Search found 141 matches
- Sun Mar 14, 2021 5:59 pm
- Forum: Administrative Questions and Class Announcements
- Topic: THANK YOU DR LAVELLE!
- Replies: 47
- Views: 6821
Re: THANK YOU DR LAVELLE!
BOOST!!! You need to see this Dr. Lavelle!
- Sat Mar 13, 2021 8:03 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Saying Thank You to Dr. Lavelle
- Replies: 490
- Views: 576637
Re: Saying Thank You to Dr. Lavelle
I'm taking a break from writing my soc paper to tell you, Dr. Lavelle, that I could not be more appreciative of you. Chem 14A and 14B would not have been the same without your amazing energy and commitment to teaching. Your music was awesome, the UA sessions were a life saver, and the only thing tha...
- Fri Mar 12, 2021 11:42 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Anode and Cathode
- Replies: 25
- Views: 1387
Re: Anode and Cathode
By their definition, the anode is being oxidized and the cathode is being reduced. So, yes.
- Fri Mar 12, 2021 11:41 pm
- Forum: Student Social/Study Group
- Topic: Planning on dorming in the Fall?
- Replies: 61
- Views: 3726
Re: Planning on dorming in the Fall?
I'm definitely planning on dorming! A weird side effect of quarantine those is I'm pretty fine with having a single now even though I so wanted a traditional dorm with roomies senior year of high school.
- Fri Mar 12, 2021 11:38 pm
- Forum: Balancing Redox Reactions
- Topic: determing n (moles of e)
- Replies: 12
- Views: 625
Re: determing n (moles of e)
The way I do it is by looking at an element and seeing what happens to its charge on the other side of the reaction. Then, I can double-check with the other element.
- Fri Mar 12, 2021 11:36 pm
- Forum: Student Social/Study Group
- Topic: Playlist
- Replies: 86
- Views: 7754
Re: Playlist
Angelica Soriano 3L wrote:My favorite song at the moment is an anime opening but it's so good!!
Shogeki - Yuko Ando
https://open.spotify.com/track/5QwAdWCn ... zzNnCQbYsg
YOOO! mad respect to this
- Fri Mar 12, 2021 11:35 pm
- Forum: Student Social/Study Group
- Topic: Playlist
- Replies: 86
- Views: 7754
Re: Playlist
By Your Side by Nito and Gengar Musique is a banger (both the vocals and guitar). Also, new Day6 album is coming out next month so I'm kinda hyped for that.
- Fri Mar 12, 2021 11:29 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Sapling #13
- Replies: 3
- Views: 302
Re: Sapling #13
Since OH- has to do with the disassociation of water, our solvent, we can include it in our final rate law because we can think of it as not a true intermediate. It comes from our solvent rather than the actual reactants.
- Fri Mar 12, 2021 11:21 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Sapling #13 W10
- Replies: 2
- Views: 175
Re: Sapling #13 W10
From Kate's final review, we clarified that even though [OH-] is an intermediate, it's included because it's a result of the disassociation of water, which is our solvent. Therefore, we can include it and don't need to consider it as a true intermediate like the other intermediates in this reaction.
- Fri Mar 12, 2021 11:17 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: catalysts and reverse rxns
- Replies: 3
- Views: 304
Re: catalysts and reverse rxns
Yes! Since the catalyst brings the Ea down for the whole reaction (you can see this when you graph a catalyzed vs. uncatalyzed reaction), both the forward and reverse rates will increase.
- Fri Mar 12, 2021 11:11 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Sapling week 9 and 10 question 15
- Replies: 4
- Views: 329
Re: Sapling week 9 and 10 question 15
Following your steps, since RT is in the denominator for Ea/RT=ln(k/A), you should multiply the value you got for ln(k/A) by RT rather than divide by RT (so RT's cancels out on the left side of my equation). Also, do be mindful of any negatives that appear in your calculations but it should all end ...
- Fri Mar 12, 2021 11:05 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Sapling Week 9/10 Question #7
- Replies: 5
- Views: 421
Re: Sapling Week 9/10 Question #7
The main thing I would recommend is to take it slow and be methodical because there's a lot of calculations going on. These were the values I was given. I want to be able to solve for N, M, and L individually in rate=k[A]^{N}_{0}[B]^{M}_{0}[C]^{L}_{0} . The only variable where I can immediately do t...
- Sun Mar 07, 2021 11:31 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Determining Anode vs Cathode
- Replies: 23
- Views: 964
Re: Determining Anode vs Cathode
For a diagram (either picture or cell notation diagrams) the anode will be on the left and the cathode will be on the right. Another sanity check is the cathode should be the oxidizing agent (i.e. being reduction) while the anode is the reducing agent (i.e. being oxidized).
- Sun Mar 07, 2021 11:21 pm
- Forum: Zero Order Reactions
- Topic: Overall order of the reaction
- Replies: 45
- Views: 2083
Re: Overall order of the reaction
The overall order of a reaction is found by summing the orders of the elementary reactions.
- Sun Mar 07, 2021 11:18 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3668487
Re: Post All Chemistry Jokes Here
pretty bare-bones skeleton structure if you ask me
- Sun Mar 07, 2021 11:05 pm
- Forum: Balancing Redox Reactions
- Topic: Oxidizing Agent
- Replies: 33
- Views: 1356
Re: Oxidizing Agent
When an oxidizing agent oxidizes a target molecule, the oxidizing agent is reduced in the process. Therefore, a strong oxidizing agent should have a high reduction potential and a poor oxidizing agent will have a low reduction potential. These values should be given in the problem or on a table.
- Sun Mar 07, 2021 11:01 pm
- Forum: Student Social/Study Group
- Topic: Note Taking
- Replies: 145
- Views: 17500
Re: Note Taking
I took typed-notes for fall quarter to test it out but I prefer handwritten. Investing in an iPad for this specific purpose was worth it in my opinion. I feel much more comfortable with handwritten notes because of the ability to draw pictures, arrows, and other symbols with ease.
- Sun Mar 07, 2021 10:57 pm
- Forum: Second Order Reactions
- Topic: Termolecular
- Replies: 43
- Views: 2396
Re: Termolecular
Ter- is a prefix for "3". Termolecular reactions involve the collision of 3 species. Probability-wise, this is a rare occurrence so they are not as common as unimolecular and bimolecular reactions.
- Sun Mar 07, 2021 10:55 pm
- Forum: Student Social/Study Group
- Topic: Classes for next quarter?
- Replies: 165
- Views: 17391
Re: Classes for next quarter?
I'll be in CHEM 14BL, MATH 33B, LS 7B, and AN N EA 10W. I ready for this quarter to be over but this class was pretty fun and wholesome.
- Sun Mar 07, 2021 10:50 pm
- Forum: Administrative Questions and Class Announcements
- Topic: [CHEM 14B KARAOKE]
- Replies: 68
- Views: 6590
Re: [CHEM 14B KARAOKE]
This was definitely the coolest event I've participated in over Zoom! No questions about it!
- Sun Mar 07, 2021 10:49 pm
- Forum: Student Social/Study Group
- Topic: What do you miss / What are you looking forward to?
- Replies: 92
- Views: 10660
Re: What do you miss / What are you looking forward to?
I'm a freshman and I can't wait to actually be in person. Seeing some of my friends who are going to UCSD (where dorming hasn't been completely destroyed) having fun with their roomies just makes me want to be on campus even more.
- Sun Mar 07, 2021 10:46 pm
- Forum: General Rate Laws
- Topic: Intermediates
- Replies: 17
- Views: 1507
Re: Intermediates
If you write out all the steps of a reaction and add them together for the overall reaction, intermediates should cancel out because they exist in both the forward and reverse reactions (i.e. they are products of one step and reactants of another step). Therefore, for the overall reaction rate, inte...
- Sun Feb 28, 2021 8:21 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: E vs E naught
- Replies: 36
- Views: 1734
Re: E vs E naught
Eo is measured at 1 atm, 298 K, and 1 M. It is used to find cell potential under nonstandard condition, E, with ) .
.
- Sun Feb 28, 2021 8:16 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: n in ∆G = -nFE
- Replies: 80
- Views: 4514
Re: n in ∆G = -nFE
n refers to the number of e- being transferred. You can find this once you balance the equation (make sure the charges are balanced as well).
- Sun Feb 28, 2021 8:13 pm
- Forum: Student Social/Study Group
- Topic: Fave movie/show
- Replies: 67
- Views: 5096
Re: Fave movie/show
I recently watched Ride Your Wave and would recommend it. The final scene lives in my head RENT FREE.
- Sun Feb 28, 2021 8:10 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Sapling #16 Wk7/8
- Replies: 8
- Views: 1749
Re: Sapling #16 Wk7/8
Looking at the equation ) , we know Eo will stay the same, so it won't result in a change of E. Both n and ln(Q) double, so this doubling would "cancel out".
, we know Eo will stay the same, so it won't result in a change of E. Both n and ln(Q) double, so this doubling would "cancel out".
- Sun Feb 28, 2021 8:06 pm
- Forum: Balancing Redox Reactions
- Topic: Determining Phases
- Replies: 28
- Views: 1108
Re: Determining Phases
The problem should explicitly state some of the initial states of the species (s, l, g, aq). When adding H+ or OH-, those ions should be aq, and H2O as a product will most likely be l.
- Sun Feb 28, 2021 8:03 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Oxidizing Agent and value of V
- Replies: 3
- Views: 254
Re: Oxidizing Agent and value of V
V (reduction potential) refers to how likely a molecule wants to be reduced. Oxidizing agents oxidize a target molecule by removing electrons from the target molecule, meaning the oxidizing agent takes on those electrons and is reduced in the process. So a higher V means a stronger oxidizing agent.
- Sun Feb 28, 2021 7:58 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: anode vs. cathode
- Replies: 12
- Views: 746
Re: anode vs. cathode
Another mnemonic that is helpful when looking at an unlabeled diagram is r eduction happens on the r ight. I really liked the anode mnemonic that someone else said but another way of thinking about the same process is cathode as a "t" in it, which looks like a positive sign. This means ele...
- Thu Feb 18, 2021 11:13 am
- Forum: Student Social/Study Group
- Topic: The Good Stuff - Justin Workshop
- Replies: 1
- Views: 325
Re: The Good Stuff - Justin Workshop
not all heroes wear capes :')
- Wed Feb 17, 2021 11:36 pm
- Forum: Van't Hoff Equation
- Topic: How deltaG affects product/reactant formation
- Replies: 6
- Views: 742
Re: How deltaG affects product/reactant formation
If we look at the equation for \Delta G at standard condition, we get \Delta G^{o}=-RTln(K) . For \Delta G to be negative, thus meaning the reaction favors the products, we need ln(K) to produce a positive number. When K > 1, ln(K) < 0. When 0 < K < 1, ln(K) > 0. Sinc...
- Wed Feb 17, 2021 11:29 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Larger mass= higher molar entropy
- Replies: 4
- Views: 326
Re: Larger mass= higher molar entropy
Larger, more complex molecules (more particles, more possible energy states) will have high entropy values.
- Wed Feb 17, 2021 11:25 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Order and Stability
- Replies: 4
- Views: 255
Re: Order and Stability
Higher entropy means higher disorder and more possible arrangements. The entropy of gases > the entropy of liquids > the entropy of solids. However, when looking at whether a molecule is likely to form, we also need to take into account enthalpy and the temperature of the reaction to see if the form...
- Wed Feb 17, 2021 11:17 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Extensive properties
- Replies: 2
- Views: 215
Re: Extensive properties
Enthalpy, Gibbs Free Energy, heat capacity (not specific heat capacity), heat, and internal energy are dependent on the amount of substance.
- Wed Feb 17, 2021 11:05 pm
- Forum: Calculating Work of Expansion
- Topic: Reversible Expansion
- Replies: 2
- Views: 177
Re: Reversible Expansion
From what I understand, all reversible reactions are isothermal because the energy lost by the system doing work is at such a slow rate that heat from the surroundings replenishes the energy deficit. However, if a reaction is isothermal, this does not necessarily mean it will be reversible. There ar...
- Sun Feb 14, 2021 11:48 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Entropy change involved in phase change of water
- Replies: 3
- Views: 463
Re: Entropy change involved in phase change of water
From what I understand, water in ice form has a fairly strong crystalline structure which prevents a lot of movement of the individual water molecules. However, liquid water molecules have much more freedom in movement. Even though liquid water might be denser, the individual molecules can possibly ...
- Sun Feb 14, 2021 11:44 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: ln of T1/T2 in deltaS equation
- Replies: 2
- Views: 444
Re: ln of T1/T2 in deltaS equation
In Lec 13, we focused on \Delta S in an isothermal system undergoing reversible expansion (T was constant but V was changing). However, if T was changing, we would refer to the equation in Lec 14 where \Delta S=nCln(\frac{T_{2}}{T_{1}}) . For C, we would use a C P value if the pressure was c...
- Sun Feb 14, 2021 11:37 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Gas Entropy
- Replies: 14
- Views: 904
Re: Gas Entropy
Going from higher energy states to lower energy states will decrease disorder in the system but the released energy from the reaction will increase disorder in the surroundings. So yes, the entropy of a gas will decrease when it transitions into a liquid state.
- Sun Feb 14, 2021 11:34 pm
- Forum: Calculating Work of Expansion
- Topic: negative vs positive work
- Replies: 21
- Views: 992
Re: negative vs positive work
Since these values are taken from the perspective of the system, yes. A negative work value means the system has to "exert" itself and do work on the surroundings. A positive work value means the surroundings are doing work on the system.
- Sun Feb 14, 2021 11:33 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: higer molar entropy
- Replies: 13
- Views: 658
Re: higer molar entropy
The pressure is inversely related to volume. Low pressure results in a higher volume, which means more space for molecules to be in. With more possible positions for a molecule to be present, the entropy increases.
- Sun Feb 14, 2021 11:31 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Units for Gibbs Free Energy Calculations
- Replies: 6
- Views: 416
Re: Units for Gibbs Free Energy Calculations
Looking at the problems from Sapling, 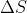 is generally J.K-1 while
is generally J.K-1 while  is in kJ. If you want you
is in kJ. If you want you  value to display as kJ, remember to dividing the entropy values by 1000. The main thing is to maintain consistency.
value to display as kJ, remember to dividing the entropy values by 1000. The main thing is to maintain consistency.
- Sun Feb 07, 2021 11:00 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Sapling #11 (Week 4-5)
- Replies: 5
- Views: 344
Re: Sapling #11 (Week 4-5)
For this problem, we will assume that all heat lost by the iron is gained by the water: 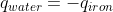 . From here, calculate
. From here, calculate  for both sides of the equation, with
for both sides of the equation, with 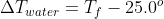 and
and  . Isolate and solve for Tf.
. Isolate and solve for Tf.
- Sun Feb 07, 2021 10:51 pm
- Forum: General Science Questions
- Topic: Study Tips for midterm
- Replies: 24
- Views: 1533
Re: Study Tips for midterm
I went to UA sessions (really good worksheets, questions, and explanations provided!) and also reviewed my discussion section problems to see I I could do them on my own.
- Sun Feb 07, 2021 10:49 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Negative Sign
- Replies: 16
- Views: 612
Re: Negative Sign
The side that starts with the higher temperature will have the negative because it will release heat as it reaches equilibrium with the other substance.
- Sun Feb 07, 2021 10:46 pm
- Forum: Student Social/Study Group
- Topic: Post Midterm 1...
- Replies: 39
- Views: 1873
Re: Post Midterm 1...
I found the UA sessions INCREDIBLY helpful, especially with bridging some gaps in knowledge because they came up with good questions. They're all really helpful UAs, but shoutout to Justin :). As said before, being able to do questions in an efficient manner does help with timing and not being stres...
- Sun Feb 07, 2021 10:39 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Sapling #20
- Replies: 9
- Views: 471
Re: Sapling #20
Looking at the equation, 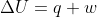 , we can figure out that w=0 since there is no change in volume. A change in volume indicates that work was either done by or on the system.
, we can figure out that w=0 since there is no change in volume. A change in volume indicates that work was either done by or on the system.
- Sun Feb 07, 2021 10:32 pm
- Forum: Student Social/Study Group
- Topic: Sapling wk 3/4 #18
- Replies: 2
- Views: 131
Re: Sapling wk 3/4 #18
From what I understand, the mathematical relationship is 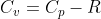 , so what you wrote would make sense as
, so what you wrote would make sense as 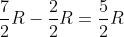 . So nice (educated) guess :)
. So nice (educated) guess :)
- Sun Feb 07, 2021 10:21 pm
- Forum: Calculating Work of Expansion
- Topic: Internal work and change in heat
- Replies: 3
- Views: 106
Re: Internal work and change in heat
To understand this, we can look at the equation 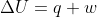 . Remember that w, whether the reaction is reversible or not, is dependent on volume change: (1)
. Remember that w, whether the reaction is reversible or not, is dependent on volume change: (1) ) , (2)
, (2) 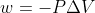 . If the volume doesn't change, w=0, so
. If the volume doesn't change, w=0, so 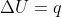 .
.
- Sun Feb 07, 2021 10:17 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Work Definition
- Replies: 33
- Views: 1185
Re: Work Definition
Work is the amount of energy needed to move something, which can be seen in the equation w=Fd . If you take this equation and F=PA , we can derive the equation for work on an irreversible reaction: w=-P\Delta V. . The negative is there because this equation looks at work from the perspective of the ...
- Sun Feb 07, 2021 10:07 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Molar Heat Capacities at Constant Volume/Pressure
- Replies: 3
- Views: 120
Re: Molar Heat Capacities at Constant Volume/Pressure
Specific heat capacities should be given in the problem. The equation sheet also gives various heat capacities for water at different physical states.
- Sun Feb 07, 2021 10:05 pm
- Forum: Calculating Work of Expansion
- Topic: Work Equations
- Replies: 3
- Views: 251
Re: Work Equations
Which wor function you'll need depends on if the reaction is reversible or not. Reversible reactions occur when a change in pressure happens at a very slow rate (the problem might also mention that the reaction is reversible). In this case, we'll use w=-nRTln(\frac{V_{2}}{V_{1}}) . A reactio...
- Sun Feb 07, 2021 9:57 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Wks 3 & 4 Sapling Q18
- Replies: 6
- Views: 383
Re: Wks 3 & 4 Sapling Q18
Kandyce Lance 3E wrote:For the constant pressure, why do we multiple 7/2?
The problem just tells us that the constant‑pressure molar specific heat for CO2(g) is equal to 7R/2. So we need to multiply the gas constant by 7/2 to get the correct constant‑pressure molar specific heat.
- Sun Feb 07, 2021 9:54 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Closed System
- Replies: 52
- Views: 2907
Re: Closed System
If a system is insulated, then no heat transfer can occur. This would be a trait on an isolated system where neither matter nor energy can be exchanged with the environment. A closed system is "isolated" in the sense that no matter can enter or exit the system, but energy can still be tran...
- Mon Feb 01, 2021 1:51 am
- Forum: Student Social/Study Group
- Topic: Work Life Balance
- Replies: 44
- Views: 1846
Re: Work Life Balance
One recommendation I have to make work/school time seem less exhausting to periodically take a short break to watch an episode of a show you are watching. A general rule you could follow is 40 minutes of studying followed up with one ~20-minute episode. Rinse and repeat. Doing this can also motivate...
- Mon Feb 01, 2021 1:47 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Taking the Anti-Log
- Replies: 37
- Views: 2685
Re: Taking the Anti-Log
Since pKa is -log(Ka), Ka would be 10^-(pKa). This process would also work for going between pKb and Kb, pH and [H3O+], and pOH and [OH-].
- Mon Feb 01, 2021 1:40 am
- Forum: Ideal Gases
- Topic: Temperature
- Replies: 99
- Views: 7030
Re: Temperature
If the temperature isn't given, I believe we just assume it's at the standard 25oC.
- Mon Feb 01, 2021 1:39 am
- Forum: Calculating Work of Expansion
- Topic: Gas Constant
- Replies: 13
- Views: 865
Re: Gas Constant
For determining which gas constant to use, look at the values given in the problem and what the unit of measurement the answer should be in. The correct gas constant will cancel out all variables present and leave you with just the unit of measurement of the answer.
- Mon Feb 01, 2021 1:37 am
- Forum: Phase Changes & Related Calculations
- Topic: endothermic/exothermic
- Replies: 43
- Views: 4769
Re: endothermic/exothermic
If a reaction requires energy, such as going from a low energy state to a high energy state (ex. solid to liquid), it is endothermic. If a reaction is going from a high energy state to a low energy state (liquid to solid), it will release energy and be exothermic.
- Sun Jan 24, 2021 11:23 pm
- Forum: Phase Changes & Related Calculations
- Topic: Define Phase Change
- Replies: 78
- Views: 5531
Re: Define Phase Change
A phase change is a general term for the various processes (sublimation, vaporization, melting/fusion, etc.) where a substance changes its state.
- Sun Jan 24, 2021 11:09 pm
- Forum: Phase Changes & Related Calculations
- Topic: when to assume x is insignificant
- Replies: 86
- Views: 7837
Re: when to assume x is insignificant
Another test to see if the approximation is acceptable is to check (after approximation) x < 5% of the initial concentration where x = the final concentration (so taking the ionization/protonation %).
- Sun Jan 24, 2021 11:04 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Hess's Law
- Replies: 58
- Views: 3158
Re: Hess's Law
Hess's Law states that enthalpy is a state function. This means that values of enthalpy can be added/subtracted because state properties are not dependent on the path taken to obtain a state.
- Sun Jan 24, 2021 11:01 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Ice Tables
- Replies: 28
- Views: 1102
Re: Ice Tables
If a concentration is given as the initial, that molecule will likely decrease. So use -x for that molecule and all other molecules on that side of the reaction. Molecules on the other side of the reaction will have a +x and have an initial concentration of 0.
- Sun Jan 24, 2021 10:59 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Pka, Ph, charged and neutral species
- Replies: 7
- Views: 420
Re: Pka, Ph, charged and neutral species
Look's good to me!
- Sun Jan 24, 2021 10:57 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: H2O as a Gas
- Replies: 69
- Views: 6872
Re: H2O as a Gas
When water is the solvent, it's ignored. When it is involved in the reaction (ex. being in gas form), it is included in calculations.
- Sun Jan 17, 2021 11:57 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: ICE Box
- Replies: 10
- Views: 540
Re: ICE Box
Use the initial value given for a molecule. This initial will most likely decrease during the reaction, so you know it will be negative. Based on which side of the equation it is on, make sure all other molecules on the same side as the molecule with the initial value will decrease and all species o...
- Sun Jan 17, 2021 11:54 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: equilibrium shifts: left of right?
- Replies: 13
- Views: 721
Re: equilibrium shifts: left of right?
Le Chatelier's principle would tell us that the reaction would shift to the right because it wants to return to equilibrium. If there is an increase in the concentration of reactants, more product must be formed to maintain the same K value at that temperature.
- Sun Jan 17, 2021 11:51 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kw Uses
- Replies: 6
- Views: 227
Re: Kw Uses
Kw can be used for problems regarding the concentrations of acids and bases and pH. So yea, but it can be used in related scenarios.
- Sun Jan 17, 2021 11:49 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kc and Kp and Keq and Q
- Replies: 6
- Views: 1273
Re: Kc and Kp and Keq and Q
All the K's are equilibrium constants. Kc denotes the equilibrium constant when using concentrations. Kp denotes the equilibrium constant when using partial pressures of gases. Keq is can denote the equilibrium constant of an equation. Q is the reaction quotient, which might not be an equilibrium. I...
- Sun Jan 17, 2021 11:45 pm
- Forum: Phase Changes & Related Calculations
- Topic: Determining Stronger Acids
- Replies: 18
- Views: 759
Re: Determining Stronger Acids
The stronger acid will have a comparatively smaller pKa.
- Sun Jan 10, 2021 9:00 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Concentrations
- Replies: 9
- Views: 494
Re: Concentrations
Concentration should be given.
If not, moles of the P/R and the volume of the solution might be given. In this case,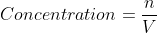 .
.
If not, moles of the P/R and the volume of the solution might be given. In this case,
- Sun Jan 10, 2021 8:55 pm
- Forum: Ideal Gases
- Topic: n/V = concentration
- Replies: 19
- Views: 2428
Re: n/V = concentration
That part of the lecture was talking about how you can convert between partial pressures and concentrations for gases. PV=nRT is the ideal gas law. If we rearrange it to P=\frac{nRT}{V} , we notice that n/V is just concentration ( M=\frac{mol}{L} ). Therefore, if we ever need to convert partial pres...
- Sun Jan 10, 2021 8:51 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Water
- Replies: 28
- Views: 1050
Re: Water
Any solvents, liquids, and solids are not included in concentration calculations. So, unless the water is in gas form, its concentration is not used because it either doesn't have a concentration (such as when it is a liquid or solid precipitate) or has a negligible concentration (such as when it is...
- Sun Jan 10, 2021 8:47 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: K and pressure
- Replies: 5
- Views: 334
Re: K and pressure
To add to what's already been said, for gases, a pressure increase can be caused by either a decrease in volume or the addition of inert gases. If the volume decreases, the side of the reaction with more moles has is favored because its concentration will increase faster. If the pressure increase is...
- Sun Jan 10, 2021 8:40 pm
- Forum: Ideal Gases
- Topic: Ideal Gas Equation units
- Replies: 4
- Views: 310
Re: Ideal Gas Equation units
P represents pressure and can be measured in atm, bar, or Torr. V represents volume and needs to be in L. n represents the number of moles. R is the gas constant and can differ depending on which unit of pressure you are using. The constants and equation sheet for this class has all of them, so you ...
- Fri Dec 11, 2020 9:09 pm
- Forum: Biological Examples
- Topic: Iron in Heme Coordination Number
- Replies: 4
- Views: 911
Re: Iron in Heme Coordination Number
In the week 9, Monday lecture, Dr. Lavelle explained that the porphyrin ring (a chelating ligand structure) fills up 4 of these sites, creating the heme structure. The fifth site is occupied by histidine, an amino acid in the myoglobin. The sixth site is what oxygen would bind to so that oxygen can ...
- Fri Dec 11, 2020 8:53 pm
- Forum: Student Social/Study Group
- Topic: Plans for Relaxing After Finals
- Replies: 98
- Views: 16872
Re: Plans for Relaxing After Finals
Lillian wrote:I'm going to sleep until my organs fail, obviously.
how can i learn this skill
- Fri Dec 11, 2020 8:38 pm
- Forum: Trends in The Periodic Table
- Topic: Finding Charge
- Replies: 4
- Views: 440
Re: Finding Charge
Because transition metals can have multiple oxidation states, the charge of a d-block atom will depend on the molecule it's in. For example, in [AgCl 2 ] - we know that the charges of all the atoms of Ag and Cl must add up to 1-. Since the charge of a chloride ion is 1-, gold must have an oxidation ...
- Fri Dec 11, 2020 8:28 pm
- Forum: Student Social/Study Group
- Topic: Struggling on topics
- Replies: 6
- Views: 439
Re: Struggling on topics
Practice and watching videos are a great way to "consume" the needed information. But to really get it down, I try to teach myself or someone else the concept to fully "digest" it. So I'd recommend not just reviewing material, but also testing if you could explain it without the ...
- Fri Dec 11, 2020 8:25 pm
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: Equilibrium constant
- Replies: 5
- Views: 271
Re: Equilibrium constant
Just to clarify, will we be expected to calculate equilibrium constants or just have a basic understanding of them? I believe that equilibrium constants are discussed further in Chem 14B. A general understanding of how and why they work should do but the basics of actually calculate them aren't too...
- Fri Dec 11, 2020 8:20 pm
- Forum: Bronsted Acids & Bases
- Topic: pH of Salt Solutions
- Replies: 2
- Views: 234
Re: pH of Salt Solutions
To add on to what's been said, weak acids will combine with water, specifically H+ ions, leaving behind OH- in solution, thus resulting in a basic solution. Weak bases will combine with water, specifically OH-, leaving behind H+/H 3 O+ in solution, thus resulting in an acidic solution. This is all b...
- Fri Dec 11, 2020 8:14 pm
- Forum: Student Social/Study Group
- Topic: Tips for Staying Focused
- Replies: 64
- Views: 4205
Re: Tips for Staying Focused
I like listening to calming music when studying, especially Lo-Fi. I have heard the opposite for some people (listen to intense music or no music at all). I know the final is in 2 days but it would be helpful to find what works for you.
- Sun Dec 06, 2020 9:41 pm
- Forum: *Molecular Orbital Theory Applied To Transition Metals
- Topic: Transition metals
- Replies: 11
- Views: 1106
Re: Transition metals
Transition metals are those listed in the d-block. According to the IUPAC, they're atoms with incomplete d sub-shell or can give rise to cations with an incomplete d sub-shell. For this class, elements of the first row of the d-block are the only transition metals to familiarize yourself with (parti...
- Sun Dec 06, 2020 9:35 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Coordination Number
- Replies: 5
- Views: 438
Re: Coordination Number
To find the coordination number, you need to find out how many times each ligand can bind with the central metal. For example, the N on NH 3 has a lone pair that can be donated. Since it has one lone pair on one atom, it can only have one coordinate covalent bond with the central atom. Therefore, (N...
- Sun Dec 06, 2020 9:19 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: HW Question coordination number
- Replies: 4
- Views: 189
Re: HW Question coordination number
SO4 is capable of having 1 coordinate covalent bond with our central metal, Co. NH3 is capable of having 1 as well. (NH3)5 therefore contributes 5 to the coordination number. This means that Co has 6 coordinate bonds to other atoms/molecules, thus a coordination number of 6.
Hope this helps.
Hope this helps.
- Sun Dec 06, 2020 9:16 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Determining Oxidation Numbers
- Replies: 6
- Views: 519
Re: Determining Oxidation Numbers
Always start by identifying the charge of the atoms/molecules directly bound to the metal. Then compare that to the overall charge of the larger complex, because the charge of the metal (oxidation number) plus the charge of the other atoms/molecules should equal the overall charge. In the case where...
- Sun Dec 06, 2020 9:09 pm
- Forum: Naming
- Topic: "(en)" Sapling
- Replies: 19
- Views: 972
Re: "(en)" Sapling
(en) is short for ethylenediamine (H2NCH2CH2NH2). It is a bidentate ligand (from the two nitrogens) and contributes 2 to the coordination number of the central atom.
Hope this helps.
Hope this helps.
- Sun Nov 29, 2020 8:32 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Sapling #20 AsO43- polarity
- Replies: 6
- Views: 304
Re: Sapling #20 AsO43- polarity
AsO 4 3- is nonpolar because it has resonance structures. In theory, any of the O could be double-bonded, so any 3 O could have that 1- charge. These electrons are delocalized and could exist on any of the oxygens at any specific time. This, in combination with the symmetrical arrangement of O aroun...
- Sun Nov 29, 2020 8:26 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Resonance Structure Definition
- Replies: 9
- Views: 519
Re: Resonance Structure Definition
Same atom connectivity refers to compounds arranging their atoms in the same way. This makes sense for resonance structures because atom arrangement is the same, only the electrons are moving. Between each resonance structure, the overall charge stays the same but FC per atom might differ between di...
- Sun Nov 29, 2020 8:09 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: How do bonds affect shapes?
- Replies: 11
- Views: 750
Re: How do bonds affect shapes?
When determining the shape, identify all regions of electron density around the central atom. These would include bonded atoms (it doesn't matter what type of bond because, in this case, they are all considered regions of electron density) and lone pairs. From there, you can figure out the most opti...
- Sun Nov 29, 2020 8:04 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bond angles
- Replies: 7
- Views: 298
Re: Bond angles
To add on to what Jeffrey said, lone pairs will slightly distort bond angles because they occupy a larger volume of electron density than a normal bond. In a normal bond, shared electrons are "confined" in-between two positively charged nuclei. Lone pairs, on the other hand, are not constr...
- Sun Nov 29, 2020 7:58 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Polarity
- Replies: 17
- Views: 736
Re: Polarity
To add on to what's been said, keep in mind resonance structures can also contribute to symmetry and nonpolar character. For example, CO 3 2- is nonpolar. Although it seems there is a highly electronegative pull between the two O 1- because there are resonance and a symmetrical structure, the molecu...
- Sun Nov 22, 2020 10:57 pm
- Forum: Resonance Structures
- Topic: Resonance Structures and Energy
- Replies: 21
- Views: 1047
Re: Resonance Structures and Energy
Yes. Molecules want to be stable, so the resonance structure with the lowest energy will be preferred, for it is most stable.
- Sun Nov 22, 2020 10:43 pm
- Forum: Ionic & Covalent Bonds
- Topic: Ionization Energy
- Replies: 19
- Views: 837
Re: Ionization Energy
The outermost electrons will be the electrons that are most affected by another atom of high electronegativity. Since the inner electrons are shielding the outermost electrons for the full nuclear attraction of the nucleus, they are the easiest to remove.
- Sun Nov 22, 2020 10:40 pm
- Forum: Trends in The Periodic Table
- Topic: Exceptions to Trends in atomic radius
- Replies: 1
- Views: 551
Re: Exceptions to Trends in atomic radius
Looking at Dr. Lavelle's lecture from 10/28/2020, Oxygen (radius = 68 pm) does have a smaller radius than nitrogen (radius = 75 pm). However, there seems to be a couple of anomalies, such as Mg being larger than Na; Pb being larger than Tl. I don't think these exceptions are too big of a deal though...
- Sun Nov 22, 2020 10:31 pm
- Forum: Lewis Structures
- Topic: Expanded Valence
- Replies: 11
- Views: 512
Re: Expanded Valence
Expanded octets can occur in p-block elements in row 3 or below. This is because they have an unoccupied d-orbital that can accept more elections than the conventional octet rule. The most common ones you'll see are Sulfur, Phosphorus, and Chlorine.
- Sun Nov 22, 2020 10:25 pm
- Forum: Bond Lengths & Energies
- Topic: Boiling/Melting Point
- Replies: 29
- Views: 1332
Re: Boiling/Melting Point
Intermolecular forces because it is the force between or "inter" molecules are responsible for holding materials together. Melting and boiling would cause these forces to weaken. Thus, we go from solid --> liquid --> gas.
- Sun Nov 15, 2020 7:09 pm
- Forum: Octet Exceptions
- Topic: Nitrogen
- Replies: 4
- Views: 167
Re: Nitrogen
Nitrogen will follow the octet rule. The main exceptions to keep in mind are H and He might only have 2 valence electrons; Li and Be might only have 4 valence electrons; B might only have 6 valence electrons; and P, S, and Cl can have octets larger than 8 due to having an empty d-orbital. Hope this ...
- Sun Nov 15, 2020 7:05 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Sapling #20
- Replies: 12
- Views: 687
Re: Sapling #20
Hydrogen bonding does not occur between CH 3 CHO molecules because there are no positively charged hydrogens present. H must be covalently bonded to an N, O, or F in its own molecule to hydrogen bond with neighboring molecules. Dipole-dipole interactions are still possible since the oxygen is slight...
- Sun Nov 15, 2020 6:58 pm
- Forum: Ionic & Covalent Bonds
- Topic: Hydrogen Bonding
- Replies: 13
- Views: 448
Re: Hydrogen Bonding
On top of the fact that N, O, and F are very electronegative, which does cause them to have a slightly negative charge, for H bonding to occur, the H must also be slightly positive. H in methane can't hydrogen bond because there isn't a sufficient electrostatic difference between C and H, but H in w...
- Sun Nov 15, 2020 6:52 pm
- Forum: Lewis Structures
- Topic: Sapling weeks 5/6 Question 4
- Replies: 6
- Views: 376
Re: Sapling weeks 5/6 Question 4
The wording of this question also confused me at first. I'll use the carbon-nitrogen bond as an example to explain what this means. In this question, overwhelming, such as in "overwhelming C-N bond character", means that the size of the actual carbon-nitrogen bond in the question was much,...

