Search found 100 matches
- Sat Mar 13, 2021 7:24 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: when to add Pt (s)
- Replies: 23
- Views: 1058
Re: when to add Pt (s)
We add Pt(s) (or a different inert conductor) if there is no conducting metal for the anode/cathode.
- Sat Mar 13, 2021 7:22 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Anode and Cathode
- Replies: 25
- Views: 1387
Re: Anode and Cathode
Yes! The anode is oxidized (loses electrons) and the cathode is reduced (gains electrons). So the electrons flow from the anode to the cathode.
- Sat Mar 13, 2021 7:18 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Enthalpy vs Entropy
- Replies: 39
- Views: 3287
Re: Enthalpy vs Entropy
Entropy refers to the disorder or microstates. Enthalpy refers to the heat exchange (absorbed or released). Both entropy and enthalpy are state functions.
- Sat Mar 13, 2021 7:15 pm
- Forum: Calculating Work of Expansion
- Topic: W= -PDeltaV
- Replies: 15
- Views: 1944
Re: W= -PDeltaV
Yes, this equation can only be used for irreversible expansion. The external pressure must also be constant!
- Sat Mar 13, 2021 7:08 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: ∆G vs ∆G˚
- Replies: 5
- Views: 492
Re: ∆G vs ∆G˚
Both ∆G and ∆Gº can tell us about spontaneity. ∆Gº does so at standard conditions, while ∆G does so at any other conditions.
- Sun Mar 07, 2021 10:11 pm
- Forum: First Order Reactions
- Topic: Slope
- Replies: 24
- Views: 943
Re: Slope
The slope for zero order and first order reactions is -k. For second order reactions, the slope is +k.
- Sun Mar 07, 2021 10:09 pm
- Forum: General Rate Laws
- Topic: Intermediates
- Replies: 17
- Views: 1507
Re: Intermediates
Intermediates are not included in the rate law because they are produced and then consumed (or consumed and then produced), meaning that they are on opposite sides of the equation so they would cancel.
- Sun Mar 07, 2021 10:02 pm
- Forum: First Order Reactions
- Topic: Equation Confusion
- Replies: 16
- Views: 733
Re: Equation Confusion
The equation used depends on the order of the reaction. The first equation is for a zero order reaction, while the second equation is for a first order reaction.
- Sun Mar 07, 2021 10:01 pm
- Forum: Zero Order Reactions
- Topic: graph for 1st, 2nd, and zero order reactions
- Replies: 8
- Views: 5329
Re: graph for 1st, 2nd, and zero order reactions
To figure out the order, you would have to plot the data against time and figure out which is the most linear. A zero order reaction would be linear with a negative slope. A first order reaction would be linear with a negative slope. A second order reaction would be linear with a positive slope.
- Sun Mar 07, 2021 9:57 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Multiple Eº & textbook #6.45
- Replies: 2
- Views: 173
Multiple Eº & textbook #6.45
Hi! I noticed that some elements have multiple standard cell potentials (for example the textbook has Fe^{2+}+2e^{-}\rightarrow Fe, E^{\circ} = -0.44 V and Fe^{3+}+3e^{-}\rightarrow Fe, E^{\circ}=-0.04 V ). How would we know which half-reaction to use if a problem just asked for the standard cell po...
- Sat Feb 27, 2021 10:31 pm
- Forum: Balancing Redox Reactions
- Topic: Oxidation vs Reduction
- Replies: 30
- Views: 1364
Re: Oxidation vs Reduction
I like using OIL RIG to remember!
O oxidation
I: is
L: loss
R: reduction
I: is
G: gain
O oxidation
I: is
L: loss
R: reduction
I: is
G: gain
- Sat Feb 27, 2021 10:26 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Sapling week 7/8 #11
- Replies: 6
- Views: 405
Re: Sapling week 7/8 #11
If Eº > 0 then the reaction is spontaneous. If Eº < 0 then the reaction is not spontaneous. So to figure out Eº we can use the equation Eº cell = Eº cathode - Eº anode.
- Sat Feb 27, 2021 10:22 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Sapling week 7/8 question 7
- Replies: 5
- Views: 425
Re: Sapling week 7/8 question 7
Ryan_Kien_1L wrote:I kept getting the line diagram wrong because I had coefficients on the right-hand side when there weren't supposed to be any.
I did this too - Is there a reason why there shouldn't be any coefficients in the cell diagram?
- Sat Feb 27, 2021 10:20 pm
- Forum: Balancing Redox Reactions
- Topic: Determining which molecule is the oxidizing agent
- Replies: 49
- Views: 1999
Re: Determining which molecule is the oxidizing agent
The oxidizing agent is being reduced and the reducing agent is being oxidized.
- Sat Feb 27, 2021 10:11 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Sapling week 7/8 #13
- Replies: 2
- Views: 611
Sapling week 7/8 #13
Hi! I am confused as to how we can determine if a substance can oxidize one substance but not another. We use the standard reduction potentials, but I am not really sure how to solve the problem from there. #13) Which of the reagents would oxidize Cr to Cr2+ , but not Ag to Ag+? Co2+ , Br2, Br-, Ca,...
- Thu Feb 18, 2021 10:40 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Delta H and Delta S both positive
- Replies: 31
- Views: 8668
Re: Delta H and Delta S both positive
If both deltaH and deltaS are positive then the reaction would be spontaneous at high temperatures.
- Thu Feb 18, 2021 10:36 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Calculating degeneracy
- Replies: 18
- Views: 3548
Re: Calculating degeneracy
Degeneracy (W) = (# of positions)^(# of molecules). Then you can just plug this into the S=KblnW equation.
- Thu Feb 18, 2021 10:24 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Cv(Heat Capacity) of Monoatomic and Diatomic Particles
- Replies: 2
- Views: 252
Re: Cv(Heat Capacity) of Monoatomic and Diatomic Particles
The Cp of a monatomic gas is 5/2 R (and it's on the equation sheet!). For a diatomic gas, we can just use the Cp = Cv + R equation and find that since Cv (for a diatomic gas) is 5/2 R, Cp would be 7/2 R.
- Thu Feb 18, 2021 10:08 pm
- Forum: Calculating Work of Expansion
- Topic: negative vs positive work
- Replies: 21
- Views: 992
Re: negative vs positive work
Yes this is correct! Work is negative when the system is expanding (because system is doing work) and work is positive when the system is compressed (since work is done on the system).
- Thu Feb 18, 2021 9:39 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Thermodynamically Favorable
- Replies: 27
- Views: 1954
Re: Thermodynamically Favorable
Thermodynamically favorable means that the reaction/process does not require an input of energy to occur, meaning it occurs naturally. When gibbs free energy is negative, the process is thermodynamically favorable.
- Sun Feb 14, 2021 1:13 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Residual Entropy
- Replies: 1
- Views: 141
Residual Entropy
Hi! I am a bit confused on residual entropy and was wondering if someone could explain it. How do we determine residual entropy / how do we know when a molecule has residual entropy?
- Sun Feb 14, 2021 1:07 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: question 18 on sapling (week 5/6)
- Replies: 5
- Views: 244
Re: question 18 on sapling (week 5/6)
Use the 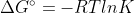 equation and then solve for K. Just make sure that you have the same units (whether that be converting deltaG to J or R to kJ).
equation and then solve for K. Just make sure that you have the same units (whether that be converting deltaG to J or R to kJ).
- Sun Feb 14, 2021 12:58 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Sapling question 4
- Replies: 9
- Views: 489
Re: Sapling question 4
To solve this problem, I used the deltaS = nRln(V2/V1) equation. I used the ratio they gave for the V2/V1 (so in your case 1/6) and used 1 mole.
- Sun Feb 14, 2021 12:50 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: week 5 and 6 sampling hw #6
- Replies: 4
- Views: 316
Re: week 5 and 6 sampling hw #6
To solve this problem, think of the the entropy as being composed to two separate events. First, find the change in entropy when there is a change in volume. Then, find the change in entropy when there is a temperature change. Use Cv for the temperature change equation. Then add both of the entropy ...
- Sun Feb 14, 2021 12:45 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Statistical Entropy vs. Thermodynamic Entropy
- Replies: 3
- Views: 306
Statistical Entropy vs. Thermodynamic Entropy
If statistical entropy (S = KlnW) and thermodynamic entropy (deltaS = q/T) lead to the same results and have similar general properties, in what ways are they different? When would we use one equation over the other?
- Sun Feb 07, 2021 10:41 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Sapling #20 Linear vs. nonlinear molecules
- Replies: 13
- Views: 602
Re: Sapling #20 Linear vs. nonlinear molecules
Yes, I would just draw out the structure and see if it is a linear or nonlinear molecule.
- Sun Feb 07, 2021 10:39 am
- Forum: Calculating Work of Expansion
- Topic: Sapling #14
- Replies: 5
- Views: 254
Re: Sapling #14
Joseph Hsing 2C wrote:For this question how do you find the number of moles?
You can use the PV=nRT equation to find the moles.
- Fri Feb 05, 2021 10:49 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: delta U= delta H
- Replies: 21
- Views: 1604
Re: delta U= delta H
- Fri Feb 05, 2021 10:41 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Postive vs. negative work
- Replies: 18
- Views: 1049
Re: Postive vs. negative work
If the value of work is positive, then work is being done ON the system. Since energy is used when a system does work, work done BY a system should have a negative value.
- Fri Feb 05, 2021 9:52 am
- Forum: Calculating Work of Expansion
- Topic: Reversible vs irreversible
- Replies: 2
- Views: 243
Reversible vs irreversible
How do we know if a reaction is reversible or irreversible? Are there certain types of reactions or conditions that will always be reversible or always be irreversible? Or will the problem have to explicitly state that it's either reversible or irreversible?
- Sun Jan 31, 2021 12:37 pm
- Forum: Calculating Work of Expansion
- Topic: Integral
- Replies: 9
- Views: 617
Re: Integral
We use volume for the integral since the volume is the thing changing.
- Sun Jan 31, 2021 12:31 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Sapling week 3 #6
- Replies: 12
- Views: 495
Re: Sapling week 3 #6
The answer would be -4 x bond energy of C-H because CH4 is being formed, meaning that bonds are being formed which is exothermic. The 4 x bond energy of C-H would imply that the bonds would be broken (endothermic) and be on the reactant side, which would be a different reaction.
- Sun Jan 31, 2021 12:26 pm
- Forum: Phase Changes & Related Calculations
- Topic: endothermic/exothermic
- Replies: 43
- Views: 4768
Re: endothermic/exothermic
Bonds are either broken or formed during a phase change. So if bonds are broken, it will be endothermic, while if bonds are formed it will be exothermic. I also like to visualize a heating curve. Solid --> liquid = endothermic liquid --> gas = endothermic solid --> gas = endothermic gas --> liquid =...
- Sun Jan 31, 2021 12:19 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Sapling wk 3/4 #8
- Replies: 5
- Views: 184
Re: Sapling wk 3/4 #8
To calculate the amount of heat absorbed from a an amount of grams, you have to convert the grams into moles using the molar mass of the substance. Then multiply it by \Delta H to get the heat (since deltaH is KJ/mol). To calculate the amount of substance produced from a certain heat, you would divi...
- Thu Jan 28, 2021 3:30 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Gas phase - Bond enthalpies
- Replies: 3
- Views: 180
Gas phase - Bond enthalpies
Hi! When we use bond enthalpies to calculate the reaction enthalpy the substance needs to be in the gaseous state, how would you make something become a gas if it were in a liquid/aqueous/solid state?
- Sun Jan 24, 2021 9:21 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: X Approximations
- Replies: 23
- Views: 936
Re: X Approximations
If Ka or Kb is less than 10^-4, then we can assume x to be negligible. We can check to make sure that x is negligible by using the percent protonation/ionized by seeing if it is less than 5%. If it is greater than 5% then we cannot assume x to be negligible.
- Sun Jan 24, 2021 9:13 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Do concentrations change at equilibrium?
- Replies: 9
- Views: 230
Re: Do concentrations change at equilibrium?
At equilibrium, the rate of the forward reaction equals the rate of the reverse reaction, so the concentrations would not change. However, if volume/pressure/temperature changed or product/reactant was added/removed, the concentrations would change and the equilibrium will adjust to these changes.
- Sun Jan 24, 2021 9:05 am
- Forum: Phase Changes & Related Calculations
- Topic: Determining Stronger Acids
- Replies: 18
- Views: 759
Re: Determining Stronger Acids
A larger Ka means a stronger acid. A smaller pKa also means a stronger acid.
- Sun Jan 24, 2021 8:59 am
- Forum: Phase Changes & Related Calculations
- Topic: No Heat Change
- Replies: 15
- Views: 590
Re: No Heat Change
The temperature remains constant during a phase change because the heat is being used to break the bonds.
- Sun Jan 24, 2021 8:55 am
- Forum: Phase Changes & Related Calculations
- Topic: H and q
- Replies: 47
- Views: 1786
Re: H and q
H represents enthalpy, while q represents the transfer of heat.
- Sun Jan 17, 2021 5:50 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Ice Box Method
- Replies: 14
- Views: 528
Re: Ice Box Method
If the problem says that you have an initial amount of the reactants and zero amount of the products, then you would subtract the x from the reactants and add x to the products. If you are given a problem where each substance has an initial amount, then I would try solving for Q to see if Q>K or Q<K...
- Sun Jan 17, 2021 4:19 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Getting two positive x values when using quadratic
- Replies: 43
- Views: 4906
Re: Getting two positive x values when using quadratic
If both the x values are positive I think we should test the values. There will might likely be a value that, when subtracted from the initial concentration, will give a negative concentration so then it cannot be that value.
- Sun Jan 17, 2021 3:49 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: X less than 5 percent
- Replies: 11
- Views: 943
Re: X less than 5 percent
The way that I understand the less than 5% rule is that if the percent ionization is less than 5%, we can approximate x. If it is larger, then we are not able to approximate x and we must solve for x algebraically.
- Sun Jan 17, 2021 3:26 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Heating an Exothermic Reaction
- Replies: 7
- Views: 397
Re: Heating an Exothermic Reaction
When doing reactions with temperature, I like to view heat as a product (exothermic) or reaction (endothermic). So by increasing the heat of the exothermic reaction, the equilibrium will shift to favor the reactants, thus causing an increase in the reactants.
- Sun Jan 17, 2021 2:55 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: increasing/decreasing a solid/liquid
- Replies: 6
- Views: 312
increasing/decreasing a solid/liquid
I know that if there is an increase/decrease in concentration, pressure, and temperature the equilibrium will adjust to minimize the effects of these changes. Does this still apply if there is an increase/decrease in the concentration of a solid or liquid? Or does the reaction not change?
- Sun Jan 10, 2021 7:16 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Units
- Replies: 27
- Views: 1011
Re: Units
I think we will either use bars or atm, since they are similar in value, but I think the units would just depend on the problem.
- Sun Jan 10, 2021 7:13 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: increasing total pressure in equilibria
- Replies: 11
- Views: 486
Re: increasing total pressure in equilibria
When the pressure increases, the equilibrium will "shift" to the side that has less moles of gas. Decreasing pressure will cause equilibrium to "shift" to the side with more moles of gas.
- Sun Jan 10, 2021 7:07 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: K vs. Q
- Replies: 53
- Views: 2260
Re: K vs. Q
Q and K are both calculated using the [p]/[r]. Q is the reaction quotient, and is used at any time where the reaction is not at equilibrium. K is for when the reaction is at equilibrium.
- Sun Jan 10, 2021 6:58 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Response of Equilibria to Change
- Replies: 8
- Views: 283
Re: Response of Equilibria to Change
The only thing that can change K is a change in temperature. If the pressure is changes or reactants/products are added, the reaction will still have the same K because the ratio will still be the same.
- Sun Jan 10, 2021 2:53 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Picking Value when doing Quadratic Equation
- Replies: 10
- Views: 464
Re: Picking Value when doing Quadratic Equation
You have to use the x value that is positive because concentrations cannot be negative.
- Sat Dec 12, 2020 5:23 pm
- Forum: Amphoteric Compounds
- Topic: Recognizing Amphoteric Substances
- Replies: 8
- Views: 591
Re: Recognizing Amphoteric Substances
Hi! There are also some amphoteric substances along the metalloid line.
- Sat Dec 12, 2020 5:20 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Ph and PKA
- Replies: 7
- Views: 474
Re: Ph and PKA
Nice name! The reason that an acid is more acidic when it has a lower pKa is that pKa = -log[Ka]. An inverse relationship would form (as Ka increases, pKa decreases). When pH < pKa (acidic conditions), the acid gets protonated as HA. It will be neutral. When pH > pKa, the acid will give off a proto...
- Sat Dec 12, 2020 5:11 pm
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: Textbook question 6C.17
- Replies: 3
- Views: 255
Re: Textbook question 6C.17
I'm not entirely sure but the way that I approached the problem was by comparing the electronegativity. I thought that since Br is more electronegative than N, it would make BrO- stronger than morphine.
- Sat Dec 12, 2020 4:47 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Textbook 6b1
- Replies: 4
- Views: 236
Re: Textbook 6b1
The way I approached this problem was to use \Delta pH = final - initial . So 0.12 [HCl] - [HCl] and then I took the -log of this value and divided by the initial [HCL] (because it is property of log functions) and then the [HCl] would cancel and I was left with just -log(0.12). \Delta pH = -log(...
- Sat Dec 12, 2020 12:26 pm
- Forum: Identifying Acidic & Basic Salts
- Topic: Sapling HW 10 Question 11
- Replies: 6
- Views: 490
Re: Sapling HW 10 Question 11
I also just wanted to add that since Cl is the most electronegative (out of Cl, Br, and I) it would stabilize the anion the most. The more stable the anion (conjugate base) is the stronger the acid is.
- Sun Dec 06, 2020 5:37 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: polydentate ligands
- Replies: 3
- Views: 173
polydentate ligands
How do we determine if a ligand is a polydentate? Does it correlate to the number of lone pairs - like would a ligand with 2 lone pairs be bidentate? Or do we just memorize which ligands are polydentate?
Re: Potassium
In cases of naming, it can be both. It just depends on if it's within the coordination sphere/ in the brackets or not. Usually, the ligand would have like a di-, tri-, tetra- prefix to indicate the number of ligands. But for example, K3[CoF6] is potassium-hexa-fluoro-cobaltate(III). The three potas...
- Sat Dec 05, 2020 5:54 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Monodentate vs. Polydentate
- Replies: 3
- Views: 355
Re: Monodentate vs. Polydentate
We would be able to find out whether or not a ligand is monodentate or polydentate when finding the coordination number by looking at their lewis structure. For example, NH3, ammonia, is monodentate because when you draw out its lewis structure, nitrogen has one pair of lone pair electrons that it ...
- Sat Dec 05, 2020 5:43 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: ligand names
- Replies: 10
- Views: 599
Re: ligand names
I think it is a good idea to memorize the ligand names (since I don't think that we will be given a chart). But I think that just doing a bunch of practice problems will make it easier to memorize!
- Thu Dec 03, 2020 4:45 pm
- Forum: Naming
- Topic: Naming metals with "-ate"
- Replies: 4
- Views: 329
Naming metals with "-ate"
I know that when the coordination compound is negatively charged we put -ate at the end of the metal. But some of the metals change like iron becomes ferrate and copper becomes cuprate, are there any other metals that change when -ate is added to them?
- Sat Nov 28, 2020 8:41 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: pi and sigma bonds
- Replies: 17
- Views: 2082
Re: pi and sigma bonds
A single bond will have 1 sigma bond. A double bond will have 1 sigma bond and 1 pi bond and a triple bond will have 1 sigma bond and 2 pi bonds. So knowing this information you just need to draw out the structure.
- Sat Nov 28, 2020 4:09 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Lone Pair VSEPR
- Replies: 5
- Views: 246
Re: Lone Pair VSEPR
Hi! So the lone pairs actually repel the bonding regions. There is more repulsion between a lone pair and a bonding region, rather than a bonding region with a bonding region. Therefore, the lone pair repels the bonding region, and it goes closer to the other bonding region. This is what makes the ...
- Sat Nov 28, 2020 4:05 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Preferential Interactions
- Replies: 4
- Views: 266
Re: Preferential Interactions
According to the whack notes I took, preferential interactions are caused when one side of the molecule is more likely to interact with a cation or anion because that side is more or less charged than the other. Non-polar molecules don't have preferential interactions, since in general they are sym...
- Sat Nov 28, 2020 3:53 pm
- Forum: Hybridization
- Topic: Hybridization in bonding groups
- Replies: 5
- Views: 345
Re: Hybridization in bonding groups
Yes, for hybridization we look at the number of regions of electron density around an atom, and double/triple bonds count as only one region of electron density.
- Sat Nov 28, 2020 3:49 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Sapling #17 Weeks 7 & 8
- Replies: 3
- Views: 179
Sapling #17 Weeks 7 & 8
Hi! For sapling #17, it asks us to draw three possible structures for C 3 H 4 . Originally, I drew three structures with a Carbon triangle (I included a photo), but sapling said it was wrong. It fixed the structures, but I am still confused as to why there would not be resonance with the Carbon tria...
- Sat Nov 21, 2020 8:26 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSEPR and isoelectronic species
- Replies: 3
- Views: 294
Re: VSEPR and isoelectronic species
I think that the way an isoelectronic species can affect VSEPR is by changing the number of regions of electron density for a particular atom. I don't think that every isoelectronic species will necessarily have the same VSEPR though.
- Sat Nov 21, 2020 8:19 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSEPR of a Radical
- Replies: 5
- Views: 333
VSEPR of a Radical
I was just wondering if unpaired electron on a radical would be considered its own electron density? When writing the AXE formula would we put a subscript of 1/2 on the E since it isn't a pair?
- Sat Nov 21, 2020 8:13 pm
- Forum: Sigma & Pi Bonds
- Topic: Sigma and Pi Bond Strength
- Replies: 4
- Views: 256
Re: Sigma and Pi Bond Strength
To my understanding, pi bonds can't rotate or they will break. Sigma bonds, however, are able to rotate without breaking, so that may be another reason/example of sigma bonds being stronger.
- Sat Nov 21, 2020 8:07 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Trends in Pi vs Sigma Bonds?
- Replies: 3
- Views: 165
Re: Trends in Pi vs Sigma Bonds?
I'm not completely sure, but I don't think that there is a specific trend for sigma and pi bonds. I think it just depends on whether there is a single, double, or triple bond.
- Wed Nov 18, 2020 3:30 pm
- Forum: Octet Exceptions
- Topic: Textbook 2C.5
- Replies: 3
- Views: 218
Textbook 2C.5
I was looking over the textbook problems and noticed that for #2D.5 part a, the book drew the Lewis structure with the unpaired electron on the chlorine atom. Does it matter which atom holds the unpaired electron? I would have thought that it would be more stable if the unpaired electron were with t...
- Sun Nov 15, 2020 10:54 pm
- Forum: Bond Lengths & Energies
- Topic: calculating bond lengths
- Replies: 7
- Views: 353
Re: calculating bond lengths
I'm not completely sure but I think that if we need the bond length it would most likely be given to us - similar to sapling where it told us the bond lengths between atoms.
- Sun Nov 15, 2020 10:38 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Hydrogen Bonding Confusion
- Replies: 3
- Views: 331
Hydrogen Bonding Confusion
Hi! I was reading the textbook and it defined hydrogen bonds are being a link between a H and F,O or N on different molecules and in different regions of the same molecule. If a hydrogen bond is an inter molecular force, how is it allowed to bond within the same molecule? Wouldn't that make it an in...
- Sun Nov 15, 2020 10:33 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Sapling # 20
- Replies: 6
- Views: 707
Re: Sapling # 20
London dispersion forces are present in all interactions and since the molecule is polar it would also have dipole-dipole interactions as well.
- Sun Nov 15, 2020 10:19 pm
- Forum: Resonance Structures
- Topic: Sapling Question
- Replies: 2
- Views: 104
Re: Sapling Question
Hi! So for this problem, you add up the bond lengths of each resonance structure (based on the lengths they give you) and divide by the amount of bonds, which is 4 for all of them. The answer is which structure's average bond length is closest to 144. Hi! I was a bit confused as to why we divide by...
- Thu Nov 12, 2020 9:47 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Sapling #12 Week 5&6 - H-bond
- Replies: 1
- Views: 67
Sapling #12 Week 5&6 - H-bond
For number 12 on the Sapling week 5/6 homework it asks to find which choice has a hydrogen bond. I know that H 3 N .... H-O-H has a hydrogen bond, but why does H 2 O ... H-CH 3 not have a hydrogen bond? I thought that H-bonds are an interaction between molecules and with H bonded with F,O, or N, so ...
- Sun Nov 08, 2020 2:28 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Formal Charge and Lewis Structures
- Replies: 10
- Views: 546
Re: Formal Charge and Lewis Structures
I think we are supposed to check the formal charge for each Lewis structure, since there may be another way to arrange the atoms to make the structure more stable.
- Sun Nov 08, 2020 12:27 am
- Forum: Lewis Structures
- Topic: Covalent Model Clarification
- Replies: 2
- Views: 202
Re: Covalent Model Clarification
I think the partial positive and partial negative are for covalent bonds only, since the atoms are sharing the electron. Because in ionic bonds the electron is gained/lost, I don't think that partial pos/neg applies.
- Sun Nov 08, 2020 12:14 am
- Forum: Resonance Structures
- Topic: Overall Structure of Ion - Sapling #4, W5
- Replies: 3
- Views: 297
Re: Overall Structure of Ion - Sapling #4, W5
Since there may be multiple structures for different formal charges, the structure with the least charge would contribute the most. At one of the UA sessions, he told us that usually the correct/most contributing structure has a central atom with a zero charge and few atoms have a charge. So to find...
- Fri Nov 06, 2020 7:26 pm
- Forum: Ionic & Covalent Bonds
- Topic: Atomic Radius
- Replies: 38
- Views: 3220
Re: Atomic Radius
Atomic radius decreases as you go across a period because the protons increase and shielding remains constant. Even though you are adding electrons, since the shielding remains constant, the effective nuclear charge is able to have a stronger pull on the electrons, thus making the atom smaller.
- Thu Nov 05, 2020 1:25 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Formal Charge & Lewis Structures
- Replies: 5
- Views: 228
Formal Charge & Lewis Structures
We use formal charge to find the most stable lewis structure, but do we have to include all possible variations of the lewis structure (like we would with resonance)? Or can we just use the most stable structure?
- Sun Nov 01, 2020 1:42 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Simplifying Electron Configurations
- Replies: 9
- Views: 985
Re: Simplifying Electron Configurations
I think it means that to simplify/use a shorthand version of the electron configuration, we can use the previous noble gas since it represents the core electrons. You would then continue adding to the electron configuration with the valence electrons.
- Sun Nov 01, 2020 1:31 pm
- Forum: Trends in The Periodic Table
- Topic: Increasing effective nuclear charge across a period
- Replies: 3
- Views: 193
Re: Increasing effective nuclear charge across a period
If I understand correctly, effective nuclear charge increases across a period because the shielding remains the same across a period. Even though the number of electrons increases across a period, the shielding stays the same because it's the core electrons that shield the valence electrons. So sinc...
- Sat Oct 31, 2020 1:48 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Angular Momentum when L=0
- Replies: 2
- Views: 104
Re: Angular Momentum when L=0
The angular momentum (l) describes the shape of the orbital. So an l = 0 would have a spherical shape (s-orbital). I don't think that the electron stops circulating around the nucleus.
- Sat Oct 31, 2020 1:35 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Atomic and Ionic Radius
- Replies: 6
- Views: 212
Re: Atomic and Ionic Radius
Both atomic radii and ionic radii increase going down a group and decrease going across a period. But for ionic radii, if you are given a list of ions from the same element, remember that the cation < original < anion.
- Fri Oct 30, 2020 9:32 pm
- Forum: Trends in The Periodic Table
- Topic: Sapling Hw 2 #20
- Replies: 3
- Views: 251
Re: Sapling Hw 2 #20
@Neel Bonthala 3E Not sure if you get notifications for this, but thanks for a great explanation! What does it mean by the word "relieved"? Does it mean that since its ionized, the oxygen now experiences electron-electron repulsion? Ionization is the energy required to remove an electron....
- Sun Oct 25, 2020 3:34 pm
- Forum: *Shrodinger Equation
- Topic: Shrodinger Equation Confusion
- Replies: 3
- Views: 284
Shrodinger Equation Confusion
I know that the Shrodinger equation on the equation sheet is V=R (1/n(final) - 1/n(initial)). But when I use this way I get a negative number, but we don't include the negative in our answer? Why is the negative not included? If the answer is positive can I switch the n(final) and n(initial) to get ...
- Sun Oct 25, 2020 3:08 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Orbitals
- Replies: 6
- Views: 342
Re: Orbitals
I believe that he was demonstrating that the upper bound of l is n-1. It can take on any possible values from 0 to n-1, with n-1 being the upper bound value. I don't think that if n=2 l could equal 1, but it could equal 0 or 1. This is a chart that I used to better understand it. When n=3, l can ha...
- Sun Oct 25, 2020 11:50 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Orbitals
- Replies: 9
- Views: 339
Re: Orbitals
I still do not understand how the energy level at 5, n = 5 and l = 0. I am still going off go how l is based off of n values like "n-1". I can't seem to find the connection. l (angular momentum) equals values based off of n-1. So if you were just given n=5, then l can equal 0,1,2,3,4. But...
- Sun Oct 25, 2020 11:37 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Electrons in Orbitals
- Replies: 5
- Views: 202
Re: Electrons in Orbitals
According to Hund's Rule, electrons are added to degenerate (same energy) orbitals with a parallel spin (so either all facing up or all facing down) before pairing up. I think that this is a result of electron electron repulsion.
- Sun Oct 25, 2020 11:23 am
- Forum: Quantum Numbers and The H-Atom
- Topic: Lecture confusion in quantum numbers
- Replies: 6
- Views: 246
Re: Lecture confusion in quantum numbers
ml is the magnetic quantum number and it can be any value between -l and l. In the lecture example l = 1, which means that the ml can equal -1,0,1. From my understanding, the px goes with -1, py with 0, and pz with 1.
- Sun Oct 18, 2020 3:14 pm
- Forum: Properties of Light
- Topic: Energy from shorter wavelength
- Replies: 5
- Views: 268
Re: Energy from shorter wavelength
To my understanding as well, if you are looking at the wavelength and frequency it will relate to the energy of a photon. For the kinetic energy you would need to look at the velocity and mass of the electron.
- Sun Oct 18, 2020 2:55 pm
- Forum: Properties of Light
- Topic: Word Problems
- Replies: 7
- Views: 242
Re: Word Problems
In word problems it might explicitly state "frequency," "wavelength," "energy" but if not look at the units. Frequency units are Hz, 1/s, s^-1; wavelength units are in some form of meter (pm, nm, etc.); energy is in joules.
- Sun Oct 18, 2020 11:30 am
- Forum: Properties of Light
- Topic: Heisenberg Uncertainty
- Replies: 4
- Views: 171
Re: Heisenberg Uncertainty
From my understanding, the Heinsenberg uncertainty principle is that you cannot know both the position and momentum of a particle at the same time. So, the more you know about the position the less you know about the momentum, and vice-versa. This uncertainty does not really apply to larger objects,...
- Sun Oct 18, 2020 11:22 am
- Forum: Properties of Electrons
- Topic: Wave properties of elecrons
- Replies: 5
- Views: 429
Re: Wave properties of elecrons
Hi! When two waves are in the same path, energy is always conserved and the end results will depend on the waves. For constructive waves, two waves will combine energies together usually because they are in phase (their crests line up), ultimately increasing the amplitude of the wave. The opposite ...
- Wed Oct 14, 2020 10:56 pm
- Forum: Photoelectric Effect
- Topic: 10/14 Lecture Threshold Energy
- Replies: 8
- Views: 339
Re: 10/14 Lecture Threshold Energy
The threshold energy/energy required to remove an electron will either be given in the problem or we will have to solve for it - so it's not a constant. You can use the KE = E (photon) - threshold energy --> then set the kinetic energy equal to zero so that solving for the threshold energy is just: ...
- Thu Oct 08, 2020 10:06 pm
- Forum: Limiting Reactant Calculations
- Topic: Percentage Yield with Limiting Reactant Calc
- Replies: 4
- Views: 273
Re: Percentage Yield with Limiting Reactant Calc
For part c, to find the grams of CO2 that should be obtained you can use the percent yield formula: actual yield/theoretical yield x 100% = percent yield. Since you know that the percent yield is 75% you can change that to a decimal (to remove the x 100%). Find the theoretical yield by using the lim...
- Thu Oct 08, 2020 9:57 pm
- Forum: Empirical & Molecular Formulas
- Topic: Fundamentals M. 19
- Replies: 6
- Views: 203
Re: Fundamentals M. 19
Not completely relevant to this problem, but a way I learned in the past to remember the 7 diatomic elements is the phrase "high on ficklebur", which corresponds to the elements H, I, O, N, F, Cl, Br (if you read it out in that order, it kind of sounds like it)! I have also heard of BrINC...
- Wed Oct 07, 2020 11:12 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: Sapling Week 1 HW_problem #9
- Replies: 9
- Views: 614
Re: Sapling Week 1 HW_problem #9
To find the molecular formula you want to use this equation: molecular formula mass/empirical formula mass. This fraction will equal a whole number that the empirical formula will be multiplied by. Since the problem states that the molecular formula mass is 110 +/- 10 just find the empirical formula...
- Wed Oct 07, 2020 10:59 pm
- Forum: SI Units, Unit Conversions
- Topic: Formula Units
- Replies: 9
- Views: 376
Re: Formula Units
Mary Shih 3J wrote:Do you calculate formula units the same way we calculate number of atoms in a molecule from molar mass and avogadros number?
To calculate the formula units you can use Avogadros number: 1 mole = 6.022 x 10^23 formula units.
Hope this helps!
- Wed Oct 07, 2020 10:27 pm
- Forum: SI Units, Unit Conversions
- Topic: Sapling Week 1 HW_problem #10
- Replies: 8
- Views: 411
Re: Sapling Week 1 HW_problem #10
For the theoretical yield I got 0.32 g. So when finding the percent yield 0.22g/0.32g x 100% I got 68.7%. Since there is only supposed to be 2 sig figs the percent yield would round to 69%. I would just double check the calculations to find the theoretical yield or sig figs.

