If I'm understanding your question correctly, searching up "Endgame" will give you the post containing the PDF of the actual worksheet, not a quizlet.
Hope that helps!
Search found 104 matches
- Mon Mar 08, 2021 2:49 pm
- Forum: Student Social/Study Group
- Topic: Review Worksheets
- Replies: 3
- Views: 378
- Mon Mar 08, 2021 2:44 pm
- Forum: General Science Questions
- Topic: Pre-Law/Pre-Med Students
- Replies: 20
- Views: 1529
Re: Pre-Law/Pre-Med Students
I am also pre-med and it's definitely not easy, like everyone else is saying. A lot of the STEM classes can be really hard and it's easy to get discouraged. But I think having a real interest in the work is what makes toughing through the hard classes/experiences doable. And so maybe then if you're ...
- Mon Mar 08, 2021 2:25 pm
- Forum: Student Social/Study Group
- Topic: Finals
- Replies: 46
- Views: 3349
Re: Finals
Do you guys think Dr. Lavelle will give us more time on the final, like he did for the second midterm? Having the extra time helped me sooo much, and I'm scared about how the time crunch will be, especially on the final. I think Lavelle said that the final is meant to be 90 min long. So there's mor...
- Mon Mar 08, 2021 2:22 pm
- Forum: Student Social/Study Group
- Topic: Note Taking
- Replies: 145
- Views: 16701
Re: Note Taking
I always handwrite my notes while watching each lecture. I think you retain information better when you write it as opposed to typing it, and I also think it's really tedious to type out equations, subscripts, etc. on a doc.
- Mon Mar 08, 2021 2:17 pm
- Forum: Student Social/Study Group
- Topic: How do you deal with burnout?
- Replies: 144
- Views: 15593
Re: How do you deal with burnout?
I can definitely relate, this quarter has been rough. Honestly my only motivation right now that's getting me through this is that it's almost over, which I think is an important reminder for yourself. There's not that much more to go, so you might as well just give it your all now so you can rest e...
- Mon Mar 01, 2021 10:15 am
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Reaction/Average Rate
- Replies: 13
- Views: 767
Re: Reaction/Average Rate
As to your second question, I'm not sure yet but I think the answer is no. At least for the textbook 7A problems I don't believe there are any with graphs. Although understanding the graphical basis of these problems is probably a good idea.
- Mon Mar 01, 2021 10:07 am
- Forum: Administrative Questions and Class Announcements
- Topic: tips if you're struggling!
- Replies: 77
- Views: 4917
Re: tips if you're struggling!
Thanks for the suggestions! I would actually also like to say that for me personally, doing the textbook problems early and figuring out what I don't understand is most helpful. Then I can ask my TA or go to a step-up and have those questioned cleared up. After I have resolved those issues, I do the...
- Mon Mar 01, 2021 10:03 am
- Forum: Balancing Redox Reactions
- Topic: Oxidizing Agent
- Replies: 33
- Views: 1316
Re: Oxidizing Agent
I agree with everyone else's answers but I would probably try to think of the reduction potentials as being more positive or more negative rather than "highest" or "lowest," because I think it's easier to mix up high and low. (But if it works for you then that's great!) Just reme...
- Mon Mar 01, 2021 9:58 am
- Forum: Student Social/Study Group
- Topic: What organizations are you guys in?
- Replies: 53
- Views: 3308
Re: What organizations are you guys in?
I've joined a couple clubs but can't really say that I've found one that's a good fit for me. I also feel like it's really hard to find clubs to join online! I think I'll be more waiting for when we're on campus to really join things.
- Mon Mar 01, 2021 9:56 am
- Forum: General Science Questions
- Topic: Midterm 2 Reactions
- Replies: 79
- Views: 6306
Re: Midterm 2 Reactions
I am hoping I do well on the final so that I can get a good grade but I think it will be difficult. Also hoping I don't make stupid mistakes! (Cuz I've definitely done that on these tests before)
- Mon Feb 22, 2021 1:39 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Can We Review our Exams?
- Replies: 69
- Views: 3367
Re: Can We Review our Exams?
I went and reviewed my midterm 1 with my TA not that long ago! You can definitely just show up during officer hours and they'll go through it with you. It can be a little daunting, but the TAs really just want to help you and I think it's important to go over what you missed.
- Mon Feb 22, 2021 1:36 pm
- Forum: Student Social/Study Group
- Topic: Fave food
- Replies: 266
- Views: 39606
Re: Fave food
I think for favorite food I'd have to say pho! Especially on cold or rainy days.
- Mon Feb 22, 2021 1:32 pm
- Forum: Student Social/Study Group
- Topic: We made it through Midterm 2!
- Replies: 71
- Views: 3963
Re: We made it through Midterm 2!
Yeah good job everyone! Just one more exam to go!
- Mon Feb 22, 2021 1:30 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Conceptual understandings
- Replies: 7
- Views: 486
Re: Conceptual understandings
I agree that the textbook is really good for explaining concepts. I'm having difficulty understanding electrochemistry too, and I think the textbook has good step-by-step breakdowns of the topics. I would also add that the step-up session are really good at solidifying your understanding of concepts...
- Mon Feb 22, 2021 1:28 pm
- Forum: Student Social/Study Group
- Topic: How to Reduce Nervousness before getting results
- Replies: 63
- Views: 5321
Re: How to Reduce Nervousness before getting results
I just like to remind myself that no matter what happens, I tried my best on the test and there's really nothing I can do about it right now. Worrying over what the score will be is not going to change anything, so it's best to just relax and let whatever's going to happen happen.
- Tue Feb 16, 2021 10:35 am
- Forum: Student Social/Study Group
- Topic: Classes for next quarter?
- Replies: 165
- Views: 16137
Re: Classes for next quarter?
I plan on taking LS 7B, Chem 14BL, and stats 10 hopefully. If I can, I'm also looking to fulfill my writing II req.
- Tue Feb 16, 2021 10:32 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: textbook 4C.3
- Replies: 2
- Views: 224
Re: textbook 4C.3
You're on the right track! For (a) use Cp, for (b) use Cv; your equations are correct. Just remember that Cv for an ideal monoatomic gas is 3/2R and Cp is 5/2R. From there you know your enthalpy change, you know your moles, and you know your initial temperature. Set up your equation as as q = n*Cp*(...
- Tue Feb 16, 2021 10:06 am
- Forum: Student Social/Study Group
- Topic: Midterm 2 Nerves
- Replies: 40
- Views: 1905
Re: Midterm 2 Nerves
I am for sure feeling the nerves as well. I want to do well but I worry that there will be tricky questions that I just won't get. So I'm right there with you!
- Tue Feb 16, 2021 9:56 am
- Forum: Ideal Gases
- Topic: Chem BL
- Replies: 107
- Views: 8241
Re: Chem BL
I plan on taking BL next quarter! (spring 2021)
I'm going to save 14C for next year, and I think that's fine because org. chem is pretty different from the other chem we've been doing so I think it's ok to take a break between.
I'm going to save 14C for next year, and I think that's fine because org. chem is pretty different from the other chem we've been doing so I think it's ok to take a break between.
- Tue Feb 16, 2021 9:50 am
- Forum: Student Social/Study Group
- Topic: Balance / Self Care Tips
- Replies: 62
- Views: 3277
Re: Balance / Self Care Tips
I like to set a half hour or an hour of time aside in the evenings to relax and do something I like, rather that just keep studying all day through. I think it's important to take a break during the day so that you're more relaxed and it reminds you that there's other things in life other than schoo...
- Mon Feb 08, 2021 11:16 am
- Forum: Student Social/Study Group
- Topic: Spring 2021
- Replies: 106
- Views: 15019
Re: Spring 2021
I was thinking of taking 14BL next quarter and waiting to take 14C until next year. Organic chem is meant to be pretty different from other chem, so there's not much harm in waiting until next year to take it, so if you have 4-5 classes you're thinking of taking but don't want to overload yourself, ...
- Mon Feb 08, 2021 11:13 am
- Forum: Administrative Questions and Class Announcements
- Topic: midterm 2
- Replies: 12
- Views: 669
Re: midterm 2
One other note to make is that even though the second midterm is not cumulative, it will probably build on past concepts or assume that you're able to do something from the last midterm as part of solving a problem on this midterm. It can't hurt to review some of the prior material :)
- Mon Feb 08, 2021 11:09 am
- Forum: Student Social/Study Group
- Topic: Book Recommendations
- Replies: 135
- Views: 14333
Re: Book Recommendations
If you're into fantasy (but not YA fantasy, stuff that's a bit denser and with less romance), read anything by Brandon Sanderson. Right now, I'm reading his Mistborn trilogy and it's amazing.
- Mon Feb 08, 2021 10:55 am
- Forum: Student Social/Study Group
- Topic: Post Midterm 1...
- Replies: 39
- Views: 1772
Re: Post Midterm 1...
I am in a similar situation to you, and I think this time around I'm going to attend more UA sessions. Especially step-up sessions! Even though you may feel comfortable with a certain concept, it's good to go because they really cement your understanding and iron out any details. I think understandi...
- Mon Feb 08, 2021 10:48 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Different R Constants
- Replies: 14
- Views: 765
Re: Different R Constants
I agree with the others, in that it definitely depends on what units you're dealing with so you just have to double check each time. Typically, though, you'll use .08206 when doing PV=nRT and 8.314 when solving for work.
- Mon Feb 01, 2021 2:25 pm
- Forum: General Science Questions
- Topic: Midterm 1 Reactions
- Replies: 70
- Views: 4796
Re: Midterm 1 Reactions
I definitely thought that midterm was pretty rough and harder than last quarter's tests. Definitely a few that I didn't know how to do. So you're totally not alone!
Also, results will probably be out at the end of the week or so @Lisa (though he hasn't said yet).
Also, results will probably be out at the end of the week or so @Lisa (though he hasn't said yet).
- Mon Feb 01, 2021 2:21 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Grading scale
- Replies: 29
- Views: 3285
Re: Grading scale
I agree with everyone else that a 93 is the cutoff for an A. In terms of our point system, I believe that means you can miss ~28 points total just fyi (assuming no rounding).
- Mon Feb 01, 2021 2:19 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Midterm #1 Results
- Replies: 18
- Views: 914
Re: Midterm #1 Results
Like the others said, I think it'll take about a week. I would also just like to remind everyone not to get too caught up or stressed out over the results, because there will be other tests and opportunities for improvement :)
- Mon Feb 01, 2021 2:14 pm
- Forum: Student Social/Study Group
- Topic: Work Life Balance
- Replies: 44
- Views: 1771
Re: Work Life Balance
One of the things I've started doing is taking a break in the evenings to do something relaxing, no matter how much more work I have left to do. I enjoy watching an episode of a TV show or playing a game. This is a good way to relax for a bit after a long day of studying and to remind yourself that ...
- Mon Feb 01, 2021 2:12 pm
- Forum: Student Social/Study Group
- Topic: Go treat yourself after MT1!
- Replies: 75
- Views: 5198
Re: Go treat yourself after MT1!
Thanks for the reminder! It's really important to take breaks and let yourself relax for a bit after tests. No matter how well you did, there is a path forward. :)
- Tue Jan 26, 2021 10:18 am
- Forum: Administrative Questions and Class Announcements
- Topic: Questions on Midterm 1
- Replies: 7
- Views: 401
Re: Questions on Midterm 1
Last quarter's range was somewhere between 12 and 16 problems I believe, so it'll likely be somewhere around there.
- Tue Jan 26, 2021 10:15 am
- Forum: Administrative Questions and Class Announcements
- Topic: Follow through points on the midterm?
- Replies: 10
- Views: 538
Re: Follow through points on the midterm?
There's no work submissions on any of his exams, everything is just multiple choice. Because of that, most of his questions do not give partial credit.
- Tue Jan 26, 2021 8:46 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: How to understand if -x is insignificant
- Replies: 13
- Views: 541
Re: How to understand if -x is insignificant
I've definitely heard people tell me different things for this so it can be kind of confusing. The most definitive answer I've heard was from lecture a couple days ago when Dr. Lavelle said that to be safe, you should only approximate when it's below 10^-4, rather than using 10^-3.
- Tue Jan 26, 2021 8:05 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Question 5.35
- Replies: 2
- Views: 129
Re: Question 5.35
So for part a, compound B goes changes partial pressure from 0 to 5, C goes from 0 to 10, which is twice the amount B does. Compound C begins with a partial pressure around 27.5 and goes down to about 17.5, which is a change of 10. Like compound C this is twice the amount B does. From this, the equa...
- Tue Jan 26, 2021 7:58 am
- Forum: General Science Questions
- Topic: Study Tips for midterm
- Replies: 24
- Views: 1482
Re: Study Tips for midterm
Definitely go over the textbook problems a couple times. A few questions from the textbook are usually on the tests, and their concepts are really important to understand. If you can do the textbook problems well, you'll be fine.
- Tue Jan 19, 2021 3:18 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Midterm 1 Inquiries
- Replies: 6
- Views: 370
Re: Midterm 1 Inquiries
I think we will just have to wait for Dr. Lavelle to send out the info for the midterm in an email. It'll probably come later this week, though!
- Tue Jan 19, 2021 3:16 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Thermodynamics on Midterm 1
- Replies: 6
- Views: 304
Re: Thermodynamics on Midterm 1
I think we just need to wait for Dr. Lavelle to send out an email on the topics that will be covered, so don't worry about it yet! I'm sure the information is coming.
- Tue Jan 19, 2021 8:47 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: State indications in Chemical Equations
- Replies: 4
- Views: 182
Re: State indications in Chemical Equations
In a correct chemical reaction all states should be indicated, yes. Either it's (s), (l), or (aq). I think you can assume it's whatever's given to you.
- Tue Jan 19, 2021 8:44 am
- Forum: Administrative Questions and Class Announcements
- Topic: Lavelle's Class Website
- Replies: 11
- Views: 379
Re: Lavelle's Class Website
Yeah because the website seems to be working for everyone else, it's likely your password. Just double check to be sure!
- Mon Jan 18, 2021 3:39 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Sapling Grading
- Replies: 8
- Views: 398
Re: Sapling Grading
I asked this question last quarter too because nobody specified this for me either. To add on to what other people are saying, you can also hit the "hint" button! Asking for hints doesn't affect your score either.
- Mon Jan 11, 2021 9:20 pm
- Forum: Student Social/Study Group
- Topic: Favorite TV shows
- Replies: 277
- Views: 40051
Re: Favorite TV shows
If you're a Star Wars fan and like animated kids' shows like Avatar, definitely check out both Star Wars Rebels and Star Wars: The Clone Wars. They're a lot of fun!
- Mon Jan 11, 2021 9:17 pm
- Forum: Student Social/Study Group
- Topic: Midterms?
- Replies: 14
- Views: 771
Re: Midterms?
I was having a hard time finding those dates too! Thanks to everyone who posted the link :)
- Mon Jan 11, 2021 3:42 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kw
- Replies: 9
- Views: 441
Re: Kw
They just mean that in general, when we talk about water we mean that's it's neutral with a pH of 7. So if that's true then [OH-] and [H3O+] are equal. Knowing these things, you can often determine how adding an acid or base to water will affect your concentrations.
- Mon Jan 11, 2021 11:14 am
- Forum: Ideal Gases
- Topic: Does temperature matter?
- Replies: 19
- Views: 601
Re: Does temperature matter?
If they just tell you the reaction takes place at some temperature, then you can ignore it a lot of the time depending on what you're looking for (for equilibrium constants it's usually not needed unless you're using PV=nRT). I think it's just there to tell you that they used a uniform temperature f...
- Mon Jan 11, 2021 11:06 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Textbook Problem 5I.19
- Replies: 5
- Views: 379
Re: Textbook Problem 5I.19
I think for this one it's saying that 60% has reacted by equilibrium, so your initial concentration is still what they gave you in the first sentence but your equilibrium concentration should be 40% of your initial (because that's what's left). Your change is the 60% of the initial concentration. O...
- Tue Jan 05, 2021 12:56 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Q vs K
- Replies: 12
- Views: 621
Re: Q vs K
It was kind of hard for me to remember how to tell if the reaction will shift to the left or right based on Q, so how I remember it is by remembering that for any fraction a bigger denominator will make the value of the fraction smaller, while a bigger numerator makes it bigger. So if Q is smaller t...
- Tue Jan 05, 2021 12:12 pm
- Forum: Student Social/Study Group
- Topic: Advice for someone who didn't take 14A with professor Lavelle
- Replies: 61
- Views: 2998
Re: Advice for someone who didn't take 14A with professor Lavelle
Definitely attend those UA sessions according to your needs, so if you want something slower because you're a little confused go to a step-up, and if you just want practice problems go to a workshop. Also, don't be shy to ask questions! The UA's are really nice and helpful.
- Tue Jan 05, 2021 12:00 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Textbook Problem 5I.19
- Replies: 5
- Views: 379
Re: Textbook Problem 5I.19
I think for this one it's saying that 60% has reacted by equilibrium, so your initial concentration is still what they gave you in the first sentence but your equilibrium concentration should be 40% of your initial (because that's what's left). Your change is the 60% of the initial concentration.
- Mon Jan 04, 2021 2:18 pm
- Forum: Ideal Gases
- Topic: Converting concentration to partial pressure [ENDORSED]
- Replies: 3
- Views: 210
Re: Converting concentration to partial pressure [ENDORSED]
The only way we have been shown to convert between concentration and partial pressure in class is using PV=nRT. So if you don't have temperature, based on what we know for this class so far, I don't think you can find partial pressure. But maybe it'll be covered in class later!
- Mon Jan 04, 2021 2:13 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Chemical Equilibrium part 4 post assessment #13
- Replies: 2
- Views: 107
Re: Chemical Equilibrium part 4 post assessment #13
I don't remember the correct answer to this problem, but I think this is how I did this problem: If you compress the volume of the system, then the pressure increases. There are more moles of gas on the left side of the reaction compared to the right side (2 HI(g) + Cl2(g) versus only 2 HCl(g)). Thi...
- Mon Dec 07, 2020 2:45 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Unable to view the lectures
- Replies: 12
- Views: 681
Re: Unable to view the lectures
Yes, I definitely had that issue while watching the lecture today. Honestly, CCLE has been iffy since yesterday. When it stops I just do something else for a few minutes and then refresh the page. Eventually it comes back up but I don't think there's any quick fix.
- Mon Dec 07, 2020 2:44 pm
- Forum: Conjugate Acids & Bases
- Topic: Alkaline
- Replies: 16
- Views: 784
Re: Alkaline
I think they're very similar, so when someone says that something is alkaline you can assume they're saying it's basic. Alkaline just means something with a pH greater than seven.
- Mon Dec 07, 2020 2:42 pm
- Forum: Student Social/Study Group
- Topic: Finals Study Things
- Replies: 27
- Views: 1398
Re: Finals Study Things
Thanks! I didn't get a chance to attend the session but I did hear about it. I'll definitely take a look at that doc!
- Mon Dec 07, 2020 2:41 pm
- Forum: Student Social/Study Group
- Topic: Calculating grade
- Replies: 24
- Views: 1562
Re: Calculating grade
I can't see my overall grade either. You can individually add up the assignments and divide it out of the 500 point total to get your grade. Or you can find a website that'll do it for you, and there are a lot of them out there. Here's a straightforward one: https://www.calculator.net/grade-calculat...
- Mon Dec 07, 2020 2:38 pm
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: pH and pKa Relation
- Replies: 3
- Views: 229
Re: pH and pKa Relation
You should also remember that pkA is the -logkA. So they are two different things, but are still related and can both help you determine the acidity of a compound.
- Tue Dec 01, 2020 10:05 pm
- Forum: Naming
- Topic: Textbook problem 9c.3
- Replies: 4
- Views: 306
Re: Textbook problem 9c.3
Like other people have been saying, the biggest thing to remember when deciding how many of each atom to have is the charges. Just make sure that when you finish writing it out that the overall charge of what's inside the brackets is equal to the overall charge of everything outside the brackets. It...
- Tue Dec 01, 2020 10:02 pm
- Forum: Student Social/Study Group
- Topic: Final Exam Study Tips
- Replies: 48
- Views: 2539
Re: Final Exam Study Tips
The textbook problems on the outlines and the sapling homework are good resources, especially since some textbook problems are recycled for the tests. Also make sure to attend the step up and workshop sessions! I heard that some of the ones for week 10 are going to be review sessions, so those shoul...
- Tue Dec 01, 2020 11:53 am
- Forum: General Science Questions
- Topic: study methods/recs
- Replies: 37
- Views: 2374
Re: study methods/recs
Other than the textbook and sapling, the workshops and step up sessions are really helpful. It's a nice review if you're already somewhat familiar with the material but want a bit more practice or have questions. You can access the worksheets again later for more studying too from the google drive.
- Mon Nov 30, 2020 1:07 pm
- Forum: Naming
- Topic: Textbook 9C.1
- Replies: 6
- Views: 326
Re: Textbook 9C.1
I think for this class I would mostly stick to using "cyano" rather than "cyanido" because that's what Dr. Lavelle uses, but I agree that both are valid.
- Mon Nov 30, 2020 1:04 pm
- Forum: Naming
- Topic: Using ido or o
- Replies: 24
- Views: 926
Re: Using ido or o
Like everyone else has basically been saying, I would stick to using -o since it's what Dr. Lavelle uses.
- Mon Nov 23, 2020 4:15 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Midterm 2 Grades Chem 14A
- Replies: 6
- Views: 447
Re: Midterm 2 Grades Chem 14A
I'm not certain that this is true, but I heard in a different thread that they're supposed to be out tomorrow. Last time it was Thursday though, so nothing is guaranteed.
- Mon Nov 23, 2020 4:12 pm
- Forum: Student Social/Study Group
- Topic: Post Midterm Blues
- Replies: 71
- Views: 3650
Re: Post Midterm Blues
505352202 wrote:Are the exam grades out yet? Does anyone know?
I read in a different thread that they should be out tomorrow.
- Mon Nov 23, 2020 4:09 pm
- Forum: Student Social/Study Group
- Topic: Big Sad: Midterm 2
- Replies: 86
- Views: 6292
Re: Big Sad: Midterm 2
Most people I've talked to felt like they didn't do very well on this midterm compared to the first, so you're not alone! I'm feeling okay about it, but I'm ~very~ anxious to know my score.
- Mon Nov 23, 2020 4:07 pm
- Forum: Student Social/Study Group
- Topic: How have your study habits changed?
- Replies: 45
- Views: 1951
Re: How have your study habits changed?
I began attending the step up sessions and actually taking notes. Before, I wouldn't write anything down and just listen, but having the extra practice of writing things down really helps a lot of people to remember things. Even if it's stuff already in my notes, I find that I remember it better to ...
- Mon Nov 23, 2020 4:05 pm
- Forum: Student Social/Study Group
- Topic: How to relax after midterms
- Replies: 54
- Views: 2936
Re: How to relax after midterms
Definitely be sure to take some time off! Spend an hour or two doing whatever you want as a break. I like to read, go for walks, and catch up on sleep. Try not to worry too much about your tests. However the test went, you can't do anything about it now.
- Tue Nov 17, 2020 11:24 am
- Forum: Bond Lengths & Energies
- Topic: Higher Melting Point
- Replies: 10
- Views: 1311
Re: Higher Melting Point
Another significant thing to remember here is that HCl is a covalent molecule, and NaCl is an ionic molecule. The intermolecular forces between ions are generally stronger than between covalent molecules.
- Tue Nov 17, 2020 11:20 am
- Forum: Student Social/Study Group
- Topic: grade worries
- Replies: 119
- Views: 19808
Re: grade worries
I think about my grades all the time, probably too much of the time. Honestly you've just got to make sure you're trying your best and taking advantage of all the resources offered to you. After that, you've basically done all you can and I think there's some reassurance in that.
- Tue Nov 17, 2020 11:11 am
- Forum: Electronegativity
- Topic: 2D.3
- Replies: 2
- Views: 163
Re: 2D.3
You're right that you have to look at electronegativity here. Barium is quite a bit less electronegative than Beryllium, since it's a lot lower on the periodic table. Therefore, the difference between Bromine and Barium is going to be greater than Bromine and Beryllium, which is why the answer is Ba...
- Mon Nov 16, 2020 12:12 pm
- Forum: Student Social/Study Group
- Topic: Exercising Our Minds and Bodies
- Replies: 120
- Views: 19335
Re: Exercising Our Minds and Bodies
I try to go for a walk every day either in the morning or evening, and then a few times a week I go jogging. I find that sitting in front of my laptop can make me really stiff so stretching or yoga is also a great idea.
- Mon Nov 16, 2020 12:09 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Ion-Dipole vs Hydrogen Bonding
- Replies: 3
- Views: 187
Re: Ion-Dipole vs Hydrogen Bonding
It was my understanding that H-bonding is generally stronger than ion-dipole, but I agree that it wasn't super clear from lecture and I'm not completely certain.
- Wed Nov 11, 2020 3:24 pm
- Forum: Lewis Structures
- Topic: Formal Charge
- Replies: 5
- Views: 218
Re: Formal Charge
I would basically always calculate formal charges on each atom. That way you'll know if there's a better way to draw the structure because sometimes there's a way to draw it with each atom having an octet, but a more stable way to draw it with double or triple bonds if the atom can accommodate an ex...
- Tue Nov 10, 2020 2:16 pm
- Forum: Lewis Structures
- Topic: Sapling HW Q4
- Replies: 9
- Views: 412
Re: Sapling HW Q4
We know that in structure with resonance, the actual bond lengths between the atoms are neither single nor double, but somewhere in between (a blend). So based on the observed data for the bond lengths that they tell you, you are meant to reference the chart of expected lengths and identify whether ...
- Tue Nov 10, 2020 2:07 pm
- Forum: Administrative Questions and Class Announcements
- Topic: missing discussion
- Replies: 4
- Views: 217
Re: missing discussion
Most TAs don't take attendance either, so I'm sure they don't mind if you couldn't make it to one.
- Mon Nov 09, 2020 11:33 am
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Polarizing Power and Covalent Character
- Replies: 3
- Views: 684
Re: Polarizing Power and Covalent Character
All ionic bonds have some covalent character, depending on how polarizable the anion is and how strong the polarizing power of the cation is. An anion that has a large radius and a lot of electrons is typically very polarizable, meaning that its electrons are more easily pulled into the shared bondi...
- Mon Nov 09, 2020 11:29 am
- Forum: Student Social/Study Group
- Topic: Making it through Midterm Results
- Replies: 13
- Views: 765
Re: Making it through Midterm Results
I would definitely recommend just to start studying early. The week before the test, you should probably just start doing some problems every day. Redoing the textbook problems a couple times is helpful, since they're also on the test, and then attend as many UA sessions as possible. You can ask que...
- Thu Nov 05, 2020 3:27 pm
- Forum: Ionic & Covalent Bonds
- Topic: configuration
- Replies: 4
- Views: 182
Re: configuration
For the ones like Cu and Ag, such as Au, they have different electron configurations because it's more stable to have a full d-orbital so they borrow an electron from the s-orbital. So whenever you see one of those you know to make that change.
- Wed Nov 04, 2020 5:49 pm
- Forum: Ionic & Covalent Bonds
- Topic: Ionic Bonding Question
- Replies: 6
- Views: 625
Re: Ionic Bonding Question
I think you might have that backwards. It was my understanding that the nonmetals are the ones that gain electrons while the metals lose them, making the nonmetal the anion and the metal the cation.
- Tue Nov 03, 2020 2:58 pm
- Forum: Einstein Equation
- Topic: E=pc vs E=hv
- Replies: 15
- Views: 854
Re: E=pc vs E=hv
I agree that it definitely depends on what you're given—just make sure you know the info the problem gives you and what you want to know. Both equations are valid.
- Mon Nov 02, 2020 5:38 pm
- Forum: General Science Questions
- Topic: Midterm Grades
- Replies: 30
- Views: 1398
Re: Midterm Grades
Does anyone know where they'll be posted? Just under grades on CCLE, right?
- Mon Nov 02, 2020 1:00 pm
- Forum: Properties of Light
- Topic: Study Advice
- Replies: 50
- Views: 2327
Re: Study Advice
The textbook problems are honestly the best practice because you know the problems might be on the test and the answers are verified. The step up sessions are good if you're really not getting a concept and you want someone to go over it slowly. The workshops, I've found, are more helpful than step ...
- Thu Oct 29, 2020 4:00 pm
- Forum: Student Social/Study Group
- Topic: How to relax
- Replies: 168
- Views: 25551
Re: How to relax
I try to go for at least one walk a day, especially since everything is virtual and we're sitting all day—it's nice to move around! I also love to read, so whenever I have free time I do that.
- Wed Oct 28, 2020 1:32 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Finding Workshop Answers
- Replies: 4
- Views: 164
Finding Workshop Answers
Does anybody know where we can get the answers to the UA workshop worksheets? Some of them are in the google drive but a lot of them aren't. Are the ones in the drive the only ones we have?
- Tue Oct 27, 2020 3:59 pm
- Forum: DeBroglie Equation
- Topic: Textbook Problem 1B.21
- Replies: 2
- Views: 100
Re: Textbook Problem 1B.21
You are right that you need to use the de Broglie wavelength equation.
First, the conversion should turn out to be .146 kg and 41.2 m/s.
Plugging in, we get (6.626*10^-34)/(.146)(41.2) = 1.10*10^-34 m
First, the conversion should turn out to be .146 kg and 41.2 m/s.
Plugging in, we get (6.626*10^-34)/(.146)(41.2) = 1.10*10^-34 m
- Tue Oct 27, 2020 12:53 pm
- Forum: *Shrodinger Equation
- Topic: Shrodinger Equation on Midterm 1
- Replies: 3
- Views: 312
Re: Shrodinger Equation on Midterm 1
I agree that you probably only have to know H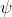 =
=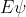 . You should probably also know generally how the equation relates to orbitals, electron probability, and the very basic concepts of the s, p, d, and f states— just the stuff covered in last Wednesday lecture.
. You should probably also know generally how the equation relates to orbitals, electron probability, and the very basic concepts of the s, p, d, and f states— just the stuff covered in last Wednesday lecture.
- Mon Oct 26, 2020 1:01 pm
- Forum: Empirical & Molecular Formulas
- Topic: Knowing chemical compounds for midterm
- Replies: 2
- Views: 232
Re: Knowing chemical compounds for midterm
I asked a similar question, and I was told that we shouldn't have to write formulas like that just from a name. They'll give us the formulas. The ones that you should know are only water, carbon dioxide, etc.
- Thu Oct 22, 2020 4:08 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Midterm
- Replies: 1
- Views: 79
Midterm
Does anybody know if we will have to be able to name compounds or write formulas for compounds on the midterm? Some of the practice textbook problems only give you the name of the compound and expect you to already know the formula. For example, F.3 asks for the formula of nitric acid.
Thanks!
Thanks!
- Thu Oct 22, 2020 4:02 pm
- Forum: Student Social/Study Group
- Topic: Midterm Prep
- Replies: 15
- Views: 645
Re: Midterm Prep
I'm just working through all the odd problems in the exercises that have been assigned in the textbook. The step-up and workshop sessions are good review too! I think the midterm doesn't have a set length yet.
- Wed Oct 21, 2020 1:31 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Balmer and Lyman series
- Replies: 2
- Views: 186
Re: Balmer and Lyman series
Balmer series ends at n=2 not n=1. It takes greater energy to jump from n=2 to n=1 (Lyman series) which corresponds to a higher frequency, which implies a lower wavelength.
- Tue Oct 20, 2020 5:29 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Sapling HW Weeks 2-4 #6
- Replies: 1
- Views: 71
Re: Sapling HW Weeks 2-4 #6
A spectral line could appear every time an electron changes energy level. So if the electron began at n=6, then there should be 5 possible lines.
- Tue Oct 20, 2020 11:39 am
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Differences in Rydberg Equation versus equation to find "En" from Friday's lecture
- Replies: 3
- Views: 152
Re: Differences in Rydberg Equation versus equation to find "En" from Friday's lecture
The Rydberg's equation is just a different version of the second equation that makes a shorter process for finding the frequency. Both of these equations rely on the Bohr frequency condition so they can be used for finding the energy or frequency of the photon that is emitted or absorbed when an ele...
- Mon Oct 19, 2020 3:23 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Atomic and Molecular Spectroscopy
- Replies: 5
- Views: 177
Re: Atomic and Molecular Spectroscopy
I think they're the same thing but atomic spectroscopy is looking at atoms and molecular spectroscopy is looking at molecules.
- Mon Oct 19, 2020 3:20 pm
- Forum: General Science Questions
- Topic: Webcams
- Replies: 17
- Views: 772
Re: Webcams
I think they're required for taking the assessments. But I think it was also mentioned that we would be able to use our phone as the webcam if we can't find one to buy or are unable to buy one.
- Fri Oct 16, 2020 1:50 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Atomic Spectra Video Module Question
- Replies: 2
- Views: 118
Re: Atomic Spectra Video Module Question
To add on based on my understanding, another way to think about this is is that as the electron is reaching higher and higher quantum levels its energy is getting closer and closer to zero. This can be seen mathematically since a constant (hR) divided by an ever-increasing number (n) approaches zero...
- Wed Oct 14, 2020 2:12 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Sapling HW Grade [ENDORSED]
- Replies: 10
- Views: 997
Sapling HW Grade [ENDORSED]
For the Sapling problems, if we click on the "Hint" button in the right corner, does our score for that problem go down? As in, will we lose points for using hints? Thanks.
- Wed Oct 14, 2020 12:02 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: notation for heisenberg indeterminancy equation
- Replies: 2
- Views: 128
Re: notation for heisenberg indeterminancy equation
H bar is shorthand for h/2pi. I'm not certain why that constant specifically is in the equation, but the textbook just says that it's a number that "occurs widely in quantum mechanics." I assume, then, that it's kind of like pi or e in math, but I'm not sure.
- Wed Oct 14, 2020 11:55 am
- Forum: Properties of Light
- Topic: Textbook Problem 1A. 3
- Replies: 7
- Views: 357
Re: Textbook Problem 1A. 3
I'm not entirely certain that this will adequately answer your question, but I read an explanation for this and it turns out the electrical field is related to the amplitude of the EM radiation. So if the frequency has decreased then the wavelength has increased because of their inverse relationship...
- Mon Oct 12, 2020 10:17 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Atomic Spectra Equation
- Replies: 1
- Views: 74
Re: Atomic Spectra Equation
I think the module mentioned that that's only for a hydrogen atom, and same with the other version of that equation.
- Fri Oct 09, 2020 11:00 am
- Forum: Molarity, Solutions, Dilutions
- Topic: G5
- Replies: 8
- Views: 311
Re: G5
a) The concentration of Na2CO3 should turn out to be around 0.07968 mol/L. Now we need to find the concentration of just Na from the concentration of Na2CO3. To do this, we know that there are 2 moles of Na per one mole of Na2CO3 (because the subscript of Na is 2). Therefore, we take the concentrati...
- Thu Oct 08, 2020 11:27 am
- Forum: Molarity, Solutions, Dilutions
- Topic: G5
- Replies: 8
- Views: 311
Re: G5
This problem uses the equation c=n/v, which we can rearrange to v=n/c. "v" being the desired volume, "n" the number of moles, and "c" the concentration. So once you have the concentration of of Na2CO3 you convert it to the concentration of just Na in the 250 mL for part...
- Wed Oct 07, 2020 2:03 pm
- Forum: SI Units, Unit Conversions
- Topic: Sapling Week 1 HW_problem #10
- Replies: 8
- Views: 405
Re: Sapling Week 1 HW_problem #10
Just tried out your problem and also got around 68%! Definitely not 69%, I'm not sure how they got that either.

