Search found 60 matches
- Thu Dec 17, 2020 1:53 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Seesaw vs. trigonal pyramidal
- Replies: 22
- Views: 2417
Re: Seesaw vs. trigonal pyramidal
No, in regards to molecular shape, see-saw and trigonal pyramidal are not the same thing. The main difference between them is that they have different amounts of regions of electron density where a see-saw molecule has 5 while a trigonal pyramidal molecule has 4, which corresponds to a steric number...
- Thu Dec 17, 2020 1:39 am
- Forum: Student Social/Study Group
- Topic: Plans for Relaxing After Finals
- Replies: 98
- Views: 20388
Re: Plans for Relaxing After Finals
I am definitely going to go into hibernation for a little bit to recover all the sleep that I lost during finals week, haha. However, I am genuinely looking forward to relaxing with my family (dogs included) during the Christmas season because it is my favorite holiday of the year. I just hope that ...
- Thu Dec 17, 2020 1:22 am
- Forum: Lewis Acids & Bases
- Topic: Lewis vs Bronsted
- Replies: 20
- Views: 1257
Re: Lewis vs Bronsted
The criteria of Bronsted acids and bases is much more restrictive in terms of classification than that of Lewis acids and bases. Namely, Bronsted acids refer to species which donate protons in solution while Bronsted bases are considered to be species which accept protons. Here, protons are referrin...
- Thu Dec 17, 2020 1:09 am
- Forum: Naming
- Topic: Latin names
- Replies: 10
- Views: 797
Re: Latin names
Like many others and Professor Lavelle said, the Latin names of metals should only be used to refer to those that are a part of an anionic coordination compound. However, do not forget that this is in addition to attaching the suffix -ate to the end of the metal's Latin name. For example, cuprate or...
- Thu Dec 17, 2020 12:59 am
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Calculating H+
- Replies: 17
- Views: 1033
Re: Calculating H+
Just as a basic understanding, know that [H3O+], [OH-], pH, and pOH are all related to each other and can be found if just one of these values are provided. In the problem you provided, we can note that the measurement was taken "at 25 degrees Celsius", meaning there should be no deviation...
- Thu Dec 17, 2020 12:44 am
- Forum: Naming
- Topic: Naming Coordination Complexes
- Replies: 4
- Views: 456
Re: Naming Coordination Complexes
There is no "easy way" of naming coordination complexes per se. I think as you continue to practice naming example coordination complexes, the easier it will get. A large part of naming them is to remember the common ligands from the chart on Professor Lavelle's website. The more effort yo...
- Sun Dec 06, 2020 6:51 pm
- Forum: Naming
- Topic: Naming [Co(CN)5(OH2)]2-
- Replies: 5
- Views: 518
Re: Naming [Co(CN)5(OH2)]2-
If you reference the ligand naming chart on either Sapling Chempendix or Professor Lavelle's website, you will see that OH2, or rather H2O, is actually aqua-, not hydro-. With cyano-, we can recognize that in terms of alphabetical arrangement, aqua- comes before. Thus, we have aquapentacyanocobaltat...
- Sun Dec 06, 2020 6:20 pm
- Forum: Naming
- Topic: Sapling Q1
- Replies: 20
- Views: 1061
Re: Sapling Q1
You are correct in your method of deducing that it was "tetraamine" from (NH3)4. I believe the first mistake made was in not recognizing the number of Cl ligands correctly. As there are two and not just one, the prefix "di" should be attached to the ligand name "chloro"...
- Sun Dec 06, 2020 6:06 pm
- Forum: Naming
- Topic: Anion Naming
- Replies: 7
- Views: 420
Re: Anion Naming
I believe that this is in fact the correct methodology behind correctly naming coordination compounds. In the case of [Co(NH_3)_5Cl]Cl_2 , the Cl2 on the end is not necessarily a part of the ligand, so you would not use a prefix to indicate the amount present and instead should opt to use it...
- Sun Dec 06, 2020 5:47 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Does pH indicate strength of an acid?
- Replies: 26
- Views: 2612
Re: Does pH indicate strength of an acid?
The things you listed all represent the characteristics of a strong acid. The lower the pH, the stronger the acid is and vice versa for bases. Thus, the stronger the acid (lower pH), the higher the concentration of hydrogen (H+) ions will be. As a result, a strong acid would reflect its ability to m...
- Sun Dec 06, 2020 4:47 am
- Forum: Resonance Structures
- Topic: Stability
- Replies: 13
- Views: 770
Re: Stability
Resonance structures are considered to be more stable due to how they allow for the delocalization of electrons, i.e. the jumping of electrons from bond to bond. This "spreading out" of electrons throughout a molecule brings its total energy down, hence making it considerably more stable t...
- Sun Dec 06, 2020 4:38 am
- Forum: Naming
- Topic: what does (en) mean?
- Replies: 23
- Views: 10610
Re: what does (en) mean?
In Br_2]) , (en) is meant to represent ethylenediamine, a bidentate ligand with the molecular formula
, (en) is meant to represent ethylenediamine, a bidentate ligand with the molecular formula 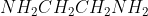 . Just for future reference, you can always look at the ligand table in Sapling's Chempendix or the one on Professor Lavelle's website if you need help as well.
. Just for future reference, you can always look at the ligand table in Sapling's Chempendix or the one on Professor Lavelle's website if you need help as well.
- Sun Nov 29, 2020 11:38 pm
- Forum: Hybridization
- Topic: Sapling #12
- Replies: 29
- Views: 1194
Re: Sapling #12
For this problem, we can observe from the Lewis structure that both carbon and oxygen have 4 areas of electron density, with carbon being bonded to 4 atoms while oxygen is bonded to 2 atoms and has 2 additional lone pairs. Hence, since they both have 4 regions of electron density, we can deduce that...
- Sun Nov 29, 2020 4:28 am
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Sapling Q.20
- Replies: 8
- Views: 1397
Re: Sapling Q.20
Like Andrew said, the ion's structure will be a resonance hybrid in the end so all of its bonds will be relatively the same. To find out the polarity of AsO_4^{3-} , it is more important to focus on the molecular geometry. We can easily recognize that each of the bonds in the molecule are polar sinc...
- Sun Nov 29, 2020 4:00 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Negative poles of molecules
- Replies: 3
- Views: 286
Re: Negative poles of molecules
To determine where the negative pole of a molecule lies, you must look at the atoms' electronegativities and compare them to one another. Negative poles will form around the most electronegative parts of a molecule. If there are two relatively equal electronegativities, the negative pole will be som...
- Sun Nov 29, 2020 3:12 am
- Forum: Student Social/Study Group
- Topic: Week 8/9 Thoughts/Worries
- Replies: 66
- Views: 4513
Re: Week 8/9 Thoughts/Worries
Although chemistry is one of the subjects I tend to struggle most with, I still feel relatively okay in this class. However, a big part of my anxiety is how the grading scale leaves little to no room for error. I am still studying as much as possible, but I definitely have to ramp it up in time for ...
- Sun Nov 29, 2020 2:57 am
- Forum: Sigma & Pi Bonds
- Topic: Delocalized π bonds
- Replies: 6
- Views: 273
Re: Delocalized π bonds
Delocalized pi bonds refer to those in which electrons can freely move between atoms, mainly referring to the bonds within resonant molecules. This is because the pi bond(s) is constantly moving throughout the molecule, hence they are "delocalized".
- Sun Nov 29, 2020 2:39 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: What is coplanar?
- Replies: 8
- Views: 5307
Re: What is coplanar?
I believe you are referring to question #19 on the Sapling homework for weeks #7 - 8. Just for starters, coplanar means that the atoms in question lie on the same plane as one another and thus, are not perpendicular. I like to think of it as being parallel. This way of thinking ties into understandi...
- Sun Nov 22, 2020 3:43 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Polarizability of anions
- Replies: 6
- Views: 1048
Re: Polarizability of anions
N^{3-} is more polarizable than O^{2-} because it has a larger ionic radius than oxygen and thus, its electron cloud is more easily distorted. Remember that ionic radius follows the pattern of atomic radius, once you separate the cations and anions. Thus, since nitrogen is to the left of oxygen on ...
- Sun Nov 22, 2020 2:06 pm
- Forum: Octet Exceptions
- Topic: Expanded Octets
- Replies: 11
- Views: 673
Re: Expanded Octets
Expanded octets can include those past 10 electrons, meaning valence shells with 12 are valid. For example, take sulfur hexafluoride (SF6). If we draw out its Lewis structure, it becomes clear that S, the central atom, has an expanded octet of 12 electrons. I am not 100% sure about valence shells wi...
- Sun Nov 22, 2020 1:45 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: London Dispersion
- Replies: 33
- Views: 1971
Re: London Dispersion
London dispersion forces (LDFs) are always occurring between molecules. They rely upon the electron's typical behavior of randomness and thus are momentary differences in charge throughout the molecule that are the result of arbitrary movement. Electrons are always randomly moving, so this is why LD...
- Sun Nov 22, 2020 4:03 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Ground State vs. Excited State
- Replies: 6
- Views: 2618
Re: Ground State vs. Excited State
Just in case, ground-state electron configuration refers to the typical electron configuration that would expect to see in atoms of elements while excited-state electron configuration refers to the electron configuration that is observed in them after additional energy is absorbed. Personally, I fin...
- Sun Nov 22, 2020 3:44 am
- Forum: Bond Lengths & Energies
- Topic: IMF vs. Intramolecular Forces
- Replies: 7
- Views: 549
Re: IMF vs. Intramolecular Forces
In the question that you referenced, I believe what they are asking about are the intramolecular forces. This is because they ask about the strength of the molecule, hinting that they are testing you on your knowledge of bond strength, not the interactions that exist between like molecules. What is ...
- Sun Nov 22, 2020 3:10 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Electron Configuration for Silver?
- Replies: 8
- Views: 962
Re: Electron Configuration for Silver?
The electron configuration for silver (Ag) is [Kr]4d^{10}5s^1 because an atom of an element is more stable (less energy) when an electron fills up the d-orbital to d^5 or d^{10} . Thus, an atom of silver prefers that one electron from the 5s-orbital be donated or passed down to the 4d-orbital to ach...
- Sun Nov 15, 2020 6:53 pm
- Forum: Ionic & Covalent Bonds
- Topic: Hydrogen Bonding
- Replies: 13
- Views: 464
Re: Hydrogen Bonding
H-N, H-O, and H-F bonds are typically associated with hydrogen bonding due to how there exists a great electronegativity difference between H and the respective atoms. Thus, H will tend to be slightly positive while N, O, and F will tend to be slightly negative. This relationship is what facilitates...
- Sun Nov 15, 2020 5:52 pm
- Forum: Lewis Structures
- Topic: Nitrate Ion Lewis Structure
- Replies: 6
- Views: 2552
Re: Nitrate Ion Lewis Structure
If drawn correctly, you should have a Lewis structure where N is the central atom, with no additional lone pairs, two O atoms that have a single bond to N, each with 3 lone pairs, and one O atom with a double bond to N, leaving it with 2 lone pairs. Referring back to the formula for formal charge, i...
- Sun Nov 15, 2020 5:36 pm
- Forum: Electronegativity
- Topic: Sapling Question 13
- Replies: 7
- Views: 303
Re: Sapling Question 13
For this question, you must focus on how electronegativity plays a role in urea. Notice that N is more electronegative than H, thus N will be slightly negative while H will be slightly positive. Seeing how this is the case, that means that in water, the 4 H in urea can technically form hydrogen bond...
- Sun Nov 15, 2020 5:22 pm
- Forum: Ionic & Covalent Bonds
- Topic: Ionic v. Covalent Bonding
- Replies: 16
- Views: 865
Re: Ionic v. Covalent Bonding
In terms of your example, you have to recognize the mechanism behind the formation of the bond. In ammonium chloride, the difference in charges between NH4+ and Cl- is what attracts the two substances to each other and causes them to ionically bond with each other. It is clear here that no sharing o...
- Sun Nov 15, 2020 4:40 pm
- Forum: Lewis Structures
- Topic: Lewis acids and Bases
- Replies: 20
- Views: 938
Re: Lewis acids and Bases
I think the most important distinction here is to determine whether a substance is more likely to act as an electron-pair acceptor or donor. If it acts as an electron-pair acceptor, you would consider it as a Lewis acid. On the other hand, if it acts an electron-pair donor, you would consider it as ...
- Sun Nov 15, 2020 3:47 am
- Forum: Student Social/Study Group
- Topic: Test Anxiety
- Replies: 62
- Views: 3970
Re: Test Anxiety
You're definitely not alone on this! I tend to have really bad test anxiety, but recently I have been getting better at suppressing it. Before the exam, I like to take breaks from studying every now and then to keep my mental health in check. I feel that this is super important, yet often overlooked...
- Sun Nov 08, 2020 4:36 pm
- Forum: Electronegativity
- Topic: Electronegativity trend
- Replies: 18
- Views: 789
Re: Electronegativity trend
Just to be clear, electronegativity is defined as the strength of which an atom pulls electrons, mainly shared ones, towards it. The periodic trend associated with this concept is that it increases as you go up columns and across periods to the right, meaning the most electronegative atoms are found...
- Sun Nov 08, 2020 4:22 pm
- Forum: Lewis Structures
- Topic: Lone Pairs Question
- Replies: 22
- Views: 1863
Re: Lone Pairs Question
Lone pairs are formally defined as pairs of valence electrons that do not partake in shared electron bonding (covalent bonds). If this still does not make sense, my personal recommendation is to think of them as isolated pairs of electrons in a sense, meaning the two dots that you typically see besi...
- Sun Nov 08, 2020 4:00 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: What do we use formal charges for?
- Replies: 15
- Views: 435
Re: What do we use formal charges for?
Formal charge is defined as the amount of charge associated with a specific atom in a molecule. This is easier to understand in the context of its usage, which is to attribute resonant structures with some degree of stability. Assuming there are multiple resonant structures, the one deemed the most ...
- Sun Nov 08, 2020 3:49 am
- Forum: Ionic & Covalent Bonds
- Topic: Atomic Radius
- Replies: 38
- Views: 3275
Re: Atomic Radius
Atomic radius decreases as you go across a period (left to right) because of how electron shielding remains constant while effective nuclear charge increases with the addition of protons. As a result, this increase in protons causes the attraction between the nucleus and electron cloud to increase, ...
- Sun Nov 08, 2020 3:29 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: n, l, mi
- Replies: 8
- Views: 592
Re: n, l, mi
n is what's known as the principal quantum number and represents the shell level. More specifically, it is the number before orbitals, such as 4s^2 where n = 4. l is the angular momentum quantum number and describes the orbital shape that is being dealt with, and although is technically n-1, I belie...
- Sun Nov 08, 2020 2:05 am
- Forum: Electronegativity
- Topic: Electronegativity vs. Electron Affinity
- Replies: 8
- Views: 522
Re: Electronegativity vs. Electron Affinity
Although they may seem the same because they share the same periodic trends (both increase as you move to the right across periods and up columns), a distinct difference does exist between the two concepts. Electronegativity refers to the attractive strength of which an atom (of an element) is capab...
- Sun Nov 01, 2020 1:58 pm
- Forum: *Shrodinger Equation
- Topic: For Ms (spin up, spin down)
- Replies: 6
- Views: 639
Re: For Ms (spin up, spin down)
I believe that the only values for the spin quantum number are m_s=\pm \frac{1}{2} . When determining the value for m_s , this is denoted by the arrows in the orbitals, where if they are pointing upwards, the spin is 1/2 while if they are pointing downwards, the spin is -1/2. On a side note, it is i...
- Sun Nov 01, 2020 1:40 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Sapling #24
- Replies: 16
- Views: 575
Re: Sapling #24
For this problem, you need to recognize that the ends of the waves must be on opposing sides of each other, meaning if the left side of the wave finishes above the line, then the right side must finish below and vice versa. The reason behind this is so that electron orbit will be comprised of only w...
- Sun Nov 01, 2020 5:28 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Shorthand Electron Configurations
- Replies: 6
- Views: 1744
Re: Shorthand Electron Configurations
The shorthand method of writing electron configuration involves using noble gas notation for simplification, meaning replacing orbitals with the most recent preceding noble gas's known electron configuration. For example, [Ne] represents 1s^22s^22p^6 . Thus, we could use this to write sodium's elect...
- Sun Nov 01, 2020 4:49 am
- Forum: Trends in The Periodic Table
- Topic: Atomic Radius
- Replies: 30
- Views: 3215
Re: Atomic Radius
The general periodic table trend for atomic radius is that it decreases as you go across a period (left to right), and increases going down a column. The reason behind the decrease in atomic radius from left to right is due to how the addition of protons, meaning the increase in atomic number, cause...
- Sun Nov 01, 2020 4:27 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Ground State Electron Configuration
- Replies: 4
- Views: 192
Re: Ground State Electron Configuration
For starters, the ground state electron configuration references when an atom is not energized, meaning it has no excess or additional energy. On the flipside, the excited state electron configuration illustrates when an atom is energized, meaning it possesses extra energy. Thus, with this input of ...
- Sun Nov 01, 2020 4:09 am
- Forum: Properties of Light
- Topic: Is c always the speed of light?
- Replies: 92
- Views: 6377
Re: Is c always the speed of light?
In reference to the equation E=\frac{hc}{\lambda } , the variable c should always be representative of the speed of light. The speed of light, c, is generally approximated to 3.00*10^8 m/s, however, more accurately is 2.99792*10^8 m/s. In terms of other cases where c is a considered a different vari...
- Sun Oct 25, 2020 5:18 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: h/4pi
- Replies: 7
- Views: 294
Re: h/4pi
I believe \frac{h}{4\pi} is written out for both convenience and accuracy. Since both Planck's constant and π are constants, there is not really a need to create another constant from them, instead just using our known values of constants instead. Also, like others said, it is much easier to typing ...
- Sun Oct 25, 2020 5:00 pm
- Forum: Properties of Electrons
- Topic: intensity vs energy
- Replies: 29
- Views: 3453
Re: intensity vs energy
This statement should be false. An increase in intensity implies an increase in the overall amount of photons present, not directly affecting the individual energy that each photon possesses. In order to increase the individual energy levels, you would need to alter the frequency.
- Sun Oct 25, 2020 4:16 am
- Forum: Photoelectric Effect
- Topic: Photoelectric Effect Problem
- Replies: 6
- Views: 582
Re: Photoelectric Effect Problem
I am not entirely 100% sure about my answer of 1.86*10^-9 m, but I am more than happy to share what I did. I started off by converting the provided 1.1 eV into joules and 810 nm to m in order to get the equivalent of 1.7622*10^-19 J and 8.10*10^-7 m, respectively. Then, I solved for the energy of th...
- Sun Oct 25, 2020 3:09 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Sampling 29
- Replies: 3
- Views: 201
Re: Sampling 29
First, you must recognize that aluminum (Al) has 13 electrons. While doing these types of problems, I would recommend referring to the "diagonal rule" for filling up orbitals, meaning 1s, 2s, 2p, 3s, etc. Remember that s-orbitals house 2 electrons while p-orbitals are capable of holding 6....
- Sun Oct 25, 2020 2:33 am
- Forum: Student Social/Study Group
- Topic: How to relax
- Replies: 168
- Views: 34713
Re: How to relax
Personally, I love to listen to music, play games with friends, or talk to my family members when I am trying to relax. Especially with the midterm coming up, I definitely feel that this is becoming increasingly important, haha.
- Sun Oct 25, 2020 2:29 am
- Forum: Administrative Questions and Class Announcements
- Topic: Disabling question randomization in Sapling
- Replies: 3
- Views: 202
Re: Disabling question randomization in Sapling
I believe that Dr. Lavelle's email regarding Sapling question randomization was him making us aware to the presence of randomized questions in homework and the fact that he was working with Sapling tech support to disable it for clarity in regards to course material progression.
- Sun Oct 18, 2020 2:08 pm
- Forum: Properties of Light
- Topic: Frequency vs Wavelength
- Replies: 22
- Views: 1361
Re: Frequency vs Wavelength
The fundamental understanding of the relationship between frequency and wavelength is that they are inversely related to each other, meaning that with a high frequency comes a short wavelength and vice versa. Going deeper into the topic, energy is also included in this as it is inversely related to ...
- Sun Oct 18, 2020 1:56 pm
- Forum: Limiting Reactant Calculations
- Topic: Difference between limiting reactant and limiting reagent?
- Replies: 14
- Views: 2404
Re: Difference between limiting reactant and limiting reagent?
Although they are not exactly the same, just know that they are used interchangeably for the most part, so you don't have to worry about different methods of calculation. At the end of the day, the most important part to understand is that they are specifying which chemical is restricting or reducin...
- Sun Oct 18, 2020 3:22 am
- Forum: Molarity, Solutions, Dilutions
- Topic: Photoelectric Effect
- Replies: 6
- Views: 382
Re: Photoelectric Effect
For starters, you must first understand the equation related to the photoelectric effect that can be used to solve for this problem which is KE (Kinetic Energy) = E_photon - E_threshold energy. Breaking it down, the individual formula for KE is (1/2)(m*v^2), where m represents mass and v is velocity...
- Sat Oct 17, 2020 9:12 pm
- Forum: Properties of Light
- Topic: Conversions of Units and Equations
- Replies: 3
- Views: 128
Re: Conversions of Units and Equations
I am not 100% sure, but I would assume that he would allow us to view the constants and equations sheet on his website during exams. Knowledge of most necessary conversions should be gained through repetition of homework problems, however, if there is an unknown conversion then the exam problem will...
- Sat Oct 17, 2020 7:56 pm
- Forum: Einstein Equation
- Topic: m vs nm
- Replies: 66
- Views: 4048
Re: m vs nm
I believe that as long as your answer is still accurate, it can in either meters or nanometers, unless explicitly stated otherwise. My TA said the preferred version is for it to be in nanometers, but leaving it in meters is also acceptable. It just comes down to it looking nicer and concise when the...
- Sat Oct 17, 2020 7:44 pm
- Forum: Significant Figures
- Topic: Sig Figs and Constants on Exams
- Replies: 9
- Views: 618
Re: Sig Figs and Constants on Exams
I would recommend using the full length of the constants when applying them in calculations because it gives a sort of "buffer" of accuracy in your final answer. Sure, it may only be one or two numbers off, but I think it is always best to be as accurate as possible with your answers. Usin...
- Sat Oct 10, 2020 7:53 pm
- Forum: Empirical & Molecular Formulas
- Topic: Ratios for empircal formulas
- Replies: 6
- Views: 468
Re: Ratios for empircal formulas
When solving for the empirical formula, you actually divide, not multiply when you find all the moles of each element in a substance. To be more specific, you divide each amount of moles by the smallest number of moles present. For example, between 0.5 mole of element X, 1 mole of element Y, and 1 m...
- Sat Oct 10, 2020 7:09 pm
- Forum: Limiting Reactant Calculations
- Topic: Theoretical yield: confused
- Replies: 8
- Views: 671
Re: Theoretical yield: confused
To help clarify, the preliminary aspect of theoretical versus actual yield is that theoretical yield is the maximum, PERFECT amount of product that you can gain from a reaction while actual yield is the practical, REALISTIC amount of product gained from a reaction after legitimate experimentation in...
- Sat Oct 10, 2020 6:47 pm
- Forum: Empirical & Molecular Formulas
- Topic: Sapling Homework 1 Q#9
- Replies: 21
- Views: 891
Re: Sapling Homework 1 Q#9
Absolutely! That is the first step in solving the problem. Using the given molecular weight of CO2, you can find the amount of carbon present in the product (it is the only substance with carbon). You can start by converting it to moles of CO2 instead, and then use that to find how many moles of car...
- Sat Oct 10, 2020 3:21 am
- Forum: Administrative Questions and Class Announcements
- Topic: Petition to Bring Music Back To Lectures [ENDORSED]
- Replies: 34
- Views: 1504
Re: Petition to Bring Music Back To Lectures [ENDORSED]
Definitely want the music introductions back! Haha, maybe we can even convince Professor Lavelle to throw in a little dancing to spice up the lectures! Who knows! But on a real note, the music really helps in creating an environment that makes it feel more like a normal classroom rather than just a ...
- Fri Oct 09, 2020 7:43 pm
- Forum: SI Units, Unit Conversions
- Topic: Sapling Week 1 HW_problem #10
- Replies: 8
- Views: 413
Re: Sapling Week 1 HW_problem #10
I do not believe that your theoretical yield is incorrect, which is already a great step in the right direction. Just as a heads up, I did experience some issues where Sapling rounded down the solution for the theoretical yield instead of up. This will undoubtedly affect your percent yield if it is ...
- Fri Oct 09, 2020 2:36 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Advice from a Medical Student - Part II [ENDORSED]
- Replies: 298
- Views: 304374
Re: Advice from a Medical Student - Part II [ENDORSED]
Wow, honestly such an inspiring and interesting story to read. It's not very often that you get access to a "behind-the-scenes" look into the world of medical school, so thank you so much for choosing to share and consistently update your story! I would be lying if I said that the journey ...

