Search found 100 matches
- Fri Mar 12, 2021 10:28 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Concentration of anodes and cathodes in Q/K
- Replies: 1
- Views: 507
Concentration of anodes and cathodes in Q/K
For the equations, E=E°-(RT/nF)lnQ or E°=(RT/nF)lnK, Q and K is [products]/[reactants] and given the concentration of the anode and cathode, why is it [anode]/[cathode] and not the other way around?
- Thu Mar 11, 2021 10:08 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Sapling Week 9/10 #19
- Replies: 2
- Views: 214
Re: Sapling Week 9/10 #19
A catalyst changes the pathway to lower the activation energy. Catalysts do not affect enthalpy or the overall reaction, it is still the same reaction just at a faster rate.
- Thu Mar 11, 2021 10:03 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Stability/Favorable
- Replies: 5
- Views: 380
Stability/Favorable
When a question is asking to state whether a reactant or product is more favorable or more stable, would it be whichever one there is more of?
- Wed Mar 10, 2021 10:12 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Catalysts and Enzymes
- Replies: 5
- Views: 357
Re: Catalysts and Enzymes
Izamary Marquez 2H wrote:Colin Juett 2F wrote:The rate is then dependent on how quickly the catalyst can catalyze a reaction.
But if it's independent of [R] does the catalyst work the same way in the sense that it lowers the activation energy?
Yes, the role of catalysts is that it lowers the activation energy so it can speed up the reaction.
- Tue Mar 09, 2021 10:00 pm
- Forum: Student Social/Study Group
- Topic: Favorite Chemistry YouTube Channels
- Replies: 39
- Views: 3611
Re: Favorite Chemistry YouTube Channels
My favorite chemistry channels are The Organic Chemistry Tutor, Professor Dave Explains, Crash Course, and Melissa Maribel! They cover a lot of the topics we learned and really clarified things for me.
- Thu Mar 04, 2021 10:52 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Order of Reaction
- Replies: 4
- Views: 265
Re: Order of Reaction
The order of a reaction is not based on the stoichiometric coefficients, there might be instances where they are the same but that is a coincidence. The order of reaction would have to be determined by looking at experiment data and adding up each order value to get the overall order of the reaction...
- Thu Mar 04, 2021 10:45 pm
- Forum: General Rate Laws
- Topic: When to use Differential Rate Law or Integrated Rate Law
- Replies: 3
- Views: 230
Re: When to use Differential Rate Law or Integrated Rate Law
Could the differential rate law also be used when time is given, like a time period/change in time because of dt?
- Thu Mar 04, 2021 10:38 pm
- Forum: General Rate Laws
- Topic: How do changing stoichiometric coefficients affect reaction rate?
- Replies: 3
- Views: 1613
Re: How do changing stoichiometric coefficients affect reaction rate?
Changing the stoichiometric coefficients does not affect the concentration or how the rate law is written rate=k[A]n but the coefficients can affect the rate constant, k, thus affecting the reaction rate.
- Thu Mar 04, 2021 10:28 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Textbook Problem 6N.13a
- Replies: 2
- Views: 148
Textbook Problem 6N.13a
Hi, for problem 6N.13 part a, it is asking us to find the value of Q. I understand the steps to find it but when I check the solution my answer is different, I got approximately 3x10 6 by doing e 15 . I don't know if the solution manual might be wrong or I am doing something else wrong but this is w...
- Mon Mar 01, 2021 10:42 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Sapling 7/8 Question 17 [ENDORSED]
- Replies: 4
- Views: 438
Re: Sapling 7/8 Question 17 [ENDORSED]
To add on, something I found useful while reading chapter 7A.3, they mentioned that the ideal gas law PV=nRT can be rewritten as n/V=P/RT. Meaning the concentration in mol/L is proportional to pressure, so pressure can be used as a measure of concentration.
- Thu Feb 25, 2021 9:03 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: difference between E and Eº
- Replies: 7
- Views: 539
Re: difference between E and Eº
E° is when the cell potential is under standard conditions 1atm, 25°C/298K, and 1 M. E is when the cell potential is not under standard conditions. If you are given a value of E or E° that is not at equilibrium, you would use the equation E = E° - (RT)/(nF) lnQ. But when the cell potential is at equ...
- Thu Feb 25, 2021 8:51 pm
- Forum: Balancing Redox Reactions
- Topic: sapling q.3
- Replies: 3
- Views: 255
Re: sapling q.3
Cr is in its natural state, so it would have an oxidation number 0. To calculate how many electron Cr is losing, calculate the oxidation numbers of CrO 4 2- , oxygen has an oxidation number of 2-(4)=8-, the overall charge of that molecule is 2-, so Cr would have to be 6+. Cr in the reactant side is ...
- Thu Feb 25, 2021 8:46 pm
- Forum: Balancing Redox Reactions
- Topic: q. 7 sapling
- Replies: 2
- Views: 171
Re: q. 7 sapling
The cathode reaction is incorrect and unbalanced. The product side should have 2Ag, not AgCl. 2AgCl(s) + 2e- ---> 2Ag(s) + 2Cl-
- Wed Feb 24, 2021 8:38 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: G from K
- Replies: 9
- Views: 524
Re: G from K
The statement above is correct. To determine the △G° you would use the equation E° = (RT/nF)lnK, for K>1 you would get a positive value but for K<1 you would get a negative value. Using the value of E° you can find △G° by using ΔG° = -nFE°, since this equation has a negative, the value of △G° would ...
- Tue Feb 23, 2021 7:29 pm
- Forum: Balancing Redox Reactions
- Topic: Sapling 3
- Replies: 1
- Views: 161
Re: Sapling 3
Since this is a basic solution, the first step is to write the half-reactions. Then balance the oxygens by adding H2O to whichever side it is needed. Then balance the hydrogens by adding H2O, one for each hydrogen that is needed (if you have 2H20 then add 4H2O on the opposite side). Once the hydroge...
- Sat Feb 20, 2021 10:36 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Clarifying question about coloumb
- Replies: 1
- Views: 159
Clarifying question about coloumb
What exactly is the work that a coloumb is doing on the galvanic cell?
- Sat Feb 20, 2021 10:33 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Ecell vs Eocell
- Replies: 7
- Views: 570
Re: Ecell vs Eocell
In Friday's lecture, Dr. Lavelle said that that day's lecture material revolves around E knot Cell, meaning that the cell is in standard conditions (pressure 1 atm, temperature at 298K (I would assume)). Next lecture we will be talking about E cell, not in standard conditions. Yes, cells in standar...
- Thu Feb 18, 2021 8:45 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Internal energy for ideal gas
- Replies: 1
- Views: 119
Internal energy for ideal gas
Where does the equation △U=3/2nRT come from and when do we need to use it? This was used in a workshop problem
- Thu Feb 18, 2021 12:21 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Irreversible vs. Reversible Work Functions
- Replies: 6
- Views: 535
Re: Irreversible vs. Reversible Work Functions
A reversible work is when there is an infinitely small change in a variable, which could be external pressure, you would use the equation w = nRTln(v2/v1). Irreversible work is when a variable does not go through infinitely small changes, so you would use the equation w = -P△V.
- Thu Feb 18, 2021 12:12 pm
- Forum: Calculating Work of Expansion
- Topic: reversiible expansion
- Replies: 2
- Views: 168
Re: reversiible expansion
In addition to what was previously said, a similar equation could be used for entropy where the reaction is an isothermal reversible expansion, △S = nRln(v2/v1)
- Sat Feb 13, 2021 2:48 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Outline Progess
- Replies: 5
- Views: 340
Re: Outline Progess
From outline 3 its only 4A, 4b, 4C right? And all of outline 4?
- Sat Feb 13, 2021 1:43 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: K Equation for ΔG
- Replies: 5
- Views: 271
Re: K Equation for ΔG
K is for when a system is at equilibrium and Q is for when it is not. You would have to use the correct equation that corresponds to whether the system is at equilibrium or not. Also, note that K is used to find △G° while Q is used to find △G.
- Sat Feb 13, 2021 1:34 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: ΔH and q
- Replies: 6
- Views: 328
Re: ΔH and q
Is there a time where it won't be at constant pressure? If so how would we calculate  ?
?
- Thu Feb 11, 2021 10:52 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Difference between constants
- Replies: 4
- Views: 200
Re: Difference between constants
In addition, Cp and Cv also relate to monatomic and diatomic ideal gases.
- Tue Feb 09, 2021 7:10 pm
- Forum: Phase Changes & Related Calculations
- Topic: Knowing reversible and irreversible
- Replies: 9
- Views: 444
Re: Knowing reversible and irreversible
Just to clarify, is an isothermal reaction and reversible reaction the same thing, or is an isothermal reaction classified as a reversible reaction?
- Thu Feb 04, 2021 11:02 pm
- Forum: Calculating Work of Expansion
- Topic: Equations
- Replies: 4
- Views: 352
Re: Equations
In addition, I understand that work is the integral from v2 to v1 (-p(delta v)), but how does it also equal -nRTln(v2/v1)?
- Thu Feb 04, 2021 10:16 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: delta U= delta H
- Replies: 21
- Views: 1625
Re: delta U= delta H
Just to clarify, delta U=delta H when pressure and volume are constant and no work is being done? yeah if we have constant volume, delta U is equal to qv and when we have constant pressure delta U is equal to qp. As a result we have delta U = delta H - PdeltaV. However, if P and V are constant then...
- Thu Feb 04, 2021 9:48 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Reversible vs. Irreversible
- Replies: 8
- Views: 345
Re: Reversible vs. Irreversible
Is an irreversible reaction only when pressure is constant or can it also be related to temperature?
- Thu Feb 04, 2021 9:37 pm
- Forum: Phase Changes & Related Calculations
- Topic: Why does steam cause severe burns?
- Replies: 22
- Views: 1844
Re: Why does steam cause severe burns?
Steam/gas/vapors have higher energy than liquid and solids. When steam touches an object it undergoes a phase change from gas to liquid (condensation), going from a gas to a liquid is an exothermic process, so energy will be released, thus, burning your skin.
- Wed Feb 03, 2021 10:59 am
- Forum: Calculating Work of Expansion
- Topic: Sapling Question 14 Week 3/4
- Replies: 2
- Views: 123
Re: Sapling Question 14 Week 3/4
You would use the initial volume (4.35) in the ideal gas, then use the volume it expands to (7.58) for the w=-nRTln(v2/v1) equation.
- Thu Jan 28, 2021 7:19 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: How to find final temperature?
- Replies: 3
- Views: 3402
Re: How to find final temperature?
You would use the equation q=mC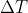 . Remember that
. Remember that 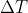 is the change in temperature so you would subtract the final temperature with the initial temperature. Then plug in values, mass*specific heat*(final temp - initial temp) and solve for the final temperature.
is the change in temperature so you would subtract the final temperature with the initial temperature. Then plug in values, mass*specific heat*(final temp - initial temp) and solve for the final temperature.
- Thu Jan 28, 2021 7:09 pm
- Forum: Phase Changes & Related Calculations
- Topic: reaction shifts
- Replies: 18
- Views: 841
Re: reaction shifts
It would be easier to think of heat as a reactant or a product in addition to the actual reactants and products. For exothermic reactions, heat would be a product, so if temperature is increased more reactants would form and more product if temperature is decreased. While for an endothermic reaction...
- Wed Jan 27, 2021 11:49 pm
- Forum: Phase Changes & Related Calculations
- Topic: States at Standard Temp / Pressure
- Replies: 6
- Views: 216
Re: States at Standard Temp / Pressure
It's good to remember that C2 (graphite) is a solid, Br2 is a liquid, and I2 is a solid. Other diatomic molecules like H2, N2, O2, etc. would be gases.
- Wed Jan 27, 2021 11:43 pm
- Forum: Phase Changes & Related Calculations
- Topic: Textbook problem 4D.17
- Replies: 3
- Views: 214
Re: Textbook problem 4D.17
For problem 15 the delta Hc was given, whereas in this problem we are told to use the appendix, so we would have to find the standard enthalpy formation of each molecule. For pure elements or elements in the most stable form their enthalpy is zero.
- Wed Jan 27, 2021 11:32 pm
- Forum: Phase Changes & Related Calculations
- Topic: Examples of sublimation
- Replies: 12
- Views: 565
Re: Examples of sublimation
Solid air fresheners are also an example of sublimation.
- Thu Jan 21, 2021 10:01 pm
- Forum: Phase Changes & Related Calculations
- Topic: How would a phase change diagram look like for the process of sublimation?
- Replies: 3
- Views: 122
Re: How would a phase change diagram look like for the process of sublimation?
The phase change diagram shown in the lecture went from solid to liquid to gas, with a positive slope in each phase and a slope of 0 when it is changing phases. Sublimation is a direct change of solid to gas, the diagram for sublimation is a positive slope at solid, a slope of 0 at solid-gas, then a...
- Thu Jan 21, 2021 9:49 pm
- Forum: Phase Changes & Related Calculations
- Topic: clarification
- Replies: 4
- Views: 235
Re: clarification
Work (w) and heat (q) depend on the path taken, so it is not a state property (state property: not dependent on the path taken to obtain that state). But heat can also be a state property if it is a constant pressure (which is enthalpy). Work and heat correlate because energy is being transferred in...
- Wed Jan 20, 2021 10:00 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Textbook Problem 5G.1
- Replies: 3
- Views: 185
Re: Textbook Problem 5G.1
Your answer is correct because the reaction will respond to the disturbance to go back to equilibrium as stated in Le Chatalier's principle. If there was an increase in product then reactants will increase, vice versa.
- Tue Jan 19, 2021 10:22 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: friday week 2 lecture question (approximation of weak acids)
- Replies: 5
- Views: 279
Re: friday week 2 lecture question (approximation of weak acids)
It is a weak acid and the Ka value is small (less than 10-4) is the x value would not change the values too much so we could approximate by omitting it. To check, if x is less than 5% of the initial concentration, then the approximation is okay.
- Tue Jan 19, 2021 10:16 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: help with textbook 5I.15
- Replies: 4
- Views: 174
Re: help with textbook 5I.15
NH3 is a product so for the change it is gaining, that is why its 0.2+x, not 0.2-x, and we leave out the NH4HS because it is a solid, we only use gases and aqueous for equilibrium.
- Thu Jan 14, 2021 4:01 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: pKA, pKB, KA, KB
- Replies: 7
- Views: 398
Re: pKA, pKB, KA, KB
Yes, the same would go for Kb and pKb. The higher the K is for Ka/Kb means there is more acid/base, so for high values of K, since pK is the -log(K) you would get smaller values for pK. Hope this helps.
- Thu Jan 14, 2021 3:52 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: pKa and pH
- Replies: 10
- Views: 602
Re: pKa and pH
pKa and pH are not the same things but they are related. pH is -log[H30+] while pKa is -log[H3O+][Conjugate Base]/[Acid]. If you know one value you can use the Henderson-Hasselbalch equation pH = pKa + log ([conjugate base]/[weak acid]) to find the other value. You can find more information on the s...
- Wed Jan 13, 2021 10:30 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Dilute Solution
- Replies: 4
- Views: 171
Re: Dilute Solution
A dilute solution is a solution that the concentration is decreased, water is typically used to dilute solutions otherwise it would be stated.
- Tue Jan 12, 2021 9:16 pm
- Forum: Ideal Gases
- Topic: Sampling hw #4
- Replies: 16
- Views: 1819
Re: Sampling hw #4
We can use the reverse reaction! Since we are starting with only product, we can flip the equation around so that PCl5 is a reactant (and the other two compounds are products). The Kp value of this reverse reaction would be 1/Kp (where Kp is the Kp of the forward reaction). Then proceed as normal, ...
- Tue Jan 12, 2021 9:10 pm
- Forum: Ideal Gases
- Topic: Lecture 3 Problem
- Replies: 7
- Views: 268
Re: Lecture 3 Problem
As stated in the previous responses, you would divide moles by the volume to find the molar concentration. The molar concentration is usually written with a capital M, which is the same as mole/liter (mol.L -1 ). If the moles of the solute is grams you would need to convert it to moles, and the volu...
- Thu Jan 07, 2021 8:56 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Finding Kc given K
- Replies: 5
- Views: 225
Re: Finding Kc given K
Is Kc not the equilibrium constant using concentrations? Kc is the equilibrium constant of concentration whereas K is the general term of an equilibrium constant. In a reaction with all gases, K is the value of partial pressure, so K=Kp. To find the value of K given Kc or vice versa you can use the...
- Thu Jan 07, 2021 3:29 pm
- Forum: Ideal Gases
- Topic: Kc for Gases
- Replies: 11
- Views: 574
Re: Kc for Gases
For Kc you would use the concentration, Kp uses partial pressure. On another note, if K is not specified as Kc or Kp and all of the molecules are gases then you would solve for Kp.
- Wed Jan 06, 2021 9:17 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Stability of reactants vs products
- Replies: 5
- Views: 624
Re: Stability of reactants vs products
Going off of the previous reply, Kc is small when it is 10-3 or less and Kc is big when it is 103 or more. When Kc is between 10-3 and 103 neither reactants or products are favored, this was mentioned in today's (1/06) lecture.
- Tue Jan 05, 2021 9:12 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Textbook Question 5H1)
- Replies: 5
- Views: 410
Re: Textbook Question 5H1)
a) they switched the placements of the reactants/products so you would just take the inverse of K, so K in this problem would be 1/41 b) the given equation is the given equation in the problem but the coefficients are just divided by 2 so you would take the square root of 41 as your answer (~6.4). ...
- Tue Jan 05, 2021 9:06 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Chemical Equilibrium Module Part 1 Q24
- Replies: 2
- Views: 96
Re: Chemical Equilibrium Module Part 1 Q24
The answer would be A because the equilibrium constant is the ratio of the concentration of products to the concentration of reactants, thus it gives the relative concentration.
- Sat Dec 12, 2020 10:58 pm
- Forum: Bronsted Acids & Bases
- Topic: determining cations or anions
- Replies: 5
- Views: 616
Re: determining cations or anions
Taha 1D wrote:You can tell it is basic because the cation is Na and that is a specatator ion. the proton transfer is just the anion pulling a hydrogen from water and leaving OH-
What is a spectator ion and how are we supposed to identify it? Was it ever mentioned in one of the lectures?
- Sat Dec 12, 2020 10:51 pm
- Forum: Bronsted Acids & Bases
- Topic: Strong acids
- Replies: 7
- Views: 491
Re: Strong acids
Memorizing them is a good way to go but if it is too difficult to remember all 7 acids, a good way is to remember the phrase "Sorry I Brought No Clean Clothese" where each of the underlined correlates to one of the strong acids.
- Sat Dec 12, 2020 10:46 pm
- Forum: Bronsted Acids & Bases
- Topic: lewis vs bronsted
- Replies: 10
- Views: 663
Re: lewis vs bronsted
Lewis acids and bases deal with electrons:
Lewis acid - accept electrons
Lewis base - donates electrons
Bronsted acids and bases deal with protons:
Bronsted acids - donates protons
Bronsted bases - accepts protons
It can be easier to remember that Bronsted is the opposite of Lewis.
Lewis acid - accept electrons
Lewis base - donates electrons
Bronsted acids and bases deal with protons:
Bronsted acids - donates protons
Bronsted bases - accepts protons
It can be easier to remember that Bronsted is the opposite of Lewis.
- Sat Dec 12, 2020 10:39 pm
- Forum: Bronsted Acids & Bases
- Topic: Acids and Bases on Final
- Replies: 2
- Views: 196
Re: Acids and Bases on Final
I would try and memorize the strong Bronsted acids and bases, anything that is not those are weak acids or bases. The strong Bronsted acids are H2SO4, HI, HBr, HNO3, HCl, HClO3, HClO4, another way to quickly memorize this is to remember the phrase " So rry I Br ought No Cl ean Clo thes". F...
- Sat Dec 12, 2020 10:29 pm
- Forum: Bronsted Acids & Bases
- Topic: Textbook Problem J.9
- Replies: 2
- Views: 302
Re: Textbook Problem J.9
For this problem, you'll need to write an equation for the given acids and bases with the product of salt and water (acid+base->salt+water). So for (a) potassium hydroxide is K(OH) (base) and acetic acid is CH3COOH (acid). The equation for this reaction should be K(OH) + CH3COOH -> CH3OOK + H2O. The...
- Sat Dec 05, 2020 7:26 pm
- Forum: Bronsted Acids & Bases
- Topic: soft salts
- Replies: 6
- Views: 332
Re: soft salts
Bases feel soapy because they react with the fatty acids and oils on our skin. I am not sure as to what soft salts are exactly, maybe it has to do with the facts that acids and bases are salts and they can be either 'soft' or 'hard' (polarizable or nonpolarizable).
- Sat Dec 05, 2020 7:00 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Final Exam Material [ENDORSED]
- Replies: 7
- Views: 501
Re: Final Exam Material [ENDORSED]
Just as the previous post stated, it is good to know what equilibrium means and how it can be used, but we will not need to know how to calculate it in this course.
- Thu Dec 03, 2020 8:51 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: homework 9 #5
- Replies: 6
- Views: 387
Re: homework 9 #5
What is (en)? Is it just a symbol for a bidentate ligand or is it a specific ligand?
- Thu Dec 03, 2020 8:44 pm
- Forum: Naming
- Topic: Naming Order
- Replies: 16
- Views: 762
Re: Naming Order
The metal atom would come first then the ligands, the cation ligand comes before the anion ligand then the neutral ligands. For more information you can check out https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Modules_and_Websites_(Inorganic_Chemistry)/Coordination_Chemistry/Structure_a...
- Thu Dec 03, 2020 8:37 pm
- Forum: Naming
- Topic: Naming Compounds-Outside of the Brackets
- Replies: 8
- Views: 461
Re: Naming Compounds-Outside of the Brackets
You would separate it with a space and sometimes the charge of the atom would separate what is outside of the bracket if it comes after.
- Fri Nov 27, 2020 9:05 pm
- Forum: Hybridization
- Topic: Bound Atoms
- Replies: 5
- Views: 164
Re: Bound Atoms
Bound atoms are atoms that are bonded to each other. Sigma bonds are single bonds that allow the bonded atoms to rotate whereas pi bonds don't allow atoms to rotate/move once they are bonded.
- Tue Nov 24, 2020 10:49 pm
- Forum: Hybridization
- Topic: Pi orbitals overlapping
- Replies: 4
- Views: 106
Re: Pi orbitals overlapping
They are perpendicular to each other because it needs to be arranged in a way that is evenly spaced from each other. And yes the carbon would have a 2sp hybridization because of the double bond and a 2p orbital.
- Tue Nov 24, 2020 10:41 pm
- Forum: Hybridization
- Topic: Finding Hybridization
- Replies: 6
- Views: 317
Re: Finding Hybridization
You could determine it by the number of lone pairs and bond pairs or you could determine it by the number of atoms it is bonded to and the number of lone pairs. I find the second way better because you wouldn't confuse it by counting the actual number of bonds (for a double/triple bond you wouldn't ...
- Tue Nov 24, 2020 10:35 pm
- Forum: Hybridization
- Topic: d hybridized orbital confusion
- Replies: 7
- Views: 379
Re: d hybridized orbital confusion
Either way is correct, it depends on your personal preference. I personally like to write spd as it in on order of energy levels.
- Tue Nov 24, 2020 10:32 pm
- Forum: Hybridization
- Topic: Sapling #18 Week7&8
- Replies: 3
- Views: 272
Re: Sapling #18 Week7&8
The three carbon atoms have two pi bonds because there is an even number of bonds/odd number of atoms the bonds would have to be perpendicular to each other. This image helped me visualized it
- Sat Nov 21, 2020 6:15 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Charge of a Molecule / Distribution of Charge
- Replies: 5
- Views: 390
Re: Charge of a Molecule / Distribution of Charge
To find the charge of the molecule, you would add up all of the formal charges from atom and that should give you the ion's charge.
- Sat Nov 21, 2020 6:09 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Dipole-Dipole vs Dipole-Induced Dipole
- Replies: 5
- Views: 970
Re: Dipole-Dipole vs Dipole-Induced Dipole
A dipole-dipole interaction is between 2 dipoles, both will have a polar charge where the positive and negative are attracted to each other. A dipole-induced dipole is an interaction between a dipole and a neutral/nonpolar atom, the dipole will induce the neutral atom to be a dipole where the opposi...
- Sat Nov 21, 2020 6:05 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: electron structure vs molecular structure
- Replies: 6
- Views: 423
Re: electron structure vs molecular structure
Electron structure can either be the Lewis structure or the electrons grouped around the nucleus drawn as an electron shell. Whereas the molecular structure is the structure of a molecule consisting of multiple atoms and where there are bonded together.
- Sat Nov 21, 2020 4:12 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Transition Metal Electrons
- Replies: 4
- Views: 331
Re: Transition Metal Electrons
I believe that transition metals can only have 2 valence electrons is because the s-block has higher energy than the d-block and "s" can only hold 2 electrons, so all transition metals only have 2 valence electrons.
- Sat Nov 21, 2020 4:03 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Importance of anions and cations
- Replies: 15
- Views: 1262
Re: Importance of anions and cations
Sana Nagori 2J wrote:Similar to what someone said I remember CATions as being PAWsitive :))
A friend told me the same thing, and I thought it was funny so I still remember it to this day and it helps a lot!
- Sat Nov 14, 2020 10:12 pm
- Forum: Lewis Structures
- Topic: Question about the central atom in PO4^-3
- Replies: 2
- Views: 181
Re: Question about the central atom in PO4^-3
More electronegative atoms should have negative charges, and phosphorus less electronegative charge than oxygen. Overall it is best to avoid any charges on the central atom when possible and for a compound with three atoms, the most stable structure would be where the negative formal charge is at th...
- Sat Nov 14, 2020 10:01 pm
- Forum: Resonance Structures
- Topic: Determining oxidation numbers?
- Replies: 9
- Views: 368
Re: Determining oxidation numbers?
You would need to find the oxidation number for each atom and it should equal the charge of the compound. The oxidation number for oxygen is -2 and there are four oxygens (-2*4). The compound has a -1 charge so x+(-2*4)=-1, x would be the charge of chlorine.
- Sat Nov 14, 2020 9:57 pm
- Forum: Student Social/Study Group
- Topic: Lecture Bops Playlist
- Replies: 4
- Views: 185
Re: Lecture Bops Playlist
Yes! Listening to Dr. Lavelle's music at the beginning of class gets me excited for class. And I'm pretty sure there are enough people listening that most of us would know some songs so we could also make the playlist ourselves.
- Sat Nov 14, 2020 9:49 pm
- Forum: Lewis Structures
- Topic: Oxidation Number
- Replies: 4
- Views: 252
Re: Oxidation Number
Oxidation numbers tell you of electrons are lost or gained. The chart posted above is a good reference. For neutral compounds (no charge) all the oxidation numbers should add up to 0. For ions, all the oxidation numbers should add up to the charge of the ion. Then for free elements its oxidation num...
- Wed Nov 11, 2020 11:06 pm
- Forum: Resonance Structures
- Topic: sapling week5/6 hw Q4
- Replies: 5
- Views: 230
Re: sapling week5/6 hw Q4
How do you determine which structure contributes the most or least to the overall structure?
- Sat Nov 07, 2020 11:21 pm
- Forum: Lewis Structures
- Topic: Negative Sign
- Replies: 12
- Views: 903
Re: Negative Sign
The charge of the overall molecule goes outside of the Lewis structure which is in a square bracket. You could also write the charge of each individual atom, Dr. Lavelle recommends underlining the formal charge of the individual atom to make it easier to distinguish.
- Sat Nov 07, 2020 11:18 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: What do we use formal charges for?
- Replies: 15
- Views: 434
Re: What do we use formal charges for?
Formal charge is used to determine whether a molecule is stable or not, a formal charge with 0 is the most stable. Since it is assumed that all electrons are shared equally there are some that break/is an exception to the octet rule.
- Sat Nov 07, 2020 11:12 pm
- Forum: Lewis Structures
- Topic: Bond Character
- Replies: 6
- Views: 304
Re: Bond Character
Bond character is determined by the difference in electronegativity of the atoms involved. It should either be ionic or covalent. I am not sure what you mean by ample or overwhelming, but I hope this helps a little.
- Sat Nov 07, 2020 11:04 pm
- Forum: Lewis Structures
- Topic: Lewis Structure for BrF3
- Replies: 6
- Views: 574
Re: Lewis Structure for BrF3
I think that that answer is not correct. Fluorine should fill its octet and the molecule must have 28 electrons, so fluorine should have another lone pair. Bromine can have an extra lone pair because its principal quantum number is n=4 so it has a d shell that can accommodate extra electrons. I agr...
- Fri Nov 06, 2020 12:34 pm
- Forum: Ionic & Covalent Bonds
- Topic: Greek Character on 11/6 Lecture at Around Minute 16:00
- Replies: 3
- Views: 136
Re: Greek Character on 11/6 Lecture at Around Minute 16:00
The Greek letter that represents the slightly negative/positive atom is the lowercase delta sign. It is written like an s but you connect the end so it's almost like an 8. The delta sign means that the more electronegative atom will pull the shared electrons towards it (delta negative) while the low...
- Fri Oct 30, 2020 12:00 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Excited State
- Replies: 3
- Views: 295
Re: Excited State
An element enters their excited state when an electron temporarily occupies an energy level greater than its ground state. This occurs when the outer electrons observe energy and jump to a higher orbital. Eventually it can release this energy and fall back to a lower state. During this process, the...
- Fri Oct 30, 2020 11:55 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Shells, Subshells, and Orbitals
- Replies: 2
- Views: 177
Re: Shells, Subshells, and Orbitals
How I visualize it is that the shell is the outer layer (n=1,2,3,4,etc.) that contains the subshells (s,p,d,f), inside the subshells are the orbitals (each orbital holds up to 2 electrons that spins up or down). Hope this helps.
- Fri Oct 30, 2020 11:34 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Electron Configurations
- Replies: 15
- Views: 559
Re: Electron Configurations
Should we specifiy 2pxpypx or 2p^3 for electron configurations? How should we write it for tests? does this apply to the d or f subshell? what letters are used for those larger subshells I believe it would apply to the d and f orbitals as well. Since p has 3 orbitals there is px, py, pz. There is a...
- Fri Oct 30, 2020 11:21 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: l=4
- Replies: 13
- Views: 570
Re: l=4
l could be 4, but Dr. Lavelle says that in this class we will only use up to l=3 since the subshell for l=3 is f, which is the highest orbital we'll use.
- Fri Oct 30, 2020 11:12 am
- Forum: Significant Figures
- Topic: Sig Figs for electron shells
- Replies: 4
- Views: 323
Re: Sig Figs for electron shells
Yes I agree with the previous post, you should base your sig figs off of the wavelength/frequency given in the question since electron shells can only have whole numbers.
- Fri Oct 23, 2020 2:45 pm
- Forum: Properties of Light
- Topic: Wavelengths in Light Spectrum
- Replies: 9
- Views: 508
Re: Wavelengths in Light Spectrum
Yes, I would try to memorize as much of the EM spectrum as you can. My TA told me that it will not be provided on the test, so it would be good to memorize it.
- Thu Oct 22, 2020 8:14 pm
- Forum: Properties of Electrons
- Topic: intensity vs energy
- Replies: 29
- Views: 3438
Re: intensity vs energy
It would be false because each photon/electron has a certain amount of energy, increasing the intensity just means that you are increasing the number of photons which increases the number of electrons with the same amount of energy.
- Thu Oct 22, 2020 8:08 pm
- Forum: Einstein Equation
- Topic: Rydberg's Equation
- Replies: 9
- Views: 320
Re: Rydberg's Equation
It would usually be final - initial, but I heard that some people switch it up. Just be sure that if you do, remember where things are and possibly have to switch the sign of your answer. There is also another way to write the equation and it is the equation given on the equation sheet linked on the...
- Thu Oct 22, 2020 7:39 pm
- Forum: Balancing Chemical Reactions
- Topic: Writing Equations [ENDORSED]
- Replies: 15
- Views: 1271
Re: Writing Equations [ENDORSED]
Not necessarily. There are 7 diatomic molecules that you will have to memorize: H2, N2, F2, O2, I2, Cl2, and Br2. Other molecules would not need 2 atoms per molecule when you only have one element in a molecule. They are pretty common though, like O2 for combustion reactions. What are diatomic mole...
- Thu Oct 22, 2020 7:32 pm
- Forum: Limiting Reactant Calculations
- Topic: General Limiting Question
- Replies: 9
- Views: 923
Re: General Limiting Question
The limiting reactant will affect how much product can be formed. It helps to calculate the theoretical yield to find the amount that could be produced, and there will side reactions so the actual yield will be less than the theoretical yield.
- Thu Oct 15, 2020 9:34 pm
- Forum: Photoelectric Effect
- Topic: Definition of "Work"
- Replies: 6
- Views: 362
Re: Definition of "Work"
Work is the threshold energy which is the minimum amount of energy needed to eject an electron from a metal. The work/threshold energy will usually be written as the Greek letter phi Φ. You will need to use the work when asked to find the energy of a photon or the kinetic energy. The energy of the p...
- Thu Oct 15, 2020 9:15 pm
- Forum: Properties of Light
- Topic: Calculator number meaning
- Replies: 6
- Views: 265
Re: Calculator number meaning
VincentLe mentioned it before, e and 10 has different values, whereas E is referring to 10 to the power of_. Just be sure to check your calculator so you don't confuse the two, but I am pretty sure most calculators will note it with a capital E.
- Thu Oct 15, 2020 1:38 pm
- Forum: SI Units, Unit Conversions
- Topic: Help Understanding SI Units?
- Replies: 7
- Views: 368
Re: Help Understanding SI Units?
Folowing the chart that was posted eariler, SI units are always usually always in factors of 3 (with the exception of die and centi). If your answer does not fit into one of the following SI units you would have to rewrite/convert it in a way that it would. An example would be 5.51x10^-7, since ther...
- Tue Oct 13, 2020 7:31 pm
- Forum: Balancing Chemical Reactions
- Topic: Mixed Numbers
- Replies: 7
- Views: 376
Re: Mixed Numbers
Usually, I start by balancing the element that occurs least then working my way to the element that occurs most. It's best if you use whole numbers for the coefficient because it will make future calculations easier. If you end up with a mixed number, there should be a number you can multiply it by ...
- Tue Oct 13, 2020 7:20 pm
- Forum: SI Units, Unit Conversions
- Topic: Rounding answers
- Replies: 44
- Views: 2368
Re: Rounding answers
When calculating I try to use as many decimal places as I can (usually four to five decimal places) to get the most accurate answer. For the answer, I would round it to however many sig figs was given in the question.
- Fri Oct 09, 2020 11:23 am
- Forum: Properties of Light
- Topic: Converting wavelength to Angstrom
- Replies: 5
- Views: 908
Re: Converting wavelength to Angstrom
Do we need to memorize these unit lengths and how to write them out? Or will there be a guide/chart of some sort to help us when calculating?
- Wed Oct 07, 2020 6:53 pm
- Forum: Balancing Chemical Reactions
- Topic: butane balancing equation question
- Replies: 6
- Views: 317
Re: butane balancing equation question
I was confused with this question at first too. All of the reactants and products are in a gas state, so the question is asking for the moles of all of the chemical compounds. To find the net number of moles produced, you would take the total number of moles of the products and subtract with the tot...
- Wed Oct 07, 2020 6:32 pm
- Forum: Balancing Chemical Reactions
- Topic: Periodic Table
- Replies: 66
- Views: 5156
Re: Periodic Table
Will the Periodic Table we receive on tests and activities have the electronegativity and other more specific details about certain elements? I believe we will be receiving a standard periodic table for the test so it will most likely not have the electronegativity or specific details about certain...
- Tue Oct 06, 2020 12:13 pm
- Forum: Significant Figures
- Topic: Sig Figs for Molar Mass
- Replies: 14
- Views: 798
Re: Sig Figs for Molar Mass
When I am calculating I like to use the most amount of numbers given (usually around four to five significant figures) so that I can get the most accurate answer. Then I would round the answers to the smallest amount of significant figures, if not I would usually keep it around four to five figures,...
- Mon Oct 05, 2020 11:13 am
- Forum: Molarity, Solutions, Dilutions
- Topic: Balancing Chemical Equations
- Replies: 12
- Views: 540
Re: Balancing Chemical Equations
If your equation has a common factor and it can be simplified it is best to do so. In the lecture, Professor Lavelle says the coefficient must be the lowest whole number. It would also make it easier to do calculations later.

