Search found 101 matches
- Sun Mar 14, 2021 10:57 pm
- Forum: General Science Questions
- Topic: Final thoughts
- Replies: 28
- Views: 4873
Re: Final thoughts
I feel a little better about the final than the midterms. The questions were more simple and straightforward.
- Sun Mar 14, 2021 10:54 pm
- Forum: Student Social/Study Group
- Topic: Planning on dorming in the Fall?
- Replies: 61
- Views: 3672
Re: Planning on dorming in the Fall?
I'm definitely dorming next fall. I need human interactions and I can't study at all when I'm at home.
- Sun Mar 14, 2021 10:51 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Salt Bridges
- Replies: 4
- Views: 460
Re: Salt Bridges
Salt bridge is represented by double vertical lines (||) and porous disk is represented by a single vertical line (|).
- Sun Mar 14, 2021 10:49 pm
- Forum: General Science Questions
- Topic: No Lavelle Chem 14C?
- Replies: 68
- Views: 5210
Re: No Lavelle Chem 14C?
I think Professor Lavelle only teaches 14A and B
- Sun Mar 07, 2021 11:51 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Cut off for K to ignore x
- Replies: 7
- Views: 434
Re: Cut off for K to ignore x
I think Professor Lavelle said 10^-4 just to be safe. You can also double check with the 5% rule.
- Sun Mar 07, 2021 11:49 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: E vs E naught
- Replies: 36
- Views: 1723
Re: E vs E naught
E naught is under standard condition and does NOT depend on how many times the reaction has occurred.
- Sun Mar 07, 2021 11:48 pm
- Forum: Student Social/Study Group
- Topic: Note Taking
- Replies: 145
- Views: 17331
Re: Note Taking
I handwrite my notes on my iPad using Goodnotes. It helps me retain more information. It's also a lot easier to draw out diagrams and figures.
- Sun Mar 07, 2021 11:45 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: What was your favorite chem topic?
- Replies: 137
- Views: 11484
Re: What was your favorite chem topic?
Chemical equilibrium
- Sun Mar 07, 2021 11:45 pm
- Forum: Student Social/Study Group
- Topic: How do you deal with burnout?
- Replies: 144
- Views: 16850
Re: How do you deal with burnout?
I have to admit that it's been a pretty tough quarter. I'm a premed so I like to watch medical dramas or read doctor stories to deal with burnouts. It's like rediscovering why I wanted to be a doctor and how much this means to me. I also like to exercise. It resets my mood and helps me find the moti...
- Sun Feb 28, 2021 11:15 pm
- Forum: Balancing Redox Reactions
- Topic: Full molecule in half reactions?
- Replies: 8
- Views: 524
Re: Full molecule in half reactions?
You would need to write the full molecule Cr2O7.
- Sun Feb 28, 2021 11:13 pm
- Forum: Student Social/Study Group
- Topic: Chem 14B Final
- Replies: 86
- Views: 5971
Re: Chem 14B Final
Go to review sessions! Last quarter, Professor Lavelle, TAs, and UAs all hosted review sessions in week 10. They were super helpful. You can also go over some homework problems and past exams.
- Sun Feb 28, 2021 10:54 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: n in ∆G = -nFE
- Replies: 80
- Views: 4451
Re: n in ∆G = -nFE
In this case, n represents the number of e- being transferred. You can find this by balancing the half-reactions.
- Sun Feb 28, 2021 9:15 pm
- Forum: Student Social/Study Group
- Topic: Book Recommendations
- Replies: 135
- Views: 15677
Re: Book Recommendations
I'm currently reading "To Live" by Yu Hua, highly recommend!
- Sun Feb 28, 2021 9:13 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Final
- Replies: 15
- Views: 876
Re: Final
Time may vary depending on your lecture but it will be on Sunday, March 14th.
- Sun Feb 21, 2021 11:38 pm
- Forum: General Science Questions
- Topic: MT 2 grades
- Replies: 34
- Views: 2026
Re: MT 2 grades
It usually comes out within a week following the midterm.
- Sun Feb 21, 2021 11:36 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: fun way to remember anode and cathode
- Replies: 16
- Views: 2682
Re: fun way to remember anode and cathode
Thank you for sharing this!
- Sun Feb 21, 2021 11:29 pm
- Forum: Student Social/Study Group
- Topic: Fave food
- Replies: 266
- Views: 41317
Re: Fave food
I love bite sized nutter butter cookies!
(definitely not a healthy choice tho)
(definitely not a healthy choice tho)
- Sun Feb 21, 2021 11:28 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Calorimeter Calibration
- Replies: 4
- Views: 600
Re: Calorimeter Calibration
To calibrate the calorimeter, all you need to do is divide heat gained by the calorimeter (or heat lost by the reaction) by the change in temperature.
Ccal=qcal/delta T
Ccal=qcal/delta T
- Sun Feb 21, 2021 11:26 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Classes for Biochem Majors
- Replies: 6
- Views: 488
Re: Classes for Biochem Majors
From my understanding, the 14 series is designed for life science majors. You probably need to take the 30 series to fulfill your major prereqs. Best of luck.
- Sun Feb 14, 2021 9:51 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: values
- Replies: 3
- Views: 265
Re: values
I don't think we'll be expected to memorize any of these. Everything we need to solve the problem should be provided on the test or the constants and equation sheet.
- Sun Feb 14, 2021 9:49 pm
- Forum: Calculating Work of Expansion
- Topic: Work Done on System?
- Replies: 12
- Views: 605
Re: Work Done on System?
I think work done on the system is when energy is being applied, like compression. Whereas, work done by the system would be expansion since the system is using energy to push against external pressure.
- Sun Feb 14, 2021 9:46 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Residual Entropy
- Replies: 5
- Views: 452
Re: Residual Entropy
From my understanding, residual entropy ignores thermal motion and can be calculated by using the Boltzmann equation (S = Kb * ln W).
- Sun Feb 14, 2021 9:43 pm
- Forum: Ideal Gases
- Topic: Chem BL
- Replies: 107
- Views: 8796
Re: Chem BL
I think people usually take it with 14C. I might take it next quarter if it doesn't fill up by second pass.
- Sun Feb 14, 2021 9:41 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Useful Summary of Thermodynamic Definitions
- Replies: 55
- Views: 18631
Re: Useful Summary of Thermodynamic Definitions
Thank you so much!
- Sun Feb 07, 2021 7:13 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Can We Review our Exams?
- Replies: 69
- Views: 3509
Re: Can We Review our Exams?
I think you can review questions you missed in your TA's office hours. Just shoot them an email.
- Sun Feb 07, 2021 7:11 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Midterm 2
- Replies: 33
- Views: 2268
Re: Midterm 2
I don't think Professor Lavelle posted an outline for midterm 2 yet.
- Sun Feb 07, 2021 6:54 pm
- Forum: Student Social/Study Group
- Topic: Chemistry Community Posts
- Replies: 12
- Views: 693
Re: Chemistry Community Posts
Use the number you see when you click on "Your posts".
- Sun Feb 07, 2021 6:40 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: deltaU
- Replies: 29
- Views: 935
Re: deltaU
Delta U is the change in internal energy. You can calculate delta U by using the following formula:
Delta U = n * Cv * delta T
Delta U = n * Cv * delta T
- Sun Feb 07, 2021 6:09 pm
- Forum: Student Social/Study Group
- Topic: Silly Mistakes?
- Replies: 72
- Views: 6459
Re: Silly Mistakes?
Thank you so much for sharing this!
- Sun Jan 31, 2021 11:16 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Taking the Anti-Log
- Replies: 37
- Views: 2670
Re: Taking the Anti-Log
Like everyone said, you'd do 10^(-pKa) to go from pKa to Ka.
- Sun Jan 31, 2021 11:13 pm
- Forum: General Science Questions
- Topic: Midterm 1 Reactions
- Replies: 70
- Views: 5050
Re: Midterm 1 Reactions
I didn't feel as confident about this midterm. I felt it was more difficult than 14A midterms.
- Sun Jan 31, 2021 11:11 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Factors that affect the equilibrium constant
- Replies: 31
- Views: 4194
Re: Factors that affect the equilibrium constant
Professor Lavelle said the only factor that affect K is temperature.
- Sun Jan 31, 2021 11:11 pm
- Forum: Student Social/Study Group
- Topic: Average amount of study hours per week
- Replies: 28
- Views: 1311
Re: Average amount of study hours per week
Usually I just watch the lectures and do a UA worksheet. So around 6 hours per week.
- Sun Jan 31, 2021 11:09 pm
- Forum: Student Social/Study Group
- Topic: Spring 2021
- Replies: 106
- Views: 16492
Re: Spring 2021
I'm planning to take 14BL next quarter!
- Sun Jan 24, 2021 10:42 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: How to find equations
- Replies: 4
- Views: 253
Re: How to find equations
Add water (H20) to the reactant and hydronium ion (H3O+) if it's an acid or hydroxide ion (OH-) if it's a base. The balance everything out.
Ex: HClO + H2O = H3O+ + ClO-
Ex: HClO + H2O = H3O+ + ClO-
- Sun Jan 24, 2021 10:38 pm
- Forum: Student Social/Study Group
- Topic: Midterm Study Tips
- Replies: 41
- Views: 1843
Re: Midterm Study Tips
Rosa told me that there's a helpful review midterm sheet from last year.
Link: viewtopic.php?f=160&t=58508&p=224708&hilit=pizza+rolls&sid=297c8e7a86a4e1bca675da3c35540d64#p224708
*if the link doesn't work, search pizza rolls
Link: viewtopic.php?f=160&t=58508&p=224708&hilit=pizza+rolls&sid=297c8e7a86a4e1bca675da3c35540d64#p224708
*if the link doesn't work, search pizza rolls
- Sun Jan 24, 2021 10:14 pm
- Forum: Student Social/Study Group
- Topic: List of UA sessions
- Replies: 3
- Views: 200
- Sun Jan 24, 2021 10:07 pm
- Forum: Student Social/Study Group
- Topic: UA Sessions
- Replies: 5
- Views: 346
- Sun Jan 24, 2021 10:06 pm
- Forum: Student Social/Study Group
- Topic: UA session ?
- Replies: 1
- Views: 155
Re: UA session ?
UA sessions are peer learning sessions hosted by undergraduate assistants (UA). These are small group discussions and Q&A.
- Sun Jan 17, 2021 11:15 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Simplifying Quadratic Equations
- Replies: 5
- Views: 271
Re: Simplifying Quadratic Equations
Professor Lavelle mentioned in lecture that some textbook uses 10^-3 but he prefers us go by 10^-4 just to be on the safe side. You can always check by dividing X by initial, if X is less than 5% of initial then it's okay to approximate.
- Sun Jan 17, 2021 11:11 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kw Uses
- Replies: 6
- Views: 220
Re: Kw Uses
You can use it for all problems involving pH I believe.
- Sun Jan 17, 2021 11:07 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: ICE Box Units
- Replies: 6
- Views: 326
Re: ICE Box Units
You can! Just make sure you convert it into molar concentration before you calculate K.
- Sun Jan 17, 2021 11:05 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Weak vs. Strong Acids and Bases
- Replies: 9
- Views: 574
Re: Weak vs. Strong Acids and Bases
There is a list of strong acids and bases in the textbook. But generally, strong acids will contain halogens (Cl, Br, I) and strong bases will contain elements from group 1 & 2 (Li, Na, K, Rb, Cs, Ca, Sr, Ba).
https://sites.google.com/site/chempendi ... #h.p_ID_32
https://sites.google.com/site/chempendi ... #h.p_ID_32
- Sun Jan 17, 2021 10:55 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: C in ICE Box
- Replies: 19
- Views: 740
Re: C in ICE Box
The change in reactants is always negative and the change in products is always positive because reactants are being used up to form products.
- Sun Jan 17, 2021 10:53 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: When would K be unchanged?
- Replies: 31
- Views: 1214
Re: When would K be unchanged?
K would also change when the coefficients of the species in a reaction are multiplied by a number. In that case, K would be raised to the power of that number.
- Sun Jan 10, 2021 9:43 pm
- Forum: Student Social/Study Group
- Topic: Chemistry Community Points
- Replies: 12
- Views: 731
Re: Chemistry Community Points
Yes, you will. Oftentimes there are less people posting questions so this is bound to happen.
- Sun Jan 10, 2021 9:38 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 10^3-10^-3
- Replies: 2
- Views: 116
Re: 10^3-10^-3
Like the Josh said, technically it would be favoring one side or the other unless K=0, just not "strongly favored."
- Sun Jan 10, 2021 9:31 pm
- Forum: Student Social/Study Group
- Topic: Chemistry Community
- Replies: 29
- Views: 1323
Re: Chemistry Community
Sunday 11:59 PM PST is the official deadline for your weekly posts but most TAs will allow you to make up for missed points in the following weeks.
- Sun Jan 10, 2021 9:20 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Substances for Calculating K
- Replies: 4
- Views: 134
Re: Substances for Calculating K
I think Professor Lavelle said that solids don't have a concentration (since it's not in liquid) and changes in solvent concentration is insignificant so it is excluded from calculations.
- Sun Jan 10, 2021 9:09 pm
- Forum: Ideal Gases
- Topic: Units of Temperature
- Replies: 82
- Views: 3949
Re: Units of Temperature
You should use Kelvin.
- Sun Dec 06, 2020 9:22 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Coordination Number
- Replies: 5
- Views: 437
Re: Coordination Number
You are really close. The coordination number for this compound is actually 6 because there are 6 coordinate covalent bond here, 1 from SO4 and 5 from (NH3)5. 1+5=6
Note: each NH3 contributes 1 coordinate covalent bond
Note: each NH3 contributes 1 coordinate covalent bond
- Sun Dec 06, 2020 9:17 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Final Zoom Link
- Replies: 6
- Views: 484
Re: Final Zoom Link
I'd assume it's the same TA link as our last midterm but I'm not 100% sure.
- Sun Dec 06, 2020 9:16 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: HW Question coordination number
- Replies: 4
- Views: 189
Re: HW Question coordination number
Coordination number = number of coordinate covalent bond
There are 6 coordinate covalent bond here, 1 from each ligand (1 SO4 + 5 NH3). Hope this helps.
There are 6 coordinate covalent bond here, 1 from each ligand (1 SO4 + 5 NH3). Hope this helps.
- Sun Dec 06, 2020 9:08 pm
- Forum: Student Social/Study Group
- Topic: Week 8/9 Thoughts/Worries
- Replies: 66
- Views: 3831
Re: Week 8/9 Thoughts/Worries
I'm a very worried about making silly mistakes and not doing well on the final. Especially since there is no partial credit and grade is heavily dependent on exam scores :<
- Sun Dec 06, 2020 8:58 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Textbook Problem 9C7
- Replies: 1
- Views: 96
Re: Textbook Problem 9C7
Chelating complexes are complexes containing a ligand that forms a ring of atoms that includes the central metal atom. In order to form a chelate, you need a ligand with the ideal geometry, which is (atom w/ lone pair) + (spacer) + (spacer) + (atom w/ lone pair) and the bonds need to be able to rota...
- Sun Dec 06, 2020 8:53 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Homework Problem 9C.7
- Replies: 5
- Views: 312
Re: Homework Problem 9C.7
In order to form chelating complexes, you need bonds that can rotate (single bonds) and the the ideal geometry, which is (atom w/ lone pair) + (spacer) + (spacer) + (atom w/ lone pair)
In this case, the answer would be b) because a) and c) have too many spacers between atoms w/ lone pairs.
In this case, the answer would be b) because a) and c) have too many spacers between atoms w/ lone pairs.
- Sun Dec 06, 2020 8:48 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3654121
Re: Post All Chemistry Jokes Here
Two chemists meet for the first time at a symposium. One is American, one is British. The British chemists asks the American chemist, "So what do you do for research?" The American responds, "Oh, I work with arsoles." The Brit responds, "Yes, sometimes my colleagues get on m...
- Sun Dec 06, 2020 8:44 pm
- Forum: Naming
- Topic: Homework Problem 9C.1
- Replies: 4
- Views: 175
Re: Homework Problem 9C.1
I think Professor Lavelle said both are acceptable.
- Sun Dec 06, 2020 8:40 pm
- Forum: Sigma & Pi Bonds
- Topic: Pi bonds
- Replies: 7
- Views: 488
Re: Pi bonds
Pi bond is the lateral or side-by-side overlap of 2 orbitals (4 orbital overlap). It do not allow bound atoms to rotate and have 1 nodal plane. It usually between p- and d- orbitals.
- Sun Dec 06, 2020 7:53 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Sapling 5
- Replies: 6
- Views: 410
Re: Sapling 5
(en) refers to NH2CH2CH2NH2, or ethylenediamine. It's a bidentate ligand (coordination number=2). And chlorine is a monodentate ligand (coordination number=1). So together, the coordination number for [Co(en)2(CO)2]Br is 2*2+2*1=6.
- Sun Dec 06, 2020 7:43 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Sapling Question #5
- Replies: 3
- Views: 375
Sapling Question #5
For each metal complex, give the coordination number for the metal species.
[Pt(en)Cl2]
Are we expected to know the name and denticity for ligands like ethylenediamine(en) and 1,2-bis(diphenylphosphino)ethane(dppe) on the midterms?
[Pt(en)Cl2]
Are we expected to know the name and denticity for ligands like ethylenediamine(en) and 1,2-bis(diphenylphosphino)ethane(dppe) on the midterms?
- Sun Nov 22, 2020 11:10 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Thanksgiving
- Replies: 26
- Views: 1321
Re: Thanksgiving
I think it's highly likely that we will have a lecture on Friday, but discussions should be cancelled. Happy Thanksgiving :3
- Sun Nov 22, 2020 11:08 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bond Angles
- Replies: 25
- Views: 1017
Re: Bond Angles
I think we will need to know the bond angles for electron pair geometries (without lone pair), like 180, 120, 109, 90; and that having lone pairs influences the bond angles between bonded atom. But I doubt we will be required to memorize specific bond angles for different molecules.
- Sun Nov 22, 2020 11:00 pm
- Forum: Ionic & Covalent Bonds
- Topic: Ionic and Covalent Bonds
- Replies: 7
- Views: 504
Re: Ionic and Covalent Bonds
Ionic
- Typically between metal and nonmetal
- Involve transfer of electrons
Covalent
- Typically between nonmentals
- Involve sharing of electrons
- Typically between metal and nonmetal
- Involve transfer of electrons
Covalent
- Typically between nonmentals
- Involve sharing of electrons
- Sun Nov 22, 2020 10:57 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Dipole-Induced-Dipole and Dipole-Dipole
- Replies: 10
- Views: 894
Re: Dipole-Induced-Dipole and Dipole-Dipole
Dipole-dipole is between two polar molecules whereas dipole-induced dipole is between a polar molecule and a nonpolar molecule.
Examples
dipole-dipole: HF-HF (HF is polar)
dipole-induced dipole: HCl-N2 (HCl is polar and N2 is nonpolar)
Examples
dipole-dipole: HF-HF (HF is polar)
dipole-induced dipole: HCl-N2 (HCl is polar and N2 is nonpolar)
- Sun Nov 22, 2020 10:52 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Optimal Formal Charge Configuration
- Replies: 3
- Views: 272
Re: Optimal Formal Charge Configuration
Typically, you want to place the negative formal charge on the more electronegative atom and the positive formal charge on the less electronegative atom. Hope this helps.
- Sun Nov 22, 2020 10:49 pm
- Forum: Sigma & Pi Bonds
- Topic: Pi Bonds with s Orbital
- Replies: 4
- Views: 204
Re: Pi Bonds with s Orbital
That would not be possible because the s orbital is spherical. You need at least two p orbitals to form a pi bond.
- Sun Nov 22, 2020 10:45 pm
- Forum: Hybridization
- Topic: Strength of Sigma Bonds vs Pi Bonds
- Replies: 5
- Views: 189
Re: Strength of Sigma Bonds vs Pi Bonds
Sigma bonds are stronger because it's a head-on overlap between the orbitals, which allows electron density to be concentrated to a much larger degree between the two nuclei.
- Sun Nov 22, 2020 10:41 pm
- Forum: Student Social/Study Group
- Topic: Finals!
- Replies: 43
- Views: 2064
Re: Finals!
Yes, it will be cumulative.
- Sun Nov 22, 2020 10:40 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: NO2- Bent Molecular Geometry
- Replies: 7
- Views: 1516
Re: NO2- Bent Molecular Geometry
NO2- have 2 electron pairs and 1 lone pairs, making it AX2E, which is bent/angular.
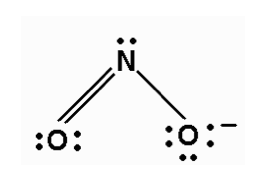

- Sun Nov 22, 2020 10:32 pm
- Forum: Sigma & Pi Bonds
- Topic: Pi Bond Name
- Replies: 5
- Views: 274
Re: Pi Bond Name
Pi is the greek version of the letter p. In this case, it's referring to the p orbital. Pi bonds are commonly formed between p orbitals, as shown in Dr. Lavelle's lecture. However, d orbitals can also engage in pi bonding.
- Sun Nov 15, 2020 11:56 pm
- Forum: Dipole Moments
- Topic: Which interactions are intermolecular?
- Replies: 7
- Views: 404
Re: Which interactions are intermolecular?
Yes, these are intermolecular forces since they are interactions between molecules rather than within molecules.
- Sun Nov 15, 2020 11:55 pm
- Forum: Student Social/Study Group
- Topic: Midterm 2 content
- Replies: 20
- Views: 903
Re: Midterm 2 content
I think this midterm will be more focused on bonding and Lewis structures. So just be ready to calculate formal charge.
- Sun Nov 15, 2020 11:52 pm
- Forum: Student Social/Study Group
- Topic: Class grade
- Replies: 18
- Views: 878
Re: Class grade
I'm on a pre-med track and am currently a pre-psychobio major. Is anyone in a similar boat to me who knows what the consequences of changing to P/NP will be? What are the pros or cons? I believe changing to P/NP gives you credit without having it affect your GPA in a negative way (if you at least p...
- Sun Nov 15, 2020 11:47 pm
- Forum: Administrative Questions and Class Announcements
- Topic: 14B and 14BL
- Replies: 6
- Views: 422
Re: 14B and 14BL
I think people usually take 14BL with 14C.
- Sun Nov 08, 2020 10:50 pm
- Forum: Photoelectric Effect
- Topic: Participation points
- Replies: 16
- Views: 762
Re: Participation points
My TA said it's fine as long as you have 50 posts by the end of the quarter.
- Sun Nov 08, 2020 10:46 pm
- Forum: Student Social/Study Group
- Topic: Mid-quarter Check in
- Replies: 67
- Views: 3829
Re: Mid-quarter Check in
I'm feeling very burnt out and my mental health is taking a hit. The quarter system makes me want to die i_i
- Sun Nov 08, 2020 10:44 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Taking Bio and Chem simultaneously
- Replies: 26
- Views: 2450
Re: Taking Bio and Chem simultaneously
I'm taking LS7B and CHEM14A this quarter along with LS30A and another GE. The workload is not too bad but it's certainly not light. It's definitely doable if you are willing to put in the effort.
- Sun Nov 08, 2020 10:39 pm
- Forum: Ionic & Covalent Bonds
- Topic: Difference Between Ionic & Covalent Bonds
- Replies: 11
- Views: 484
Re: Difference Between Ionic & Covalent Bonds
Aside from what everyone mentioned, ionic bonds tend to be stronger than covalent bonds due to the coulombic attraction between ions of opposite charges. However, covalent bonds are stronger than ionic bonds in water.
- Sun Nov 08, 2020 10:35 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Midterm 1 answer key
- Replies: 13
- Views: 758
Re: Midterm 1 answer key
Dr. Lavelle said that he'll go over some of the questions but we won't be able to see which question we missed.
- Sat Oct 24, 2020 9:25 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Constants in the tests
- Replies: 5
- Views: 304
Re: Constants in the tests
You can use the "Constants and Equations" sheet from Dr. Lavelle's website during the midterm.
- Sat Oct 24, 2020 9:12 pm
- Forum: Properties of Electrons
- Topic: Question 12 on sapling week 2
- Replies: 4
- Views: 248
Re: Question 12 on sapling week 2
I think it's just different units. It can also be:
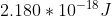
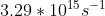
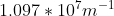
- Sat Oct 24, 2020 8:56 pm
- Forum: Photoelectric Effect
- Topic: Photoelectric Effect Post-Module Assessment #29
- Replies: 4
- Views: 281
Re: Photoelectric Effect Post-Module Assessment #29
luludaly2B wrote:Thank you! I did this and then multiplied by 1000, in order to convert the number into J from kJ. Is this correct?
Yes :)
- Sat Oct 24, 2020 8:51 pm
- Forum: DeBroglie Equation
- Topic: Textbook Question 1B.15
- Replies: 6
- Views: 340
Re: Textbook Question 1B.15
In part b, you are given the threshold frequency, which is the minimum frequency of radiation required to remove an electron from a metal surface. To solve this problem, just calculate the work function from threshold frequency using: \phi =hf_{o} \phi = work function h = Planck constant f_{o} = thr...
- Sat Oct 24, 2020 8:38 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Roman Numerials Next to Metals
- Replies: 3
- Views: 524
Re: Roman Numerials Next to Metals
The roman numerals indicate the charge and oxidation state of transition metal ions. Typically, you want the overall charge to be zero.
Ex: if sulfide have a charge of -2, you'd want the iron ion to have a charge of +2, written as iron (II) or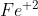
Ex: if sulfide have a charge of -2, you'd want the iron ion to have a charge of +2, written as iron (II) or
- Sat Oct 24, 2020 8:29 pm
- Forum: Student Social/Study Group
- Topic: Topic 1.C
- Replies: 2
- Views: 244
Re: Topic 1.C
Dr. Lavelle told us that the midterm covers everything up the materials discussed on Wednesday's lecture :)
- Sat Oct 24, 2020 8:24 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Amplitude
- Replies: 16
- Views: 747
Re: Amplitude
Increasing amplitude = increasing intensity. I don't think it's related to wavelength and/or frequency. But I could be wrong.
- Sat Oct 24, 2020 8:17 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Bohr Frequency Condition: v
- Replies: 3
- Views: 218
Re: Bohr Frequency Condition: v
I think they are both referring to frequency.
- Sat Oct 24, 2020 8:08 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Textbook Question 1B.25
- Replies: 4
- Views: 262
Re: Textbook Question 1B.25
I think it stands for reduced Planck constant, which is equal to 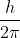 .
.
Edit: spelling
Edit: spelling
- Sat Oct 24, 2020 8:05 pm
- Forum: Photoelectric Effect
- Topic: Calculations
- Replies: 18
- Views: 713
Re: Calculations
I usually keep all of my digits and round at the very end, not sure if it's correct. But, I don't think it will be a big deal for midterm since it's multiple choice.
- Sat Oct 24, 2020 8:00 pm
- Forum: Photoelectric Effect
- Topic: Photoelectric Effect Post-Module Assessment #29
- Replies: 4
- Views: 281
Re: Photoelectric Effect Post-Module Assessment #29
The work function is basically the minimum E needed to remove an electron from a metal surface. In this case, it is already given to you in the unit kJ/mol. To find the energy required to remove an electron from ONE sodium atom, just divide 150.6 kJ/mol by Avogadro's number.
- Sat Oct 24, 2020 7:55 pm
- Forum: Photoelectric Effect
- Topic: Photoelectric Effect Problem
- Replies: 6
- Views: 565
Re: Photoelectric Effect Problem
2878.68 nm? I'm not too sure.
- Sat Oct 24, 2020 7:47 pm
- Forum: DeBroglie Equation
- Topic: Sapling Weeks 2-4 #22
- Replies: 4
- Views: 168
Re: Sapling Weeks 2-4 #22
Your approach to the question sounds right to me, maybe double check your calculations?
- Sat Oct 24, 2020 7:42 pm
- Forum: Balancing Chemical Reactions
- Topic: combustion
- Replies: 4
- Views: 414
Re: combustion
I think it indicates that the reaction is to be heated.
- Sat Oct 24, 2020 7:39 pm
- Forum: Trends in The Periodic Table
- Topic: Electron Affinity and Electronegativity
- Replies: 9
- Views: 467
Re: Electron Affinity and Electronegativity
Electronegativity: how well an atom can attract electrons toward itself
Electron affinity: amount of energy released when an electron is added to a molecule or atom
Electron affinity: amount of energy released when an electron is added to a molecule or atom
- Sat Oct 24, 2020 7:36 pm
- Forum: Student Social/Study Group
- Topic: Chem Community Questions
- Replies: 8
- Views: 829
Re: Chem Community Questions
They are due at 11:59 PM PST/PDT every Sunday :)
- Sat Oct 24, 2020 7:22 pm
- Forum: Properties of Light
- Topic: Sapling HW question 12
- Replies: 3
- Views: 164
Re: Sapling HW question 12
1. Calculate the energy of electron in ground state and 5th energy level. E_{n}=-\frac{hR}{n^{2}} h = 6.626*10^{-34} R = 3.29*10^{15} n = energy level 2. Calculate the energy emitted. \Delta E=E_{final}-E_{initial} \Delta E = E_{1}-E_{5} 3. Calculate the wavelength from \Delta E \lambda =\frac{hc}{E...
- Sat Oct 24, 2020 7:09 pm
- Forum: Photoelectric Effect
- Topic: Textbook 1B.3A problem
- Replies: 2
- Views: 435
Re: Textbook 1B.3A problem
The term "work function" refers to the minimum E required to remove an electron from a metal surface. Remember, longer wavelength = lower frequency = lower energy. So when the problem asks you to find the longest wavelength, you can just calculate the wavelength of the electromagnetic radi...
- Sat Oct 24, 2020 6:36 pm
- Forum: Properties of Electrons
- Topic: Textbook problem Quantum World 1A 15
- Replies: 3
- Views: 231
Re: Textbook problem Quantum World 1A 15
The unit for wavelength in E=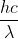 is meter (m), not nanometer (nm). Therefore, you'd need to convert 102.6 nm to m before plugging it into the equation.
is meter (m), not nanometer (nm). Therefore, you'd need to convert 102.6 nm to m before plugging it into the equation.
(6.63*10^{-34})}{102.6*10^{-9}}=1.94*10^{-18}J)
Edit: formatting
Edit: formatting
- Sat Oct 24, 2020 6:22 pm
- Forum: Einstein Equation
- Topic: Week 2-4 Sapling HW Quesiton 25
- Replies: 6
- Views: 493
Re: Week 2-4 Sapling HW Quesiton 25
From my understanding, E=hv is only used for calculating energy of photons whereas the de Broglie's equation is used for calculating energy of any moving particles with momentum.

