Search found 123 matches
- Fri Mar 12, 2021 9:31 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: When to add Enaught of cell
- Replies: 2
- Views: 323
Re: When to add Enaught of cell
E naught is a state function? That's why it's capitalized? The only things we've learned that aren't state functions are heat (q) and work (w)? I'm not sure what you mean exactly. Edit: I see what you mean. I did a little research and apparently E naught isn't a state function. Idk when you'd add an...
- Fri Mar 12, 2021 9:28 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Help with UA Thermochem Problem
- Replies: 1
- Views: 226
Re: Help with UA Thermochem Problem
I might be wrong, but don't you just take the specific heats and multiply them by the moles to cancel the per mole unit and by the temperature in kelvin to cancel the per kelvin unit to get delta H in terms of J and then use the Hess type approach (products-reactants) to find the overall delta H rea...
- Fri Mar 12, 2021 9:23 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Sapling 17
- Replies: 7
- Views: 676
Re: Sapling 17
You really gotta think of this while looking at a reaction profile (energy vs time as the reaction progresses). Ea is the energy barrier so the height of the bump to the energy of the reactants. delta H is the difference in energy between the reactants and products. If you flip the reaction profile/...
- Fri Mar 12, 2021 12:33 pm
- Forum: Balancing Redox Reactions
- Topic: Textbook 6K.5a
- Replies: 6
- Views: 490
Re: Textbook 6K.5a
I believe O3 --> O2 and then Br- --> BrO3- + 6e-? I didn't check so my numbers may be off but that's who you should be dividing it. The oxygens have an oxidation state of 0. The Br- gets oxidized into BrO3.
- Fri Mar 12, 2021 10:12 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Textbook Question 6L.7
- Replies: 2
- Views: 165
Re: Textbook Question 6L.7
If you look at the anode reaction, Cd (s) + 2OH- (aq) --> Cd(OH) 2 (s) + 2e-, there is OH- in the solution with the anode, Cd(s). OH- isn't stable by itself (in solid form) so in order to make that solution, you'd need to have it attached to something. At the same time, you don't want the cation to ...
- Fri Mar 12, 2021 10:08 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Book 6L.7
- Replies: 2
- Views: 225
Re: Book 6L.7
Cell reactions are kind of like the overall reaction while the half-reaction kind of tells you what's happening and what's reducing/oxidizing. It's kind of similar to elementary steps/reactions vs overall reactions in kinetics. If you look at part a, the Ag in the AgBr is being oxidized and becomes ...
- Fri Mar 12, 2021 9:52 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Textbook 6L 7
- Replies: 2
- Views: 225
Re: Textbook 6L 7
If you look at the anode reaction, Cd (s) + 2OH- (aq) --> Cd(OH) 2 (s) + 2e-, there is OH- in the solution with the anode, Cd(s). OH- isn't stable by itself (in solid form) so in order to make that solution, you'd need to have it attached to something. At the same time, you don't want the cation to ...
- Thu Mar 11, 2021 11:33 pm
- Forum: Balancing Redox Reactions
- Topic: 6K.5 Part D
- Replies: 3
- Views: 230
Re: 6K.5 Part D
If you're referring to P4 --> H2PO2- + PH3 as the half reaction, it's not? That's the skeletal and you're supposed to break it up into two half reactions:
P4 --> H2PO2- + e-
P4 +3e- --> PH3
And then balance it like a regular redox.
P4 --> H2PO2- + e-
P4 +3e- --> PH3
And then balance it like a regular redox.
- Sun Mar 07, 2021 12:32 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Final on 3/13
- Replies: 18
- Views: 997
Re: Final on 3/13
Reminder that it's the start of Daylight Savings in CA on that day! Please don't be late!
- Sun Mar 07, 2021 12:29 pm
- Forum: Balancing Redox Reactions
- Topic: What is the tip to balance basic and acidic redox reactions with the x and H/OH and H20?
- Replies: 2
- Views: 264
Re: What is the tip to balance basic and acidic redox reactions with the x and H/OH and H20?
I personally found Chemistry Libre Texts very helpful!
https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Supplemental_Modules_(Analytical_Chemistry)/Electrochemistry/Redox_Chemistry/Balancing_Redox_reactions
https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Supplemental_Modules_(Analytical_Chemistry)/Electrochemistry/Redox_Chemistry/Balancing_Redox_reactions
- Sun Mar 07, 2021 12:27 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: what does E notch tell us compared to the E of a cell?
- Replies: 8
- Views: 379
Re: what does E notch tell us compared to the E of a cell?
If you're referring to E naught (like E with the degree symbol), it's basically the E of the cell under standard conditions (298.15 K and 1 atm). E of the cell (without the degree symbol) means the E of the cell not under standard conditions.
- Sun Mar 07, 2021 12:24 pm
- Forum: Student Social/Study Group
- Topic: How do you deal with burnout?
- Replies: 144
- Views: 20093
Re: How do you deal with burnout?
I try to have a set time every day to myself (no homework or studying during this time) to destress or relax. Like I set aside at least two hours a day for myself that is strictly reserved for doing fun stuff and stuff that I wanna do. You can do selfcare during this time or watch a quick episode or...
- Sun Mar 07, 2021 12:18 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: when can we not use -0.0592/n when finding the E of a cell?
- Replies: 10
- Views: 875
Re: when can we not use -0.0592/n when finding the E of a cell?
I believe when the reaction is not under standard conditions. Bc the -0.0592 is calculated by plugging in standard temp and stuff into the equation.
- Sat Feb 27, 2021 1:06 pm
- Forum: Balancing Redox Reactions
- Topic: Sapling HW Week 8 Q4
- Replies: 5
- Views: 373
Re: Sapling HW Week 8 Q4
That helped a lot! Thank you Zoe and Sophia!
- Thu Feb 25, 2021 11:35 pm
- Forum: Balancing Redox Reactions
- Topic: Balancing Redox: Coefficients
- Replies: 2
- Views: 193
Balancing Redox: Coefficients
In the beginning, one of the first things you're supposed to do is balance out the elements on either side of the half reaction EXCLUDING oxygen and hydrogen right? And then you don't really mess with the coefficients afterwards (like after adding H2O, you don't go back and multiply the whole reacti...
- Thu Feb 25, 2021 11:32 pm
- Forum: Balancing Redox Reactions
- Topic: Balancing Redox: Leftover H+ or OH-
- Replies: 4
- Views: 281
Balancing Redox: Leftover H+ or OH-
At the end of balancing (when you add the two half equations together), are you allowed to have leftover H+ on either side? Are you allowed to have leftover OH- on either side? Does this depend on if the reaction occurs in acidic or basic solution?
- Thu Feb 25, 2021 11:32 pm
- Forum: Balancing Redox Reactions
- Topic: Balancing Redox Method
- Replies: 2
- Views: 212
Balancing Redox Method
So I looked up how to balance redox reactions and was curious about a few things: 1. Can you add H+ to any side as long as there is another H on the other side? Does that H have to also be an H+ or can it be in a compound? 2. Similarly, can you add e- to any side as long as the charges are balanced?
- Thu Feb 25, 2021 11:27 pm
- Forum: Balancing Redox Reactions
- Topic: Sapling HW Week 8 Q4
- Replies: 5
- Views: 373
Sapling HW Week 8 Q4
Gold has always been a highly prized metal, and it has been widely used from the beginning of history as a store of value. It does not rust like iron and does not become tarnished like silver. It is so chemically inert that it will not react with even the strongest concentrated acids. But it can be...
- Wed Feb 17, 2021 12:05 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Textbook 4I1
- Replies: 2
- Views: 164
Re: Textbook 4I1
Thank you!!!
- Wed Feb 17, 2021 11:50 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Textbook 4I1
- Replies: 2
- Views: 164
Textbook 4I1
4I.1 What is the total entropy change accompanying a process in which 40.0 kJ of energy is transferred as heat from a large reservoir at 800. K to one at 200. K? text.jpg The solutions manual uses q/t which makes sense but can someone explain why one of the calculations is negative and the other is...
- Mon Feb 15, 2021 11:48 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Calculating Change in Enthalpy for Constant Volume
- Replies: 4
- Views: 2979
Re: Calculating Change in Enthalpy for Constant Volume
Hi, yes I got that part. I was asking how to find the change in enthalpy for constant volume.
- Mon Feb 15, 2021 10:17 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Calculating Change in Enthalpy for Constant Volume
- Replies: 4
- Views: 2979
Calculating Change in Enthalpy for Constant Volume
For Textbook Problem 4C3 Part B: 4C.3 Calculate the final temperature and the change in enthalpy when 765 J of energy is transferred as heat to 0.820mol Kr(g) at 298 K and 1.00 atm (a) at constant pressure (b) at constant volume. Treat the gas as ideal. For constant pressure we know that q p = delta...
- Mon Feb 15, 2021 9:59 am
- Forum: Calculating Work of Expansion
- Topic: Constant Pressure and Constant Volume Constants for Ideal Gases
- Replies: 3
- Views: 214
Constant Pressure and Constant Volume Constants for Ideal Gases
Does anyone know what the constants for calculating heat of an ideal gas at both constant volume and at constant pressure are (Cv and Cp)?
- Sun Feb 14, 2021 12:23 pm
- Forum: Calculating Work of Expansion
- Topic: Difference between reversible and irreversible work of expansion
- Replies: 10
- Views: 492
Re: Difference between reversible and irreversible work of expansion
To add to everything that's been said, reversible work of expansion is theoretical (correct me if I'm wrong) because it is based off of the idea that all energy can be turned into heat which is not possible since some energy/heat will be lost to the surroundings.
- Sun Feb 14, 2021 12:19 pm
- Forum: Student Social/Study Group
- Topic: 14BL next quarter
- Replies: 7
- Views: 535
Re: 14BL next quarter
I think the only professor teaching it next quarter is Dr. Casey... she's alright. I'll let those who also have her elaborate.
- Sun Feb 14, 2021 12:15 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Entropy Equations
- Replies: 3
- Views: 126
Re: Entropy Equations
Hi, if you're in the groupme for the chem 14B class, there is a link in the bio with an equations list (i'm not going to post the link though bc the forum is viewable to the public). you could also use the equations worksheet on Dr. Lavelle's site.
- Wed Feb 10, 2021 12:32 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: 4A13 Textbook Problem
- Replies: 2
- Views: 172
4A13 Textbook Problem
A constant-volume calorimeter was calibrated by carrying out a reaction known to release 3.50 kJ of heat in 0.200 L of solution in the calorimeter (q=−3.50kJ), resulting in a temperature rise of 7.32C. In a subsequent experiment, 100.0 mL of 0.200 M HBr(aq) and 100.0 mL of 0.200M KOH(aq) were mixed...
- Sat Feb 06, 2021 4:02 pm
- Forum: Calculating Work of Expansion
- Topic: Is reversible expansion just theoretical?
- Replies: 11
- Views: 450
Re: Is reversible expansion just theoretical?
Yes, just theory but we will still be expected to know it (see lecture from Friday).
- Sat Feb 06, 2021 3:56 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Sampling hw #4
- Replies: 7
- Views: 453
Re: Sampling hw #4
It's not a rule that single reactant decomposing into products is endo or exothermic. You need to look at the bonds being broken (in the reactants) and the bonds that are being formed (in the products) to see if it's exo or endothermic (which is why they gave you the table). When the enthalpy in pro...
- Sat Feb 06, 2021 3:51 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: enthalpy and heat
- Replies: 10
- Views: 458
Re: enthalpy and heat
Q refers to heat while H refers to enthalpy. They (for the most part) are not interchangeable.
- Sat Feb 06, 2021 3:50 pm
- Forum: Student Social/Study Group
- Topic: zoom study group
- Replies: 1
- Views: 190
- Tue Feb 02, 2021 11:26 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Week 3/4 Sapling #18
- Replies: 2
- Views: 176
Week 3/4 Sapling #18
The question states A 0.201 mol sample of N 2 (g), initially at 298 K and 1.00 atm, is held at constant pressure while enough heat is applied to raise the temperature of the gas by 15.1 K. Calculate the amount of heat q required to bring about this temperature change, and find the corresponding tota...
- Sun Jan 31, 2021 12:14 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: isolated system examples
- Replies: 4
- Views: 159
Re: isolated system examples
An insulated thermos was an example given in lecture (i think). I would say stick to open/closed systems because Dr. Lavelle mentioned that nothing really happens in isolated systems since we are assuming no matter or energy is being exchanged. If nothing is being exchanged, we can't really do any c...
- Sun Jan 31, 2021 12:12 pm
- Forum: Ideal Gases
- Topic: Reversing Reactions
- Replies: 68
- Views: 2687
Re: Reversing Reactions
When you reverse a reaction, K is flipped. Ie. Kreverse = 1/Kforward
- Sun Jan 31, 2021 12:07 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Increasing volume and effect on K
- Replies: 4
- Views: 148
Re: Increasing volume and effect on K
Increase in pressure/volume has no effect on K. The only thing that affects K is temperature. That is the reason why the reaction shifts to the right or left when there is a change in pressure. When there is increased volume/decreased pressure, the reaction wants to go back to equilibrium and thus i...
- Sun Jan 31, 2021 10:18 am
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Isothermal, Reversible, and Irreversible Processes
- Replies: 2
- Views: 173
Re: Isothermal, Reversible, and Irreversible Processes
So from the lecture: A reversible process happens when the pressure of a system is similar to the pressure of its surroundings. The example given is the piston and how the two pressures were 2 atm/bar and something very close to two atm/bar. In a sense, the system and surroundings were in equilibriu...
- Sun Jan 31, 2021 10:02 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Entropy vs Enthalpy
- Replies: 11
- Views: 589
Re: Entropy vs Enthalpy
We haven't learned this yet, but from what I remember in high school, enthalpy is a measure of heat while entropy is a measure of "chaos"/randomness/disorder. This probably didn't help you very much,,, but we should learn entropy soon.
- Sat Jan 23, 2021 12:00 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Midterm 1 Allowed Materials and Procedure
- Replies: 6
- Views: 360
Midterm 1 Allowed Materials and Procedure
Hi, I was wondering what materials we are allowed to bring in for the midterm (ie. calculator, periodic table, and constants and equations sheet) and how the midterm will work. Will the process be similar to last quarter where we use respondus and have a separate web cam for zoom? Or will we be atte...
- Sat Jan 23, 2021 11:56 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Sapling Week 2 #7
- Replies: 3
- Views: 263
Re: Sapling Week 2 #7
Hi, the only thing that seems off here is your Ka. I believe you calculated it wrong (Kw = Ka x Kb).
- Sat Jan 23, 2021 11:53 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Sapling Week 2 #8
- Replies: 3
- Views: 241
Re: Sapling Week 2 #8
The only thing that seems odd to me right now is your Ka value. Did you perhaps miss something? Because Kw = Ka x Kb and I don't think that Ka is correct? Because you didn't need to use the quadratic equation.
- Sat Jan 23, 2021 10:35 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Sapling Week 2 Hw 5
- Replies: 6
- Views: 364
Re: Sapling Week 2 Hw 5
I'm not sure about the first part of the question but I can take a stab at the second part. Formal concentration is the same as initial concentration but different from concentration at equilibrium. The [B] calculated using the [OH-] and the K b expression is the concentration of B at equilibrium. S...
- Sat Jan 23, 2021 10:29 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: sapling question 5 week 2
- Replies: 2
- Views: 106
Re: sapling question 5 week 2
To add to what was said above, this problem is an example of working backwards (which is interesting since we don't get to do those a lot). I think that to find the percent protonation, the equation was [BH+]/[B formal ] where [B formal ] is [BH+] + [B at equilibrium ]. The [B] calculated using the ...
- Sat Jan 23, 2021 10:19 am
- Forum: Administrative Questions and Class Announcements
- Topic: Midterm 1 Outline 3
- Replies: 3
- Views: 191
Re: Midterm 1 Outline 3
Dr. Lavelle should have sent out an email detailing what will be covered. I think he gives us the corresponding problems to which topics he's stopping on.
- Sat Jan 16, 2021 12:24 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Sapling Week 2 question 10
- Replies: 2
- Views: 251
Re: Sapling Week 2 question 10
So you would basically solve an ICE table for the initial values: [N2O4] = 0.429 and [NO2] = 3.19 and solve for x. Here you are trying to find the final concentration after equilibrium has been reestablished. When equilibrium is reestablished, you will have the same ratio of reactants to products bu...
- Sat Jan 16, 2021 12:13 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6D 17: pH of salts
- Replies: 1
- Views: 130
Re: 6D 17: pH of salts
The reason why Na+ stays an ion in water is because it's stable as is without having to react with water. CH3CO2- would be a proton acceptor and generate OH- ions because it's a weak base and reacts with water. If it were a salt of/ a strong acid or base, it would stay dissociated. Since it is a wea...
- Sat Jan 16, 2021 11:59 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Homework Problem #3 7
- Replies: 3
- Views: 215
Re: Homework Problem #3 7
Using [N2]=[O2]=0.100 M and [NO]=0.600 M, calculate the Ka value. This value will allow you to calculate the values at equilibrium should the reaction be disturbed. Then solve an ICE table with the values [N2]=[O2]=0.100 M and [NO]=0.900 M for the initial concentration. Reminder: solving for x doesn...
- Sat Jan 16, 2021 11:51 am
- Forum: Administrative Questions and Class Announcements
- Topic: Acids and Bases Equilibrium Questions
- Replies: 3
- Views: 233
Re: Acids and Bases Equilibrium Questions
You could, since I think acid/base equilibrium is part of Chem 14A.
- Fri Jan 15, 2021 9:10 am
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: 6D.5 part d
- Replies: 3
- Views: 677
Re: 6D.5 part d
I don't know if this helps but: Since you are give the pKa of the conjugate acid of codeine (pKa = 8.21), you can calculate the pKb through the equation pKw = pKa + pKb. The pKa of the conjugate acid also tells you that codeine is a pretty strong base (please correct me if I'm wrong), since the pKa>...
- Sat Jan 09, 2021 10:46 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Question doesn't match answer key
- Replies: 4
- Views: 290
Re: Question doesn't match answer key
Yea, for some reason I got the same equation as Garmani.
- Sat Jan 09, 2021 10:38 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Textbook 5H.3
- Replies: 3
- Views: 114
Re: Textbook 5H.3
You would have to calculate the K value from two equations: H2 (g) + Cl2 (g) <--> 2HCl (g) and 2BrCl (g)<--> Br2 (g) + Cl2 (g).
I didn't include exact values in case you wanted to try it out, but the answer is in the solutions manual.
I didn't include exact values in case you wanted to try it out, but the answer is in the solutions manual.
- Sat Jan 09, 2021 10:17 am
- Forum: Ideal Gases
- Topic: R constant
- Replies: 4
- Views: 245
Re: R constant
As the previous answer stated, I think it really depends on the units. If you look at the constants and equations (there a new one for Chem 14B located on the class website), the R constant has units so just make sure that they cancel when you plug everything in.
- Sat Jan 09, 2021 10:12 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Le Chatelier's Principle
- Replies: 4
- Views: 204
Re: Le Chatelier's Principle
It's definitely possible, but it depends on the question being asked. I think here it's just a theoretical question as to which would increase or decrease, but in the real world, there will be factors that limit/affect the reaction.
- Sat Jan 09, 2021 10:10 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Inert Gas Pressure Change
- Replies: 3
- Views: 132
Re: Inert Gas Pressure Change
The volume doesn't decrease, from what I know, because 1) you aren't really changing the container even if pressure does increase and 2) inert gases don't react with the reactants. So theoretically, it's like the inert gas isn't there or doesn't make a difference in the space that the reactants of a...
- Sun Dec 13, 2020 9:10 am
- Forum: Identifying Acidic & Basic Salts
- Topic: Question 7 sapling
- Replies: 5
- Views: 288
Re: Question 7 sapling
You distinguish acidic, basic, or neutral salts based on if it carries a acid or base. For example, NH 4 ClO 4 carries an acid (NH 4 + ). When put into water, the salt will dissociate and the NH 4 + will donate a proton to H 2 0 to create H 3 0 + and NH 3 . Because of the H 3 0 + s, the concentrati...
- Sun Dec 13, 2020 9:06 am
- Forum: Identifying Acidic & Basic Salts
- Topic: Question 7 sapling
- Replies: 5
- Views: 288
Re: Question 7 sapling
You distinguish acidic, basic, or neutral salts based on if it carries a acid or base. For example, NH 4 ClO 4 carries an acid (NH 4 + ). When put into water, the salt will dissociate and the NH 4 + will donate a proton to H 2 0 to create H 3 0 + and NH 3 . Because of the H 3 0 + s, the concentratio...
- Sun Dec 13, 2020 8:58 am
- Forum: Amphoteric Compounds
- Topic: How to figure amphoteric compounds
- Replies: 4
- Views: 404
Re: How to figure amphoteric compounds
Typically, I would draw one just to be safe, but theoretically speaking, you don't if you know it well? Sorry, if this doesn't help|||-_-
- Sat Dec 12, 2020 12:47 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Textbook 6C19
- Replies: 4
- Views: 296
Textbook 6C19
Why is HClO 2 a stronger acid than HBrO 2 ? The solution manual said that it was because Cl had a stronger electronegativity, but didn't we say that the strength of an acid was inversely related to bond length? ie. HBr is a stronger acid than HCl because the bonds are longer and weaker. Why is the o...
- Sat Dec 12, 2020 12:30 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Oxyacids
- Replies: 2
- Views: 152
Oxyacids
Do we need to know oxyacids for the exam?
- Fri Dec 11, 2020 1:11 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Textbook 6B3
- Replies: 4
- Views: 406
Textbook 6B3
A careless laboratory technician wants to prepare 200.0 mL of a 0.025 M HCl(aq) solution but uses a volumetric flask of volume 250.0 mL by mistake. (a) What would the pH of the desired solution have been? (b) What will be the actual pH of the solution as prepared? The answer was: The pH of the desi...
- Tue Dec 08, 2020 11:01 pm
- Forum: Lewis Structures
- Topic: N2H4
- Replies: 7
- Views: 593
N2H4
Why is the preferred Lewis Structure for N2H4:
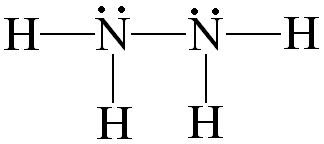
instead of a structure with a triple bond in between the two nitrogens?
instead of a structure with a triple bond in between the two nitrogens?
- Tue Dec 08, 2020 9:39 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: T-shaped
- Replies: 2
- Views: 242
T-shaped
Would T-shape molecular geometry be the same thing as trigonal planar? I mean, it's in the same plane (?) but the bond angles are different?
- Mon Dec 07, 2020 2:42 pm
- Forum: Conjugate Acids & Bases
- Topic: Alkaline
- Replies: 16
- Views: 843
Alkaline
Is the word alkaline synonymous to basic? ie. when you say a solution is more alkaline, it's more basic?
- Sun Dec 06, 2020 8:25 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Sapling Week 9
- Replies: 6
- Views: 496
Re: Sapling Week 9
I believe pentagonal pyramidal is wrong (correct me if I'm wrong) because it's not the most efficient way. Ie, you want your electrons as far away from each other as possible. Putting five electrons together in one plane (pentagonal) doesn't minimize the electron-electron repulsion. Hexagonal planar...
- Sun Dec 06, 2020 8:23 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Linear Molecular Shape and Bond Angle of 180.5
- Replies: 1
- Views: 126
Re: Linear Molecular Shape and Bond Angle of 180.5
In general, bond angles are determined by electron-electron repulsions. Lone pair electrons exert a "larger" repulsion than bound electrons which is probably the reason (?) why you got an unexpected bond angle. I don't think that we will ever be asked to determine exact bond angles(correct...
- Sun Dec 06, 2020 8:11 pm
- Forum: Bronsted Acids & Bases
- Topic: HF
- Replies: 15
- Views: 929
Re: HF
HF is weak(er) when compared to HI etc. bc of fluorine's high electronegativity and thus strong bond to hydrogen. The stronger the bond, the harder it is to break or dissociate when added to water. How strong an acid or base here is actually inversely related to the bond strength and consequently th...
- Sun Dec 06, 2020 8:07 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Types of Ligands
- Replies: 3
- Views: 373
Re: Types of Ligands
To add to the answers above, a ligand is basically any molecule/compound bound to a transition metal. A way to quickly scan for how many bonds a ligand can form would be to look out for electron rich species in a ligand. Someone above mentioned NH 2 CH 2 CH 2 NH 2 and two nitrogens leading to two bo...
- Sun Dec 06, 2020 7:58 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Naming Coordination Compounds
- Replies: 7
- Views: 393
Re: Naming Coordination Compounds
Yes. You would need the individual oxidation numbers to figure out the transition metal's oxidation number for naming. Easy way to find oxidation numbers: For the s block, it's helpful to look at the numbers above each group (ie. group 1 is +1 while group 2 is +2). Similarly, for the p-block, just l...
- Sun Dec 06, 2020 7:50 pm
- Forum: Lewis Acids & Bases
- Topic: Weak/ Strong Acids and Bases
- Replies: 6
- Views: 464
Re: Weak/ Strong Acids and Bases
Yes! Strong acids and bases will most likely show up on the test since Dr. Lavelle went over how to figure out if an acid/base is strong. To add to the previous answers: -the higher the electronegativity, the stronger the bond (the less strong of an acid/base it is) -the smaller the electronegativit...
- Tue Dec 01, 2020 12:09 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Transition Metal
- Replies: 3
- Views: 117
Transition Metal
In the previous lecture and today's lecture, how were we able to figure out that nickel was Ni2+ and cobalt was Co2+?
- Sat Nov 28, 2020 1:11 pm
- Forum: Student Social/Study Group
- Topic: Midterm/Final Success?
- Replies: 17
- Views: 877
Re: Midterm/Final Success?
Everyone's already said the key points, but I also wanted to mention that the difference between a 90% and an 85% is like one question. I know my numbers are off, but please remember to be kind to yourself if you are beating yourself over one question. I'd also like to mention that a lot of exam que...
- Sat Nov 28, 2020 1:02 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Sapling Wk8 Q. 18, Determining Molecular Shape
- Replies: 10
- Views: 542
Re: Sapling Wk8 Q. 18, Determining Molecular Shape
To add, typically hydrocarbons (molecules with only hydrogens and carbons) are linear/chains. So all you really need to do is draw the carbons, put in the hydrogens, and then fill in the double bonds of the carbons where necessary.
- Sat Nov 28, 2020 1:00 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSEPR identification of carbonate ion (CO3(2-))
- Replies: 4
- Views: 1461
Re: VSEPR identification of carbonate ion (CO3(2-))
To add to the answers above: Double bonds are indeed "shorter" than single bonds, but the structure you are looking at right now is a resonance structure so all of the bonds will be equal (ie. less than a single but more than a double bond). So I believe the angles will all be equal ( but ...
- Sat Nov 28, 2020 12:51 pm
- Forum: Student Social/Study Group
- Topic: Final Study
- Replies: 32
- Views: 1758
Re: Final Study
I would suggest redoing the textbook problems that were assigned to us and reading the textbook. Something that I like to do is create flashcards to quiz myself on key concepts. If more people are interested, we could also form a study group to discuss difficult topics and work on problems together...
- Sat Nov 28, 2020 12:48 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Double/Triple Bonds and Polarity
- Replies: 8
- Views: 419
Re: Double/Triple Bonds and Polarity
While double and triple bonds may effect polarity, electronegativity is the main factor that affects polarity. Since Arsenic is surrounded by 4 oxygens, and the oxygens are more electronegative than As, there will be diploe vectors pointing towards those 4 oxygens. However, all of them cancel out m...
- Sat Nov 28, 2020 12:42 pm
- Forum: Sigma & Pi Bonds
- Topic: Sapling HW Q20
- Replies: 2
- Views: 140
Sapling HW Q20
Select the correct statement about the relative positions of the hydrogen atoms in the three structures: a) The hydrogen atoms of H 2 CCH 2 and H 2 CCCCH 2 lie in the same plane. b) The hydrogen atoms of H 2 CCH 2 and H 2 CCCH 2 lie in the same plane. c) The hydrogen atoms of H 2 CCCH 2 and H 2 CCC...
- Thu Nov 19, 2020 12:09 pm
- Forum: Electronegativity
- Topic: Electronegativity versus atomic radius
- Replies: 8
- Views: 1256
Re: Electronegativity versus atomic radius
To add to the post above, the smaller the atom/molecule is, the less polarizable it is which describes the character of the bond.
- Thu Nov 19, 2020 10:36 am
- Forum: Lewis Structures
- Topic: Textbook Q 2.C.5
- Replies: 3
- Views: 155
Re: Textbook Q 2.C.5
Hey David, Yea it's kind of weird because for the textbook problems, it seemed like formal charge wasn't what determined which atom has the unpaired electron. However, from what I've seen it seems like the most electronegative atom will want the full octet, so the less electronegative species will ...
- Thu Nov 19, 2020 10:20 am
- Forum: Lewis Structures
- Topic: Textbook 2B.1
- Replies: 4
- Views: 272
Re: Textbook 2B.1
for fluorine:
Fc double bond= 7-((4/2) + 4)= -1
Fc single bond= 7-((2/2) + 6)=0
for nitrogen:
Fc triple/three bonds = 5 - ((6/2) + 2) = 0
Fc four bonds = 5 - (8/2) = +1
Fc double bond= 7-((4/2) + 4)= -1
Fc single bond= 7-((2/2) + 6)=0
for nitrogen:
Fc triple/three bonds = 5 - ((6/2) + 2) = 0
Fc four bonds = 5 - (8/2) = +1
- Thu Nov 19, 2020 10:11 am
- Forum: Octet Exceptions
- Topic: Expanded Octet and the 3d orbital
- Replies: 3
- Views: 264
Re: Expanded Octet and the 3d orbital
In general, expanded octet is when the central atom has more than 8 valence electrons/can make more than 4 bonds. The reason certain elements can do this is because of the d orbital. The element accepts extra electrons and puts them in the d orbital therefore only elements with n>2 can have an expan...
- Thu Nov 19, 2020 10:06 am
- Forum: Electronegativity
- Topic: Most Electronegative Element
- Replies: 5
- Views: 335
Re: Most Electronegative Element
He is considered an inert gas because it has 2 electrons in the s orbital. Therefore adding electrons to its shell is not favorable while fluorine desperately wants to fill the p-orbital/is about to complete its octet.
- Thu Nov 19, 2020 9:59 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Textbook 2A.9
- Replies: 2
- Views: 94
Textbook 2A.9
Which M+ ions are predicted to have the following ground state e- configurations?
a) [Ar] 3d7
b) [Ar] 3d6
Could someone explain this? I didn't really understand why a) was Co2+ and b) was Fe2+. I thought a) was copper and b) was nickel.
- Tue Nov 17, 2020 7:49 pm
- Forum: Octet Exceptions
- Topic: Textbook 2C7 Iodine
- Replies: 2
- Views: 317
Textbook 2C7 Iodine
Determine the number of electron pairs (both bonding and lone pair) on the iodine atom in: ICl 2 + , ICl 4 - , ICl 3 , and ICl 5 How do you figure out how many electron pairs iodine has? I noticed that sometimes iodine has more than four bonds but also has lone pairs and other times it has only two...
- Sun Nov 15, 2020 8:53 pm
- Forum: Electronegativity
- Topic: Partial vs Formal charge
- Replies: 8
- Views: 1080
Re: Partial vs Formal charge
Found this post in a previous year: A formal charge has a value of either a positive integer, a negative integer, or zero. A partial charge usually has a positive or negative non-integer. Each atom in a molecule has both, but in this course, we're only able to calculate the formal charge. For the pa...
- Sun Nov 15, 2020 8:49 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Polarizability
- Replies: 3
- Views: 210
Re: Polarizability
Polarizability is a measure of how easily an electron cloud is distorted by an electric field. I.e. can I pull all the electrons to one side of the atom to create a slightly positive side and a slightly negative side. Basically, the more tightly held the electrons are, the less polarizable they are....
- Sun Nov 15, 2020 8:42 pm
- Forum: Dipole Moments
- Topic: Hydrogen Bonding
- Replies: 3
- Views: 82
Re: Hydrogen Bonding
Hydrogen bonding acts more like a dipole-dipole bond or electrostatic attraction. Hydrogen bonding isn't actual bonding in the way that covalent bonds are bonds. Hydrogen bonding is just attraction/intermolecular force.
- Sun Nov 15, 2020 8:39 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Probability?
- Replies: 1
- Views: 92
Re: Probability?
Hi, I believe that this refers to the electron density. Like, the atomic orbitals are drawn as spheres or balloon looking structures (the very first lecture on orbitals) to show that there is a higher chance the electron could be found in this area. But I could also be wrong |||-_-
- Sat Nov 14, 2020 10:02 am
- Forum: Photoelectric Effect
- Topic: Microwaves
- Replies: 2
- Views: 354
Microwaves
It's been on my mind for a while but... when you microwave food, are you just exciting the electrons in the food and is the heat the energy that the electrons give off when they return to ground state?
- Wed Nov 11, 2020 1:58 pm
- Forum: Bond Lengths & Energies
- Topic: Energy per mole
- Replies: 2
- Views: 122
Energy per mole
In ion-ion interactions (say, Na+ and Cl-), the energy of the interaction is -250kJ/mol. Dr. Lavelle said that it's 250kJ per mole of INTERACTIONS. Does this mean that for one mole of Na+ and one mole of Cl-, the energy of their interactions is 250kJ?
- Wed Nov 11, 2020 1:54 pm
- Forum: Dipole Moments
- Topic: Hydrogen Bonds
- Replies: 6
- Views: 332
Hydrogen Bonds
Are hydrogen bonds (from hydrogen bonding) an actual bond or is it just an attraction force/electrostatic attraction?
- Sat Nov 07, 2020 11:27 am
- Forum: Octet Exceptions
- Topic: Group 13
- Replies: 3
- Views: 230
Group 13
Are Group 13 elements always electron deficient or does it depend? Also, does electron deficient (the way that Dr. Lavelle uses it) mean that the atom/element can be without an octet?
- Thu Nov 05, 2020 10:33 am
- Forum: Lewis Structures
- Topic: NO3-
- Replies: 4
- Views: 175
NO3-
Why does NO3- have nitrogen as the center atom? I know that center atoms are the ones with lowest ionization energy but doesn't oxygen have a lower ionization energy than nitrogen due to its lone pair and electron-electron repulsion?
- Tue Nov 03, 2020 2:03 pm
- Forum: Lewis Structures
- Topic: Monday Lecture
- Replies: 3
- Views: 284
Monday Lecture
In Monday's lecture, why did Dr. Lavelle say that nitrogen had a lower ionization energy than oxygen? Didn't he say that oxygen had a lower ionization energy due to the one electron pair and electron electron repulsion?
- Tue Nov 03, 2020 2:01 pm
- Forum: Resonance Structures
- Topic: Limitations of Lewis Structures
- Replies: 6
- Views: 949
Limitations of Lewis Structures
Lewis structures have limitations on how well they can represent molecules. Is resonance the name of a limitation or is it a way to overcome the limitation?
- Sat Oct 31, 2020 11:49 am
- Forum: Lewis Structures
- Topic: Octet Rule
- Replies: 12
- Views: 516
Re: Octet Rule
I think there are more elements that are exceptions but Dr. Lavelle has only talked about the first four elements being exceptions to this rule.
- Sat Oct 31, 2020 11:46 am
- Forum: Properties of Electrons
- Topic: Sapling #21: Electron Affinity
- Replies: 2
- Views: 144
Re: Sapling #21: Electron Affinity
Electron affinity is how much energy is produced/absorbed when an atom accepts an electron. This is based on how much an atom "wants" an electron and electronegativity. For example, F is very electronegative and will release energy when it accepts an electron. Ne or Li on the other hand wo...
- Sat Oct 31, 2020 11:39 am
- Forum: Ionic & Covalent Bonds
- Topic: Sampling 28
- Replies: 10
- Views: 309
Re: Sampling 28
I think you'd have to look at the periodic table too. Like n=1 only has the s-orbital, n=2 only has the s and p orbital, and then n=3 has s and p and d orbital, etc. If I remember correctly, l = (n-1), like the highest possible l.
- Sat Oct 31, 2020 11:38 am
- Forum: SI Units, Unit Conversions
- Topic: SI Units & US
- Replies: 4
- Views: 289
Re: SI Units & US
Because the US wanted to be special and declare their sovereignty... and then we never bothered to think how much easier it would be to just go to SI and just switch.
- Sat Oct 31, 2020 11:32 am
- Forum: SI Units, Unit Conversions
- Topic: eV to Joules (Defining these Units) Question
- Replies: 12
- Views: 1588
Re: eV to Joules (Defining these Units) Question
Most relevant units and conversions will be provided on the constants and equations sheet that Dr. Lavelle provided us with.
- Sat Oct 31, 2020 11:24 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Ground State vs. Excited State Question
- Replies: 8
- Views: 1579
Re: Ground State vs. Excited State Question
Ground state is when the electrons are all in the configuration you would expect them to be in (ie. carbon would be 1s 2 2s 2 2p 2 ). Like you could accurately find the electron configuration just by looking at its position. The excited configuration is a bit different since the electron configurati...
- Sat Oct 31, 2020 11:06 am
- Forum: Ionic & Covalent Bonds
- Topic: Salt
- Replies: 4
- Views: 271
Salt
Why is (NH4)sSO4 a salt? I thought salts were a metal and a nonmetal? ie. NaCl is a salt because Na+ is a metal and Cl is a nonmetal.

