
Gibbs free energy, ΔG, is the maximum amount of energy from a system that can be used to perform useful work, in other words, work not associated with the expansion of a system.
- Fri Feb 28, 2020 8:50 am
- Forum: Balancing Redox Reactions
- Topic: Oxidation Number
- Replies: 2
- Views: 215
Re: Oxidation Number
To add on, as a general rule, H is +1, O is generally -2 (except for peroxides), and halogens (i.e. F) are typically -1.
- Fri Feb 28, 2020 8:36 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: 4J.5
- Replies: 2
- Views: 479
Re: 4J.5
Yes, you're supposed to use ∆G˚=∆H˚-T∆S˚ with the values provided for ∆H˚ and ∆S˚ within that table. The purpose here is to show that the calculated answer should be pretty close to the actual ∆G˚ provided in that table, but it will likely be off by a few decimals.
- Fri Feb 28, 2020 8:26 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Entropy units
- Replies: 7
- Views: 445
Re: Entropy units
As a general rule of thumb, try to write out the units as you're working through the problem since problems often give you a value in kJ, but the constants you use involve J. Otherwise unless the problem specifies, you can leave your answer in J or kJ. The answer key likely leaves the answer in kJ w...
- Fri Feb 28, 2020 8:21 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Question 4J.7
- Replies: 5
- Views: 370
Re: Question 4J.7
Just to list a few more, H2, Cl2, Br2, and C(s, graphite) also have enthalpies of formation equal to 0.
- Fri Feb 28, 2020 8:14 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Go=0
- Replies: 7
- Views: 413
Re: Go=0
∆G˚=0 when products and reactants are in their standard state and K=1. Note that this is not common.
- Wed Feb 19, 2020 7:11 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: C in nCv ln (T2/T1)
- Replies: 8
- Views: 1235
Re: C in nCv ln (T2/T1)
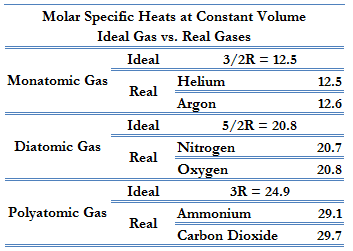
To add on, the relationship between Cv and Cp is Cp = Cv + 1. The values for Cv are shown below (know that we look at the ideal gas values):

- Wed Feb 19, 2020 7:04 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: U vs H
- Replies: 15
- Views: 1265
Re: U vs H
To add on, the equation we often use to relate the two is ∆U = q + w. At constant pressure, ∆H = qp. Thus, we can rewrite the equation as ∆U = ∆H - P∆V when pressure is constant.
- Wed Feb 19, 2020 6:58 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Reversible Reaction
- Replies: 6
- Views: 468
Re: Reversible Reaction
Something to note is that the work a system can do is greatest in a reversible process, compared to an irreversible process. This is because a reversible process is one that can be reversed by an infinitely small change in a variable, i.e. pressure such that when a gas expands reversibly, the extern...
- Wed Feb 19, 2020 6:42 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: ∆G, ∆H, ∆S
- Replies: 6
- Views: 560
Re: ∆G, ∆H, ∆S
Here's a table that relates the three fairly well, with respect to T in order to determine spontaneity:


- Wed Feb 19, 2020 3:48 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: How to interpret reversible/irreversible graphs
- Replies: 6
- Views: 702
Re: How to interpret reversible/irreversible graphs
Something to note is that the work a system can do is greatest in a reversible process, hence the larger area under the curve for the reversible graph. The graphs are attached to this for your convenience. http://chemwiki.ucdavis.edu/@api/deki/files/9998/workdone.JPG?size=bestfit&width=454&h...
- Mon Feb 10, 2020 2:13 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Spontaneous delta G
- Replies: 7
- Views: 440
Re: Spontaneous delta G
For this problem, we're using the equation: ∆G˚ = ∆H˚ - T∆S˚. The key point to keep in mind here is that the reaction is spontaneous when ∆G˚ is negative. Thus, we're trying to find the temperature, T, at which T∆S˚ > ∆H˚ such that ∆G˚ is negative. To do so, we can set ∆G˚ = 0 to find the minimum te...
- Mon Feb 10, 2020 1:48 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: S=kB*lnw equation
- Replies: 4
- Views: 904
Re: S=kB*lnw equation
To add on, the Boltzmann's constant, k = 1.38 x 10–23 J·K-1.
- Mon Feb 10, 2020 1:32 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Is spontaneity determined by entropy or free energy?
- Replies: 9
- Views: 1972
Re: Is spontaneity determined by entropy or free energy?
As others have said, check for whether the question is asking for ∆S or total entropy. That being said, a useful chart to check for spontaneity with ∆S, provided ∆H is given, is (through the use of ∆G = ∆H - T∆S): https://s3mn.mnimgs.com/img/shared/discuss_editlive/1643930/2012_01_23_14_07_42/FOR_2....
- Mon Feb 10, 2020 1:14 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: What is residual entropy?
- Replies: 16
- Views: 4915
Re: What is residual entropy?
To add on, the only time when a molecule would have no entropy is when you have a molecule at 0 K that’s perfectly ordered. Otherwise, molecules at 0 K would still have entropy due to the concept of residual entropy, which is explained by others above.
- Mon Feb 10, 2020 1:09 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Why is delta U = 0 for isothermal reactions?
- Replies: 11
- Views: 4375
Re: Why is delta U = 0 for isothermal reactions?
To add on, I believe the 3/2 in your equation came from 'Ideal gas, Cv = (3/2) R' (which can be found on the formula sheet). Note that this 'C' value varies depending on what you're trying to solve for, i.e. 'Ideal gas, Cp = (5/2) R'.
- Mon Feb 03, 2020 1:50 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Internal Energy/Open System
- Replies: 3
- Views: 354
Re: Internal Energy/Open System
To clarify, adding or removing the amount of substance in a system correlates to changing the energy of an open system. Heating/cooling the system or doing work on the system are examples provided in class for a closed system.
- Mon Feb 03, 2020 1:38 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Isolated// Energy
- Replies: 11
- Views: 609
Re: Isolated// Energy
No, in an isolated system there can be no exchange of energy with the surrounding. The combustion of glucose in a bomb calorimeter is an example. To clarify, a closed system can have energy exchange with surroundings by heating/cooling or compression/expansion.
- Mon Feb 03, 2020 1:22 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Closed Systems
- Replies: 14
- Views: 949
Re: Closed Systems
In a closed system, energy can be exchanged with surroundings, unlike an isolated system in which nothing can be exchanged with the surroundings. The energy of a closed system can be changed by heating/cooling or compression/expansion (i.e. compressing a piston).
- Mon Feb 03, 2020 1:15 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Closed System
- Replies: 7
- Views: 470
Re: Closed System
Just to add on, other examples of closed systems that can be found in the textbook (4A.1) are:
1) A coolant in a refrigerator coil
2) Mercury in a thermometer
1) A coolant in a refrigerator coil
2) Mercury in a thermometer
- Mon Feb 03, 2020 1:12 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: closed vs isolated?
- Replies: 7
- Views: 1316
Re: closed vs isolated?
An example of a closed system (energy can exchange with surroundings) is a sealed beaker of water, in which the beaker does not insulate. An example of an isolated system (nothing exchanges with surroundings) is the combustion of glucose in a bomb calorimeter. These are the examples provided in clas...
- Mon Jan 27, 2020 1:29 pm
- Forum: Phase Changes & Related Calculations
- Topic: Unrelated
- Replies: 3
- Views: 592
Re: Unrelated
For evaporation to occur, the temperature does not necessarily need to reach the boiling point. One thing to keep in mind is that evaporation is a natural process (boiling usually isn't) and while similar to boiling, the water can change from liquid to vapor form when there is an increase in either ...
- Mon Jan 27, 2020 1:16 pm
- Forum: Phase Changes & Related Calculations
- Topic: steam at 100ºC burn worse
- Replies: 7
- Views: 947
Re: steam at 100ºC burn worse
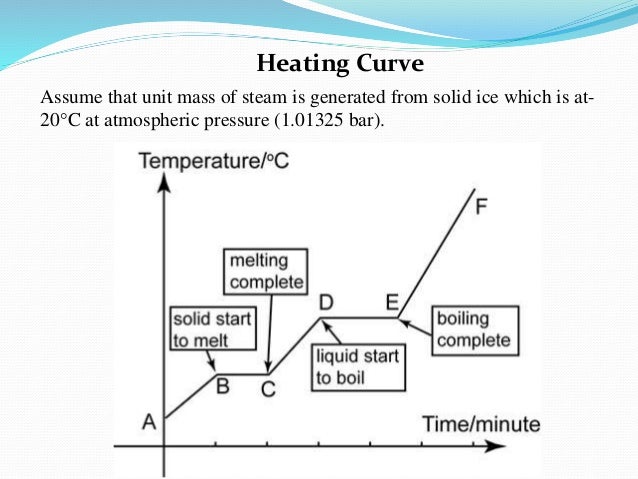
To help clarify with the help of the water heating curve, you can see in the diagram that it takes much more energy for liquid water to transition to steam than for ice to transition to liquid water. Thus, steam at 100ºC holds more energy than liquid water at 100ºC, causing worse burns. https://chem...
- Mon Jan 27, 2020 1:12 pm
- Forum: Phase Changes & Related Calculations
- Topic: boiling points
- Replies: 4
- Views: 598
Re: boiling points
I found this diagram online which will hopefully help clarify things. You can see that it takes some time before the liquid is fully vaporized.

- Mon Jan 27, 2020 1:08 pm
- Forum: Phase Changes & Related Calculations
- Topic: Why does steam cause burns?
- Replies: 29
- Views: 1275
Re: Why does steam cause burns?
Showing the actual diagram, which is the heating curve of water, you can see that it takes a lot more energy to transition from liquid to vapor than it does from solid to liquid. Therefore, steam carries much more heat for worse burns than boiling water. https://chem.libretexts.org/@api/deki/files/6...
- Mon Jan 27, 2020 1:05 pm
- Forum: Phase Changes & Related Calculations
- Topic: Steam?
- Replies: 8
- Views: 338
Re: Steam?
For a more visual representation through the heating curve of water, you can see that transitioning from liquid to vapor requires a lot more energy than it does from solid to liquid. Thus, steam carries more heat than boiled water. https://chem.libretexts.org/@api/deki/files/61002/6c6b33a839c7613ad3...
- Tue Jan 21, 2020 10:32 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Q and K
- Replies: 8
- Views: 632
Re: Q and K
Q and K are calculated essentially the same way. However, Q expresses the relative ratio of products to reactants at a given instant not necessarily at equilibrium, which is why you can compare Q and K to determine the direction of a reaction. The reaction shifts right if Q<K, shifts left if Q>K, an...
- Tue Jan 21, 2020 10:28 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: units for pressure
- Replies: 5
- Views: 558
Re: units for pressure
My TA said bar would be the most likely unit used. However, it's always a good idea to check your units and ensure the correct cancelation.
- Tue Jan 21, 2020 10:23 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Reaction Quotient
- Replies: 10
- Views: 811
Re: Reaction Quotient
Q expresses the relative ratio of products to reactants at a given instant not necessarily at equilibrium, which is why you compare Q and K to determine the direction of the reaction. The reaction shifts right if Q<K, shifts left if Q>K, and is at equilibrium when Q=K.
- Tue Jan 21, 2020 10:12 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Reaction Q
- Replies: 7
- Views: 407
Re: Reaction Q
Q expresses the relative ratio of products to reactants at a given instant not necessarily at equilibrium, which is why you can compare Q with K to determine the direction of the reaction. The reaction shifts right if Q<K, shifts left if Q>K, and is at equilibrium is Q=K.
- Tue Jan 21, 2020 10:09 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Using Kc Vs Kp
- Replies: 22
- Views: 1071
Re: Using Kc Vs Kp
Kp can only be used when reactants and products are gases. Kc can be used for gases or aqueous substances, but you should check the units involved to ensure you're using the right K.
- Mon Jan 13, 2020 10:48 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Partial Pressure to Concentration
- Replies: 3
- Views: 365
Re: Partial Pressure to Concentration
Just to add on, when using PV=nRT, you can rearrange the formula as such: P=(n/V)RT. (n/V) then represents the concentration, mol/L, and using the constant R and the given temperature, you can convert between partial pressure and concentration.
- Mon Jan 13, 2020 8:47 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Equilibrium constants
- Replies: 4
- Views: 960
Re: Equilibrium constants
Solids have an essentially constant concentration because they are practically incompressible. As a result, it takes enormous pressure to cause even a tiny reduction in volume. As such, solids can be excluded from the equilibrium constant.
- Mon Jan 13, 2020 8:43 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: solids and liquids
- Replies: 6
- Views: 227
Re: solids and liquids
Liquids and solids have an essentially constant concentration because they are practically incompressible. As a result, it takes enormous pressure to cause even a tiny reduction in volume. As such liquids and solids can be excluded from the equilibrium constant.
- Mon Jan 13, 2020 8:38 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Q
- Replies: 10
- Views: 396
Re: Q
Q expresses the relative ratio of products to reactants at a given instant not necessarily at equilibrium. This is mainly useful to compare to K in order to determine the direction of the reaction. When Q=K, the system is at equilibrium.
- Mon Jan 13, 2020 8:29 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Calculating K
- Replies: 5
- Views: 183
Re: Calculating K
Liquids and solids have an essentially constant concentration because they are practically incompressible. As a result, it takes enormous pressure to cause even a tiny reduction in volume. As such liquids and solids can be excluded from the equilibrium constant.
- Thu Jan 09, 2020 9:40 pm
- Forum: Ideal Gases
- Topic: Difference between K and Q
- Replies: 9
- Views: 334
Re: Difference between K and Q
To add on, since Q expresses the relative ratio of products to reactants at a given instant not necessarily at equilibrium, you know the reaction shifts right if Q<K and shifts left if Q>K.
- Thu Jan 09, 2020 9:29 pm
- Forum: Ideal Gases
- Topic: pressure [ENDORSED]
- Replies: 13
- Views: 1118
Re: pressure [ENDORSED]
Yes, by PV=nRT, pressure (P) can be increased by increasing the temperature (T) or increasing the moles (n) of the substance involved (assuming no other variables are changed for simplicity's sake).
- Thu Jan 09, 2020 9:25 pm
- Forum: Ideal Gases
- Topic: Ideal Gases
- Replies: 7
- Views: 535
Re: Ideal Gases
As others have mentioned, an ideal gas is theoretical. Most gases do behave closer to ideal with high temperature and low pressure, however. For most problems, unless stated otherwise, ideal gases should be the ones involved such that we can simplify calculations and use i.e. PV=nRT (ideal gas law).
- Thu Jan 09, 2020 9:17 pm
- Forum: Ideal Gases
- Topic: Definition of Ideal Gases?
- Replies: 3
- Views: 365
Re: Definition of Ideal Gases?
Ideal gases have negligible volume, no attractive or repulsive forces, random movement, and perfectly elastic collisions. Most gases behave close to the ideal with high temperature and low pressure; hence an ideal gas is theoretical since PV=nRT shows that pressure typically increases with temperatu...
- Thu Jan 09, 2020 9:05 pm
- Forum: Ideal Gases
- Topic: R in PV=nRT
- Replies: 34
- Views: 6720
Re: R in PV=nRT
R is the universal gas constant, sometimes known as the Regnault constant. The value of the R constant is 8.3144598 J/mol·K.
- Tue Dec 03, 2019 10:50 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Saying Thank You to Dr. Lavelle
- Replies: 490
- Views: 575411
Re: Saying Thank You to Dr. Lavelle
Dear Dr. Lavelle, Thank you for an incredible winter quarter! I really appreciate all of the resources you provide on your website and the many, many hours of review and study sessions you offer. You definitely help to build a stable foundation for future courses in chemistry. With that, I look forw...
- Tue Dec 03, 2019 10:38 pm
- Forum: Bronsted Acids & Bases
- Topic: Bronsted Versus Lewis
- Replies: 4
- Views: 485
Re: Bronsted Versus Lewis
The Lewis definition is more consistent in classifying most acids-bases, since a molecule might not have hydrogen to donate for the definition of a Bronsted acid. However, my TA said, at least for now, the Bronsted definition will be primarily used (i.e. for writing chemical equations in acid-base r...
- Tue Dec 03, 2019 10:32 pm
- Forum: Bronsted Acids & Bases
- Topic: Conjugate acids and bases
- Replies: 5
- Views: 488
Re: Conjugate acids and bases
Hopefully, this will help you visualize the acid-base pairings in a reaction (if you're a visual learner like me):


- Tue Dec 03, 2019 10:27 pm
- Forum: Bronsted Acids & Bases
- Topic: bronsted vs lewis
- Replies: 2
- Views: 129
Re: bronsted vs lewis
Just to add on, if you think of a Bronsted acid as donating a proton, it's consistent with the idea of receiving electrons by definition of a Lewis acid (donates H + , gets e- back). Apparently, the Lewis definitions are more consistent for most acids/bases, especially for those without hydrogen whe...
- Tue Dec 03, 2019 10:14 pm
- Forum: Bronsted Acids & Bases
- Topic: Strong Acids and Bases
- Replies: 4
- Views: 399
Re: Strong Acids and Bases
I agree! Knowing strong acids and bases would be very helpful in writing out the equation or for calculations, especially for our current level of chemistry which focuses mainly on strong acids and bases. Table 6C.3 in the textbook, page 464, might be helpful.
Re: Oxidation
To add on, if the problem asks for the oxidation number of the metal, use:
(# of metal ion)*(oxidation number) + (# if each ligand)*(charge of each ligand) = charge of ion
(# if each ligand)*(charge of each ligand) = charge of ion
The charge of the ion should be given, allowing you to isolate and solve for the oxidation number of the metal.
(# of metal ion)*(oxidation number) +
The charge of the ion should be given, allowing you to isolate and solve for the oxidation number of the metal.
- Wed Nov 27, 2019 12:39 pm
- Forum: Bronsted Acids & Bases
- Topic: Strong acid
- Replies: 6
- Views: 833
Re: Strong acid
Strong acids almost completely ionize in solution. HCl, which dissociates completely, is a strong acid. Therefore, 0.1 M HCl(aq) implies 0.1 M H3O+(aq) and 0.1 M Cl-(aq).
- Wed Nov 27, 2019 12:33 pm
- Forum: Bronsted Acids & Bases
- Topic: Definition
- Replies: 5
- Views: 187
Re: Definition
A Bronsted acid is a proton donor (i.e. HCl, HBr), while a Lewis acid is an electron acceptor (i.e. BF3, H+).
- Wed Nov 27, 2019 12:27 pm
- Forum: Bronsted Acids & Bases
- Topic: Bronsted Acids and Bases
- Replies: 3
- Views: 482
Re: Bronsted Acids and Bases
Bronsted acids are proton donors. I believe that H2SO3 is a Bronsted acid because it can donate a proton to become HSO3-, its conjugate base.
- Wed Nov 27, 2019 12:01 pm
- Forum: Bronsted Acids & Bases
- Topic: Bronsted acid
- Replies: 9
- Views: 1039
Re: Bronsted acid
By definition, Bronsted acids are proton donors. HBr is a Bronsted acid because it can donate its H+, to which it would become Br-.
- Fri Nov 22, 2019 12:57 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Polarity
- Replies: 7
- Views: 323
Re: Polarity
To add on, a good rule of thumb is that if the structure is symmetrical, it's typically nonpolar, and if the structure is asymmetrical, it's typically polar. You may have to look at electronegativity differences and dipole-dipole interactions to check.
- Fri Nov 22, 2019 12:42 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: bent v. angular
- Replies: 27
- Views: 1530
Re: bent v. angular
Bent and angular refer to the same shape with VSEPR formulas AX2E or AX2E2.
- Fri Nov 22, 2019 12:40 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: polar vs non polar
- Replies: 6
- Views: 417
Re: polar vs non polar
To add on, sometimes you can tell just by looking at the formula where it may be obvious that hydrogen bonds are present, etc. Often times, it's better to draw out the structure to be sure, during which looking at the symmetry of the structure helps to determine whether or not it's polar/nonpolar. N...
- Fri Nov 22, 2019 12:27 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Why is CH2Cl2 polar?
- Replies: 12
- Views: 793
Re: Why is CH2Cl2 polar?
To add on, typically speaking, a molecule with a tetrahedral shape is only nonpolar if all four atoms bonded to the central atom are the same.
- Fri Nov 22, 2019 12:22 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Removing Non-Axis Atoms First
- Replies: 4
- Views: 396
Re: Removing Non-Axis Atoms First
Referencing back to the seesaw shape, the lone pair in the equatorial plane is removed first to minimize repulsion. By doing so, the more repulsive lone pair interacts with only 2 bonds at 90° instead of three bonds if the axial lone pair was removed. Hence, removing the lone pair in the equatorial ...
- Sat Nov 16, 2019 8:29 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: What is VSPER
- Replies: 14
- Views: 1019
Re: What is VSPER
VSEPR stands for Valence-Shell Electron-Pair Repulsion. The VSEPR Model explains the experimentally observed shape of molecules and can predict distortions qualitatively but not quantitatively. It may be helpful to memorize the shapes and general angles, though you probably don't need to memorize ex...
- Sat Nov 16, 2019 8:14 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSEPR
- Replies: 2
- Views: 424
Re: VSEPR
Lewis structures are 2-D representations of molecular shape and indicate the approximate location of bonding e- and lone pair e-. The VSEPR Model, or the Valence-Shell Electron-Pair Repulsion Model, explains the experimentally observed shape of molecules. Note that the VSEPR Model can predict distor...
- Sat Nov 16, 2019 7:59 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: seesaw
- Replies: 9
- Views: 672
Re: seesaw
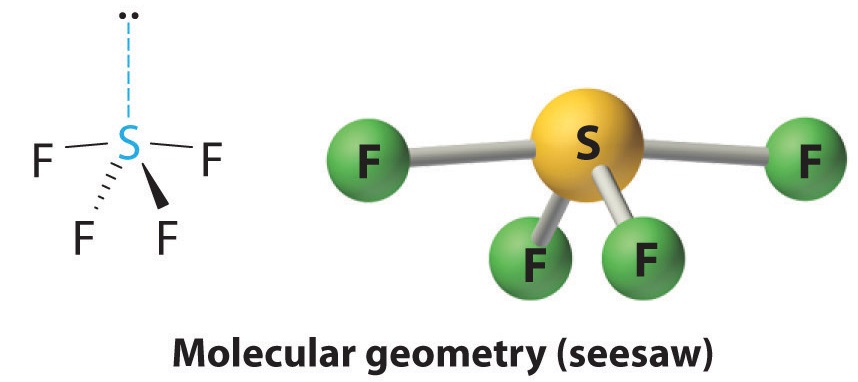
If you're a visual learner like I am, here's the image for SF4, AX4E with 4 bonding pairs and 1 lone pair:

- Sat Nov 16, 2019 7:56 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bond Angles
- Replies: 8
- Views: 345
Re: Bond Angles
Like what others have said, it will probably be helpful to memorize the angles. As a side note, trigonal planar angles are 120 degrees. The 107 degrees was for, i.e. NH3, a molecule with 1 lone pair and 3 bonding pairs.
- Sat Nov 16, 2019 7:50 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Polar vs. Nonpolar
- Replies: 12
- Views: 823
Re: Polar vs. Nonpolar
While not always the case, symmetrical shapes typically indicate the molecule is nonpolar; there are dipole-dipole interactions, but they cancel out for a nonpolar molecule. Asymmetrical shapes typically indicate the molecular is polar since the dipole-dipole interactions can't cancel out.
- Fri Nov 08, 2019 1:07 pm
- Forum: Ionic & Covalent Bonds
- Topic: Ionic vs. Covalent
- Replies: 4
- Views: 914
Re: Ionic vs. Covalent
Just to add on, for the more in-between electronegativity differences, where it's uncertain whether the bonds are covalent or ionic, it's based on properties. For example, if the substance can dissociate in water, it may be more ionic in character.
- Fri Nov 08, 2019 12:23 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Quantum Numbers
- Replies: 6
- Views: 523
Re: Quantum Numbers
ms is usually pretty arbitrary unless the question specifies that the electron is +1/2 or -1/2. The most important thing to remember is that no two electrons in an atom can have the exact same four quantum numbers, so if one electron is +1/2, the other in the pair is -1/2.
- Fri Nov 08, 2019 12:17 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Formal names for each letter
- Replies: 5
- Views: 324
Re: Formal names for each letter
n = Principle Quantum Number l = Angular Momentum Quantum Number m l = Magnetic Quantum Number m s = Spin Magnetic Quantum Number n defines l and m l and as such is the principle quantum number. For l, I tend to think of it as the odd one out; it's not the principle and it's not magnetic so it must ...
- Fri Nov 08, 2019 12:03 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: n, l ,ml, ms
- Replies: 13
- Views: 1518
Re: n, l ,ml, ms
As mentioned, ms can be either +1/2 or -1/2, and it's pretty arbitrary unless the question specifically provides the spin. The most important thing to note is that no two electrons in the same atom can have the exact same four quantum numbers.
- Fri Nov 08, 2019 12:00 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Quantum numbers
- Replies: 12
- Views: 775
Re: Quantum numbers
To add on, the angular momentum quantum number (l), or the secondary quantum number, describes the shape of the orbital that an electron occupies with allowed values of n-1.
- Sat Nov 02, 2019 9:16 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Angular Momentum Quantum Number
- Replies: 3
- Views: 487
Re: Angular Momentum Quantum Number
The Angular Momentum Quantum Number (l) describes shape by corresponding with a certain sub-shell, each of which with a unique arrangement. I hope this diagram will help you better picture what the second quantum number describes; note that not all possible variations of each sub-shell are shown in ...
- Sat Nov 02, 2019 9:07 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Ms and ML
- Replies: 6
- Views: 2063
Re: Ms and ML
Magnetic Quantum Number (m l ) labels different orbitals or sub-shells with allowed values of l, l-1,..., 0,..., -l. For example: l=2; m l can be -2, -1, 0, 1, 2 Spin Magnetic Quantum Number (m s ) denotes the spin of an electron with values of +1/2 or -1/2, depending on if the electron is spin up o...
- Sat Nov 02, 2019 9:01 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Quantum Number
- Replies: 3
- Views: 145
Re: Quantum Number
As said previously, the spin magnetic quantum number (ms) can be +1/2 or -1/2. It's usually pretty arbitrary, so long as you're aware that no two electrons in the same atom have the same four quantum numbers.
- Sat Nov 02, 2019 8:58 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Magnetic Quantum Number
- Replies: 5
- Views: 174
Re: Magnetic Quantum Number
You would only say 5 if the question asks for the number of possible ml values. Otherwise, given that l=2, you can't say that 5 is a possible value of ml since the allowed values are l, l-1,..., 0,..., -l.
- Sat Nov 02, 2019 8:51 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Quantum Numbers
- Replies: 7
- Views: 266
Re: Quantum Numbers
The spin number, or the spin magnetic quantum number (m s ), is +1/2 or -1/2 since an electron can be spin up or down. The significance is that no two electrons in the same atom can have the same four quantum numbers. It tends to be pretty arbitrary as long as the combination of the four quantum num...
- Thu Oct 24, 2019 5:18 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Hunds Rule
- Replies: 2
- Views: 568
Re: Hunds Rule
Here's a visual aid for Hund's Rule, which may help you understand a bit better (if you're visual learner like I am):
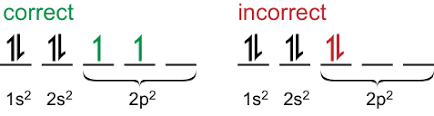
- Thu Oct 24, 2019 5:04 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Spin Quantum Number
- Replies: 4
- Views: 385
Re: Spin Quantum Number
An important thing to note with the inclusion of the spin quantum number is that no two electrons in the same atom can have exactly the same four quantum numbers. The spins are indicated, as everyone said, by +1/2 or -1/2 since the electron can spin up or down.
- Thu Oct 24, 2019 5:02 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Orbital Angular Momentum
- Replies: 3
- Views: 153
Re: Orbital Angular Momentum
The angular momentum quantum number (l) describes "shape" and is also known as the sub-shell. The allowed values are l = 0,1,2,...,n-1. Note that n is the principle quantum number which determines energy and size, or shells.
l = 0 s-orbital
l = 1 p-orbital
l = 2 d-orbital
l = 3 f-orbital
l = 0 s-orbital
l = 1 p-orbital
l = 2 d-orbital
l = 3 f-orbital
- Wed Oct 23, 2019 9:55 am
- Forum: Properties of Light
- Topic: Photons
- Replies: 3
- Views: 171
Re: Photons
Hello! Just to add on a little more information, certain energy formulas will not work for photons due to photons having zero mass. E=mc^2, for example, will not work for the photon as it would for an electron; photons have energy, yet the formula E=mc^2 would result in an answer of 0 J for the phot...
- Wed Oct 23, 2019 9:40 am
- Forum: Properties of Light
- Topic: Destructive Interference
- Replies: 3
- Views: 1653
Re: Destructive Interference
Just to add on, destructive interference occurs when waves come together in a way that they cancel each other out. While usually in between for a result of smaller amplitude, destructive interference can produce zero amplitude in the right conditions. Note that when two waves interfere destructively...
- Wed Oct 16, 2019 5:25 pm
- Forum: Photoelectric Effect
- Topic: Work function units
- Replies: 4
- Views: 658
Re: Work function units
Just to add on: the work function is the energy needed to remove an electron, also known as the threshold energy. The unit for energy is J (joule); as such, the unit for work function is J.
- Wed Oct 16, 2019 4:58 pm
- Forum: SI Units, Unit Conversions
- Topic: Joules
- Replies: 2
- Views: 160
Re: Joules
The SI units for Joules (J) are kg * m^2 * s^-2. Not sure if this will be the best method to help you, but my TA explained how he remembers the SI units for Joule: W = F * d; where W is work (unit is J), F is force, and d is distance = [m(a)] * d; since F=ma = [kg(m*s^-2)] * m; where kg is SI unit f...
- Wed Oct 16, 2019 4:46 pm
- Forum: SI Units, Unit Conversions
- Topic: Conversion
- Replies: 2
- Views: 132
Re: Conversion
I agree with the previous post! Just to make it clearer (if you're a visual learner like I am): \frac{2.26*10^-46 m}{1} * \frac{1 nm}{1*10^-9 m} Since meters (m) is in both the numerator and denominator, you can cross them out. Then you'll be left with nanometers (nm), the unit you want. Again, conv...
- Wed Oct 16, 2019 2:38 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: spin magnetic quantum number
- Replies: 3
- Views: 215
Re: spin magnetic quantum number
Just to add on to previous replies, the spin magnetic quantum number was deemed necessary after the Stern and Gerlach Experiment, where the electrons were found to have two different spins (hence +1/2 or -1/2). It's important to note that no two electrons in the same atom have the same four quantum ...
- Wed Oct 16, 2019 2:10 pm
- Forum: Properties of Light
- Topic: Lyman Series & Balmer Series
- Replies: 4
- Views: 237
Re: Lyman Series & Balmer Series
Just to add on, the Balmer Series corresponds to visible light, n1=2; Lyman series corresponds to ultraviolet light, n1=1. This is good to know for some problems that just provide what type of electromagnetic radiation is involved, i.e. 1A.15.
- Sat Oct 12, 2019 1:13 am
- Forum: SI Units, Unit Conversions
- Topic: Conversion
- Replies: 6
- Views: 583
Re: Conversion
The unit for joules (J) in SI units is kg⋅m^2⋅s^−2. Like what others have said, meters and joules are units of measure for different criteria; meters are units of length and joules are units of energy. A possible equation that relates the two is: Kinetic energy = 1/2 * mass * velocity ^2.
- Sat Oct 12, 2019 12:56 am
- Forum: SI Units, Unit Conversions
- Topic: knowing how many sig figs to use
- Replies: 17
- Views: 812
Re: knowing how many sig figs to use
To keep your work as accurate as possible, try not to round until the final answer. On the basis that you do round, keeping a certain number of significant figures should be enough to result in the final answer. For example, if the answer needs to have two significant figures, keeping four significa...
- Sat Oct 12, 2019 12:52 am
- Forum: SI Units, Unit Conversions
- Topic: Formula units vs molecule
- Replies: 7
- Views: 375
Re: Formula units vs molecule
Formula units work the same way as do molecules and atoms through Avogadro's number. According to the book, the key difference is that formula units pertain to ionic compounds, whereas molecules are of molecular compounds and atoms are of elements. If you want to check it out yourself, the informati...
- Sat Oct 12, 2019 12:41 am
- Forum: Balancing Chemical Reactions
- Topic: Writing an Equation for the Reaction
- Replies: 9
- Views: 3691
Re: Writing an Equation for the Reaction
Just to add onto the topic of combustion, O2 (oxygen gas) is reacted with the given fuel for the products of CO2 (carbon dioxide) and H2O (water). Depending on the fuel, you may need to balance the equation.
- Sat Oct 12, 2019 12:33 am
- Forum: SI Units, Unit Conversions
- Topic: Practice Problems?
- Replies: 11
- Views: 617
Re: Practice Problems?
There is a list of practice problems, for fundamentals and further topics, on the class syllabus. Just scroll down to the last pages.
Here's the link: https://lavelle.chem.ucla.edu/wp-conten ... SYLL_1.pdf
Here's the link: https://lavelle.chem.ucla.edu/wp-conten ... SYLL_1.pdf
- Fri Oct 04, 2019 10:30 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: E.16
- Replies: 2
- Views: 325
Re: E.16
Thanks!
- Fri Oct 04, 2019 3:59 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Avogadro's number
- Replies: 9
- Views: 545
Re: Avogadro's number
Avogadro's number, 6.022 x 10^23, represents the number of "particles" within one mole of a substance. These particles can be, for example, the number of atoms per mole of a given compound.
- Fri Oct 04, 2019 3:54 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Rounding
- Replies: 12
- Views: 880
Re: Rounding
It's best to only round at the end of the calculations. If you round throughout the series of calculations, the answer could be different depending on the number of significant figures needed. Generally speaking, using 7 instead of 6.94 for Lithium can likely get you the right answer for say multipl...
- Fri Oct 04, 2019 3:47 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Theoretical vs. Actual Yield
- Replies: 38
- Views: 14107
Re: Theoretical vs. Actual Yield
Theoretical yield is the amount of product that can be obtained if a chemical reaction has 100% efficiency, the maximum amount of yield possible. Actual yield is the amount of product actually produced by the reaction. Due to side reactions, impurities, some of the product sticking on to the sides o...
- Fri Oct 04, 2019 3:39 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: E.16
- Replies: 2
- Views: 325
E.16
E.16 The molar mass of the metal oxide M2O is 231.74 g.mol^-1. What is the molar mass of the chloride of this metal?
For this question, what exactly is the chloride within M2O, and how would you go about finding the molar mass of said chloride?
For this question, what exactly is the chloride within M2O, and how would you go about finding the molar mass of said chloride?

