Search found 102 matches
- Fri Mar 12, 2021 3:02 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Lecture Review Question
- Replies: 2
- Views: 308
Re: Lecture Review Question
I couldn't think of any way to solve it unless we are given more information. We either need values for the enthalpy and entropy or the values for Gibbs free energy of formation for the products and reactants. Depending on what we are given, we take one of those approaches to the problem.
- Fri Mar 12, 2021 2:59 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Orders of reactants
- Replies: 14
- Views: 819
Re: Orders of reactants
The reaction order also tells us what units we will expect for k as well as how much the rate will change when the concentration of one of the products is changed.
- Fri Mar 12, 2021 2:57 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Change in k in response to temperature change
- Replies: 2
- Views: 171
Re: Change in k in response to temperature change
Think about how the reaction with the lower Ea can proceed easier without an increase in temperature because it takes less energy than the reaction with the higher Ea that cannot. So when you increase the temperature, it makes much more of a difference to the reaction with the higher Ea because it w...
- Fri Mar 12, 2021 2:52 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Calculating standard cell potentials
- Replies: 4
- Views: 422
Re: Calculating standard cell potentials
We did use this equation, but since Mg2+(aq) + 2e- -> Mg (s) has a lower reduction potential, it is going to be oxidized meaning it is the anode. So when we subtract the values: -1.66V - (-2.36V), the sign changes because we are subtracting a negative number. So it ends up being -1.66+2.36 which giv...
- Fri Mar 12, 2021 2:49 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: cell diagram from lecture
- Replies: 3
- Views: 252
Re: cell diagram from lecture
I'm not quite sure, but regardless of its state, the AgBr still has an Ag+ ion but the ion is solid. So in this case the ion is solid, but regardless is an ion.
- Thu Mar 04, 2021 11:27 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagrams and Amount of Species
- Replies: 1
- Views: 139
Re: Cell Diagrams and Amount of Species
Since in the example you mentioned the MnO4- and Mn2+ are both part of the cathode so they are written on the same side, and because they are both in the same phase (aq) they are separated by a comma. Because neither of them is a solid, we see the need to write Pt(s) in the reaction as well in order...
- Thu Mar 04, 2021 11:24 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagrams Shorthand
- Replies: 2
- Views: 185
Re: Cell Diagrams Shorthand
My TA had this helpful overview
• Salt bridge: Two vertical lines (||)
• Different phase: One vertical line (I)
• Same phase: One coma (,)
Diagram order:
Inert Metal – Anode – salt bridge - Cathode – Inert Metal
• Salt bridge: Two vertical lines (||)
• Different phase: One vertical line (I)
• Same phase: One coma (,)
Diagram order:
Inert Metal – Anode – salt bridge - Cathode – Inert Metal
- Thu Mar 04, 2021 11:18 am
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: first order of rxns
- Replies: 14
- Views: 762
Re: first order of rxns
Yes, that is the definition of a first oder reaction because the n value has to be one or else it is not first order.
- Thu Mar 04, 2021 11:16 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: E naught
- Replies: 4
- Views: 308
Re: E naught
No, because the cell potential is the potential an electron experiences, the coefficients of the equation have no effect on this.
- Thu Mar 04, 2021 11:13 am
- Forum: Balancing Redox Reactions
- Topic: Oxidation and Reduction
- Replies: 11
- Views: 832
Re: Oxidation and Reduction
For example, if you have Au +(aq) --> Au(s) + Au 3+(aq), you can see how Au+ is both reduced to Au(s) because it gains an electron but also is oxidized to Au3+ because it loses 2 electrons. Both of these instances occur in the same reaction.
- Fri Feb 26, 2021 10:58 am
- Forum: Interesting Applications: Rechargeable Batteries (Cell Phones, Notebooks, Cars), Fuel Cells (Space Shuttle), Photovoltaic Cells (Solar Panels), Electrolysis, Rust
- Topic: Corrosion Prevention
- Replies: 5
- Views: 412
Re: Corrosion Prevention
Since corrosion happens when a substance is oxidized or exposed to oxygen and it loses electrons and gets smaller, it helps to put a surface in between the substance and the surroundings so that the air will not oxidize the substance. A common example is paint, but for a more chemical approach you c...
- Fri Feb 26, 2021 10:55 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Anode/Cathode
- Replies: 45
- Views: 1622
Re: Anode/Cathode
Yes, even if the cell itself might have the two flipped, you always put the anode on the left and the cathode on the right to show the order the electrons are flowing.
- Fri Feb 26, 2021 10:54 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Question About Cell Diagram Order Sapling #7 Week 7/8
- Replies: 3
- Views: 234
Re: Question About Cell Diagram Order Sapling #7 Week 7/8
This essentially means that you write in order of what touches what. In other words, in this case, the solid electrode is touching the crystals that are forming on the metal, and those are touching the aqueous solution ions. So for example you might put Pb(s)|PbCl2(s)|Cl-(aq) since from the diagram,...
- Fri Feb 26, 2021 10:49 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Writing Cell Diagrams
- Replies: 5
- Views: 358
Re: Writing Cell Diagrams
The general outline is that the anode is always placed on the left side, and the cathode is placed on the right side. The salt bridge is represented by double vertical lines. So it will look like : Electrode material being oxidized (anode) | aqueous species || Aqueous species | Electrode material be...
- Fri Feb 26, 2021 10:46 am
- Forum: Balancing Redox Reactions
- Topic: Balancing Redox: Leftover H+ or OH-
- Replies: 4
- Views: 275
Re: Balancing Redox: Leftover H+ or OH-
Yes, you are allowed to have leftover OH- and H+. Which one you have is determined by if it is in acidic or basic solution, and to convert from acidic to basic you just add OH- which becomes H2O on one side and then OH- on the other indicating basicity.
- Tue Feb 16, 2021 12:48 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Entropy Change with Pressure
- Replies: 3
- Views: 291
Re: Entropy Change with Pressure
Since delta S = nR*ln(V2/V1) and pressure and volume are inverses in the ideal gas law equation, you can substitute the inverse for pressure into the equation which gives delta S = nR*ln(P1/P2).
- Tue Feb 16, 2021 12:43 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: When to Use An Equation
- Replies: 3
- Views: 277
Re: When to Use An Equation
We use this equation (delta U = 3/2 nR delta T) when we are solving for the total internal energy of an ideal gas. But if we are trying to find the internal energy for any other system that is not an ideal gas, we use delta U = w + q.
- Tue Feb 16, 2021 12:39 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Difference Between Delta G Knot and Delta G
- Replies: 3
- Views: 285
Re: Difference Between Delta G Knot and Delta G
Detla G naught implies standard conditions (25 C, 1atm) but if that is not the case then you would just refer to it as delta G.
- Tue Feb 16, 2021 12:38 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Textbook 4H.9
- Replies: 2
- Views: 202
Re: Textbook 4H.9
Container A is the highest because it has the most particles because that one mol is monoatomic so there are 6.022x10^23 atoms in that container which makes it higher than B and C who are both diatomic meaning there are 3.011x10^23 atoms in each. But since C is vibrationally active, it is higher tha...
- Tue Feb 16, 2021 12:35 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: 4G3
- Replies: 2
- Views: 234
Re: 4G3
To add on to what was already said, the BF3 molecule only has one appearance regardless of how you draw it, it will always look the same. But if you think about the possibilities for COF2, the orientation of the O and 2 Fs around the carbon has more options which means greater disorder even though t...
- Wed Feb 10, 2021 1:36 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: ΔG° vs ΔG
- Replies: 20
- Views: 825
Re: ΔG° vs ΔG
Every time you see the ° that means the system is standard (25 C or 298 K and 1 atm) but whenever the system has a different temperature or pressure than you just use G not G°.
- Wed Feb 10, 2021 1:33 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Today's lecture
- Replies: 5
- Views: 281
Re: Today's lecture
Yes, to add on to what others have said entropy of the universe is the same as entropy of the system plus entropy of the surroundings which is the total entropy.
- Wed Feb 10, 2021 1:32 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Sapling 4I.1
- Replies: 1
- Views: 237
Re: Sapling 4I.1
Yes, that was the same reasoning I used when solving this. Basically when it's lost its negative and the colder temp one (200K) gains it so it is positive.
- Wed Feb 10, 2021 1:05 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Entropy of Surroundings
- Replies: 4
- Views: 278
Re: Entropy of Surroundings
From what I remember, the temperature of which he spoke was the temperature the surroundings approach after the reaction goes to completion.
- Wed Feb 10, 2021 1:03 pm
- Forum: Student Social/Study Group
- Topic: Week 6
- Replies: 5
- Views: 359
Re: Week 6
The best way for me to make sure I pay attention is to watch the lecture and write down not just what is on the slides but more so the things he says because that is what really helps understand. Forcing yourself to copy the material while listening to it is a really good way to solidify information.
- Wed Feb 03, 2021 12:30 pm
- Forum: Calculating Work of Expansion
- Topic: Work Formulas
- Replies: 5
- Views: 182
Re: Work Formulas
I agree with what was stated above, the V can never be 0 because all matter takes up space, but the delta V can be 0 if the system is isolated.
- Wed Feb 03, 2021 12:21 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Work Equation
- Replies: 3
- Views: 239
Re: Work Equation
The work function is negative because the system is doing work, using energy, and losing it to the surroundings. So the work function for the system will be negative if the gas expands. But if the gas is compressed, work is done on the system (not by the system itself) so it will be a positive value.
- Wed Feb 03, 2021 12:18 pm
- Forum: Calculating Work of Expansion
- Topic: How does an ideal gas expand?
- Replies: 4
- Views: 260
Re: How does an ideal gas expand?
Basically, the system responds to a change in pressure to match the pressure of the surroundings. So yes, the two will be equal but only if the system adjusts to the surroundings, even if infinitesimally.
- Wed Feb 03, 2021 12:15 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Degeneracy (W)
- Replies: 7
- Views: 279
Re: Degeneracy (W)
I agree, I don't think it does.
- Wed Feb 03, 2021 12:13 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Microstates
- Replies: 16
- Views: 1118
Re: Microstates
In Lavelle's lecture from today (2/3), he talks about a 2 particle system with 4 microstates and says the degeneracy would be W=4=2^2. I'm a little confused by this, because in the discussion above, it says that W=X^n, where X is the number of microstates and n is the number of particles. So, would...
- Thu Jan 28, 2021 12:00 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kc vs Kp
- Replies: 3
- Views: 94
Re: Kc vs Kp
Adding on, to convert between them is super simple if you are given one but need the other. Kp=Kc(RT)^(delta_n) where delta_n is the change in the number of moles (moles products - moles reactants)
- Thu Jan 28, 2021 12:00 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kc vs Kp
- Replies: 3
- Views: 94
Re: Kc vs Kp
Adding on, to convert between them is super simple if you are given one but need the other. Kp=Kc(RT)^(delta_n) where delta_n is the change in the number of moles (moles products - moles reactants)
- Thu Jan 28, 2021 11:58 am
- Forum: Phase Changes & Related Calculations
- Topic: when to assume x is insignificant
- Replies: 86
- Views: 7804
Re: when to assume x is insignificant
We can assume x is insignificant when the equilibrium constant is less that 10^-4.
- Thu Jan 28, 2021 11:56 am
- Forum: Phase Changes & Related Calculations
- Topic: Define Phase Change
- Replies: 78
- Views: 5525
Re: Define Phase Change
To add on to what everyone else has said, when you look at a phase change graph, each new state is represented as a line with a new slope. So when the slope of the line changes, the phase is changing for that substance.
- Thu Jan 28, 2021 11:54 am
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Chem 14B Midterm Week 4
- Replies: 3
- Views: 164
Re: Chem 14B Midterm Week 4
Just study lectures from weeks 1-3, the cutoff is Friday the 22nd.
- Wed Jan 27, 2021 10:06 pm
- Forum: Student Social/Study Group
- Topic: Spring 2021
- Replies: 106
- Views: 16714
Re: Spring 2021
I am taking it right now with 14B and it's really not too hard. The online format involves a lot of simulations but I feel as though I understand the ideas even though we don't get the "hands on" aspect.
- Thu Jan 21, 2021 10:24 am
- Forum: Phase Changes & Related Calculations
- Topic: Steam vs. Water
- Replies: 9
- Views: 413
Re: Steam vs. Water
So basically the temperature does not change during the phase change but after the liquid is completely switched to vapor and more heat is still added then the temperature changes? You can see this by looking at the slope of the heating curve graph. During phase changes, the slope of the graph is f...
- Thu Jan 21, 2021 10:20 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Acids and Bases
- Replies: 8
- Views: 370
Re: Acids and Bases
Since we are focused on the donation or acceptance of protons (not electrons) at least with regard to pH and Ka calculations, I would assume that yes, we are more focused on Bronsted-Lowry which donate and accept protons instead of Lewis which donate and accept electrons.
- Thu Jan 21, 2021 10:09 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: C in ICE Box
- Replies: 19
- Views: 752
Re: C in ICE Box
The change for products is negative because they are going to form reactants. Because reactants are being formed, their C is positive.
- Thu Jan 21, 2021 10:08 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: When would K be unchanged?
- Replies: 31
- Views: 1224
Re: When would K be unchanged?
Since K is the equilibrium constant at a certain temperature, changing the temperature changes K to a new value for that new temperature.
- Thu Jan 21, 2021 10:06 am
- Forum: Phase Changes & Related Calculations
- Topic: phase transition
- Replies: 13
- Views: 426
Re: phase transition
When a substance is heated, the heat has to go somewhere. So for phase changes, the heat goes into breaking the bond. We might think at first that heating raises its temperature, but that is actually not true because all the heat is going into breaking the bonds.
- Fri Jan 15, 2021 5:07 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Using Kw
- Replies: 5
- Views: 212
Re: Using Kw
The most helpful for me was to think Kw constant allows us to find hydronium ion concentration or hydroxide concentration when only one is given.
- Fri Jan 15, 2021 5:05 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Textbook Problem 5I #15
- Replies: 4
- Views: 441
Re: Textbook Problem 5I #15
It would be an ice table but instead of starting with 0 for your initial concentration of products, you start with .2M. Then you add to the left and subtract from the right and solve as you would normally! Don’t forget that you only ever include aqueous and gaseous in your equilibrium constant.
- Fri Jan 15, 2021 5:03 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Sapling Week 1 #9
- Replies: 7
- Views: 396
Re: Sapling Week 1 #9
Be sure you are using 2x and also remembering to square the concentration of NO!! I forgot that the first time around.
- Fri Jan 15, 2021 5:01 pm
- Forum: Administrative Questions and Class Announcements
- Topic: acids and bases on the midterm
- Replies: 6
- Views: 697
Re: acids and bases on the midterm
The acids and bases we learned in 14A are those typically suggested to memorize and have a solid understanding of. I would say that knowing the more common ones can only help you!
- Thu Jan 14, 2021 3:10 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: pKA and pH
- Replies: 8
- Views: 246
Re: pKA and pH
Instead of equating the two (pKa and pH), the main idea is that they have the same relationship as each other with regard to pKb and pOH respectively.
- Fri Jan 08, 2021 9:37 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Sapling Week 1&2 Homework Question
- Replies: 8
- Views: 509
Re: Sapling Week 1&2 Homework Question
If you want a full explanation for this question, the chemical equilibrium part 4 video on Lavelle's website was helpful and describes the relationship between temperature increase, product and reactant increase, and the value of Q. It starts at 37:50 and the link is https://lavelle.chem.ucla.edu/wp...
- Fri Jan 08, 2021 9:32 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Chemical Equilibrium Part 2 Post Module #29
- Replies: 1
- Views: 170
Re: Chemical Equilibrium Part 2 Post Module #29
To set up this problem, you need to make an ICE table so for initial conditions BrCl=1.84 x 10^-4 M Br2=0M and CL2=0 M. Then, you know that 18.3% of BrCl gas remains at equilibrium, so you can find the equilibrium concentration of BrCl by multiplying its initial concentration by 0.183. Doing so you ...
- Fri Jan 08, 2021 9:26 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Water
- Replies: 28
- Views: 1046
Re: Water
Only include aqueous and gaseous substances in an ICE table, never a liquid or a solid. So just make sure you double check which phase it is in.
- Thu Jan 07, 2021 9:30 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: R value
- Replies: 5
- Views: 377
Re: R value
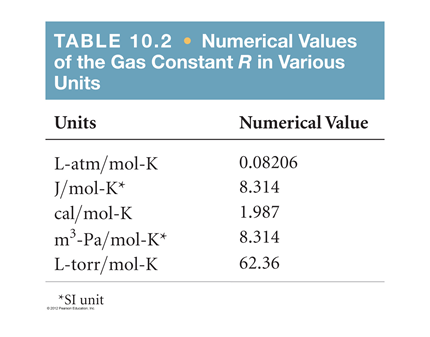
These are some useful tables to keep track of R values and units given.
- Thu Jan 07, 2021 9:25 pm
- Forum: Ideal Gases
- Topic: Finding x for K using quadratic equation
- Replies: 6
- Views: 267
Re: Finding x for K using quadratic equation
When you get two values for X, ignore a value that is negative as well as a value that is greater than the initial reactant concentration or pressure. In other words, the value of X should be positive and smaller than the initial concentrations on the left side of the reaction.
- Fri Dec 11, 2020 10:49 pm
- Forum: Hybridization
- Topic: AsF³ Hybridization
- Replies: 6
- Views: 741
Re: AsF³ Hybridization
I think you are correct that it is sp3. As has 5 valence electrons and F has 7. So 5+7x3= 26 electrons. In general, you can think of having a central atom and know that you only need to pair three atoms around it for this problem because there are only 3 Fs. So knowing that the valence for each F wi...
- Fri Dec 11, 2020 10:43 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bent vs Angular
- Replies: 20
- Views: 968
Re: Bent vs Angular
Angular and Bent refer to the same molecular geometry and can be used interchangeably.
- Fri Dec 11, 2020 10:40 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Why is CH2Cl2 polar
- Replies: 6
- Views: 481
Re: Why is CH2Cl2 polar
Rachel Kho Disc 1I wrote:Wait so does this also mean that tetrahedral molecules are usually polar? Are there any examples of nonpolar tetrahedral molecules?
CCl4 is an example of a molecule that is tetrahedral and nonpolar.
- Fri Dec 11, 2020 10:37 pm
- Forum: Dipole Moments
- Topic: identifying polarity from a dipole moment
- Replies: 2
- Views: 469
Re: identifying polarity from a dipole moment
It helps to identify when the molecule is not symmetrical, because this usually means it has a dipole moment. To see where the arrow points (towards the more negative) you have to think about which element is more electronegative. Hope this helps! https://www.chemtopper.com/myblog/wp-content/uploads...
- Fri Dec 11, 2020 10:23 pm
- Forum: Student Social/Study Group
- Topic: Final
- Replies: 18
- Views: 941
Re: Final
There's typically at least 2 or 3 textbook problems so it helps to make sure you understand them. But also many of the problems are written by the TA's too so it's a balance.
- Sat Dec 05, 2020 10:12 am
- Forum: General Science Questions
- Topic: study methods/recs
- Replies: 37
- Views: 2436
Re: study methods/recs
Something I find helpful for conceptual questions is going back through those learning outcomes sheets on the chem 14A Lavelle website and making sure I not only understand but can explain the list of concepts on the bottom and actually have a grasp of what they mean and how they can be applied.
- Sat Dec 05, 2020 10:10 am
- Forum: Naming
- Topic: Naming Order
- Replies: 16
- Views: 743
Re: Naming Order
The order of the ligands does not really matter because they will be alphabetized when naming the compound anyways. As long as they come after the transition metal cation (central atom) then you should be okay.
- Sat Dec 05, 2020 10:04 am
- Forum: Naming
- Topic: what does (en) mean?
- Replies: 23
- Views: 10502
Re: what does (en) mean?
en stands for ethylenediamine, is a bidentate ligand (involves 2 electron pairs), is a neutral molecule, and has the molecular formula NH2CH2CH2NH2.
- Sat Dec 05, 2020 9:58 am
- Forum: General Science Questions
- Topic: Enrolling in Chem 14B and BL simultaneously?
- Replies: 14
- Views: 1741
Re: Enrolling in Chem 14B and BL simultaneously?
I heard from someone in 14B and BL currently that with classes online, it is not as challenging as it might normally be to balance both, so if you are up for the workload and have a solid foundation in chemistry she suggested to go for it. But honestly, most people take BL with 14C so you would be t...
- Sat Dec 05, 2020 9:55 am
- Forum: General Science Questions
- Topic: Tricks for Knowing Locations of Metals and Nonmetals on Periodic Table
- Replies: 8
- Views: 5853
Re: Tricks for Knowing Locations of Metals and Nonmetals on Periodic Table
I think memorizing where metalloids are located is the most important because if you know where metalloids are, you also know that metals are to the left of it and nonmetals are to the right. The way I memorized the location of metalloids on the periodic table is that I imagine a mental staircase t...
- Sun Nov 29, 2020 10:12 am
- Forum: Student Social/Study Group
- Topic: How to study for class
- Replies: 30
- Views: 1400
Re: How to study for class
I have found that the textbook simply gives too much information and the lectures are more to the point of what we need to know. It is also too time consuming to try to learn from the book and from the lectures, so I typically watch lectures and either check khan academy or consult the book if I am ...
- Sun Nov 29, 2020 10:08 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSEPR Chart
- Replies: 18
- Views: 1017
Re: VSEPR Chart
I agree, pretty sure we are expected to memorize these shapes and know their names, how to draw them, and the bond angles.
- Sun Nov 29, 2020 10:03 am
- Forum: Hybridization
- Topic: Confusion on certain carbon hybridizations
- Replies: 5
- Views: 392
Re: Confusion on certain carbon hybridizations
Since each carbon is bonded to three other atoms (double to carbon, single to hydrogen, and a single to hydrogen) it would be sp2 hybridization.
- Sun Nov 29, 2020 10:00 am
- Forum: General Science Questions
- Topic: Electron Affinity
- Replies: 4
- Views: 180
Re: Electron Affinity
Since electron affinity is based on how much each electron wants to achieve an a stable octet, noble gasses with a stable octet therefore do not desire to gain or lose electrons.
- Sun Nov 29, 2020 9:57 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: 120 degree bond angles
- Replies: 3
- Views: 191
Re: 120 degree bond angles
I found this table to be really useful for studying shapes and angles. https://8dd969e7-a-ff053077-s-sites.googlegroups.com/a/coe.edu/principles-of-structural-chemistry/chemistry-and-climate-change/molecular-shapes/CD%20Spectrum.png?attachauth=ANoY7crF6LZuLn5XS4eaA7LRqj1A37qe5pqikDjjrIUlsarDWzmRkpK...
- Wed Nov 18, 2020 11:36 pm
- Forum: Lewis Structures
- Topic: Why is CO2 a lewis acid.
- Replies: 1
- Views: 381
Re: Why is CO2 a lewis acid.
Because CO2 has two double bonds, there is space for the molecule to accept electrons and shift them to the O, breaking the double bond and replacing it with a single bond. In this manner, CO2 can act as an electron acceptor and bond to an electron rich atom or molecule.
- Wed Nov 18, 2020 11:31 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Hydrogen Bond
- Replies: 6
- Views: 501
Re: Hydrogen Bond
If two H2SeO4 molecules were to interact, hydrogen bonds would take place between the hydrogens in one molecule (which are bound to an O) and the oxygen in the other which gives rise to hydrogen bonds.
- Wed Nov 18, 2020 11:25 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Polarizing Power vs Polarizability
- Replies: 4
- Views: 348
Re: Polarizing Power vs Polarizability
It's fundamentally the same idea (how the charge on one atom affects the charge on another) just different terms because the atoms have opposite charges. For cations with a positive charge, their ability to distort an anion and skew the electron cloud is known as their polarization power. The tenden...
- Wed Nov 18, 2020 11:21 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Electron Configuration of O-
- Replies: 2
- Views: 149
Re: Electron Configuration of O-
The oxygen anion has a charge of 2-, not 1-, so you would add two electrons and get 2p^6. Maybe the 1- charge was a typo, because when oxygen forms an anion it carries a 2- charge.
- Wed Nov 18, 2020 11:17 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Schrodinger's Wave Function Equation
- Replies: 4
- Views: 298
Re: Schrodinger's Wave Function Equation
I think the main point of Schrodinger's wave function equation is how it helps describe atomic orbitals. I remember it being a double derivative but other than that I'm pretty sure there was nothing else calculation-based he went over.
- Thu Nov 12, 2020 12:09 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Liquid or Solid molecules
- Replies: 1
- Views: 114
Re: Liquid or Solid molecules
Being more polarizable is tied to having more electrons, so the bigger the atom (the further down the periodic table) the more electrons it has. Consequently, it has greater interaction potential energy because the charges are greater so with more interaction attraction energy, the larger molecules ...
- Thu Nov 12, 2020 12:03 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: 2.A.21
- Replies: 2
- Views: 196
Re: 2.A.21
To determine the number of unpaired electrons, you have to draw the electron configuration for each ion (adding or subtracting electrons depending on the charge) and then filling in each subshell, spin up then spin down. If ever you are left with an subshell that is not full where there are not enou...
- Mon Nov 09, 2020 1:46 pm
- Forum: Bond Lengths & Energies
- Topic: Negative energies? [ENDORSED]
- Replies: 7
- Views: 752
Re: Negative energies? [ENDORSED]
Negative energies imply that energy is given off by what is taking place. In this case, the more negative energy forces for ion-ion attractions and hydrogen bonds are more favorable and give off more energy than dipole-dipole and dipole induced-dipole. The reverse is true that a greater energy must ...
- Mon Nov 09, 2020 1:26 pm
- Forum: Electronegativity
- Topic: Finding electronegativity values
- Replies: 6
- Views: 238
Re: Finding electronegativity values
No, we do not have to memorize electronegativity values. They will be given to us in a table when we need to perform certain calculations. However, you should familiarize yourself with the electronegativity trends and know that F, O, and Cl are highest.
- Mon Nov 09, 2020 12:32 pm
- Forum: Ionic & Covalent Bonds
- Topic: Dipole moments / covalent bonds
- Replies: 3
- Views: 168
Re: Dipole moments / covalent bonds
From my understanding, the calculation for a dipole moment involves multiplying the charge by the distance between atoms. So the longer the bond length (ie. single bond), the greater the dipole value will be. A shorted bond length (double or single bond) will have a smaller value for distance betwee...
- Fri Nov 06, 2020 9:48 pm
- Forum: Lewis Structures
- Topic: Lewis Bases and Lone Pair Electrons
- Replies: 3
- Views: 153
Re: Lewis Bases and Lone Pair Electrons
You should always put the lone pairs on the central atom when you are drawing a lewis structure. That way you can ensure that the outer electrons have filled valence shells with a low formal charge and consequently know you are finding the most stable configuration.
- Fri Nov 06, 2020 9:43 pm
- Forum: Electronegativity
- Topic: Shielding Effect
- Replies: 6
- Views: 197
Re: Shielding Effect
What helped me most understand shielding was the example of people standing around a fire pit. Those closest to it block you from some of the heat, but you can still feel the warmth of it even if there are people standing between you and the fire. So because s-orbital electrons are closer to the nuc...
- Mon Nov 02, 2020 1:08 pm
- Forum: Lewis Structures
- Topic: Two different SO4^-2 structures
- Replies: 2
- Views: 121
Re: Two different SO4^-2 structures
To add on to Nika's explanation, by adding two double bonds, the formal charges lowered for 4 of the 6 atoms which we learned today is much more stable. When drawing Lewis structures, the one with the lower formal charges is a better representation of the molecule.
- Mon Nov 02, 2020 12:39 pm
- Forum: Lewis Structures
- Topic: Delocalized Definition
- Replies: 4
- Views: 302
Re: Delocalized Definition
Delocalized electrons means that the electrons in a molecule are not paired to just one atom. They are able to move freely because they are shared equally between different atoms.
- Mon Nov 02, 2020 12:31 pm
- Forum: Resonance Structures
- Topic: Lecture 11/2
- Replies: 5
- Views: 166
Re: Lecture 11/2
The extra electron added at the end is due to the overall -1 charge of the nitrate ion (NO3-). Nitrate is one of the polyatomic ions and is pretty common, so I would say you should be familiar with the formula and the overall charge.
- Thu Oct 29, 2020 11:28 am
- Forum: *Shrodinger Equation
- Topic: Sapling week 2/3
- Replies: 12
- Views: 1330
Re: Sapling week 2/3
You can find the molar mass of a Helium atom on the periodic table: 4.002 g/mol. Since we are using Joules with Planck's constant, convert grams to kilograms, so 4.002 x 10^-3 kg. And make sure to multiply by Avogadro's number so 6.002 x 10^23 He atoms/mol in this question so that you get the value...
- Thu Oct 29, 2020 11:25 am
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Atomic Spectra
- Replies: 4
- Views: 189
Re: Atomic Spectra
The photons are essentially packets of light energy that help the electrons move between energy levels. To move up in energy level, an electron must absorb a specific amount of energy in the form of a photon. In the same way, to drop to a lower energy level, it must lose a specific amount of energy,...
- Thu Oct 29, 2020 11:19 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Textbook Problem 1E.13 part a
- Replies: 3
- Views: 127
Re: Textbook Problem 1E.13 part a
To add on to Alex's response, it is most stable to have a full electron shell and second most stable to have a half filled shell. It is least stable to have almost filled levels. Thus having a full d shell (10d) and a half full s shell (1s) is a more stable configuration for Ag (as opposed to an alm...
- Thu Oct 29, 2020 11:16 am
- Forum: Einstein Equation
- Topic: E=pv and E=pc
- Replies: 6
- Views: 2200
Re: E=pv and E=pc
E=pv comes from a simple combination of units. Knowing that E is in J which is kg*m^2/s^2, you can multiply momentum (kg*m/s) by velocity (m/s) to get kg*m^2/s^2. E=pc comes from knowing the velocity for a photon is the speed of light. So you essentially input C as the velocity for a photon because ...
- Thu Oct 29, 2020 11:12 am
- Forum: Empirical & Molecular Formulas
- Topic: Review for Midterm
- Replies: 11
- Views: 761
Re: Review for Midterm
Jacquelyn Challis 1H wrote:Regarding the midterm, can I use a graphing calculator or does it need to be scientific? I can't remember if Lavelle told us this info already or not.
I believe you can use a graphing calculator. If you have a TI-84 or TI-89 you should be fine.
- Wed Oct 21, 2020 12:26 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Heisenberg Diagram from Lecture
- Replies: 5
- Views: 189
Re: Heisenberg Diagram from Lecture
It is necessary to have 2 detectors to ensure you have both final and initial values so that you can calculate the total distance travelled as well as how long it took. Distance/time = velocity.
- Wed Oct 21, 2020 12:21 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Sapling Homework Order
- Replies: 3
- Views: 168
Re: Sapling Homework Order
They are all still in order and do a good job of paralleling his lectures. As Dr. Lavelle mentioned today in his lecture, we only need to know how to solve up to question 19 for the midterm. We still have to complete the rest of the assignment but it is not due until after the midterm, so complete t...
- Mon Oct 19, 2020 12:07 pm
- Forum: *Black Body Radiation
- Topic: Black Body Radiation Clarification
- Replies: 3
- Views: 209
Re: Black Body Radiation Clarification
From what I recall, that is more of a physics concept so we really don't need to know much more besides that. I think you are all set with the knowledge that black body radiation absorbs light frequencies, so don't worry about it too much!
- Mon Oct 19, 2020 12:04 pm
- Forum: Student Social/Study Group
- Topic: How are you studying?
- Replies: 204
- Views: 21713
Re: How are you studying?
For me, I start by watching the lectures and making sure to attend them around the time when we normally would if they were live. This helps me stay on top of watching the videos and also feel like I have to still "go to class" and be accountable for my participation. During lecture I take...
- Mon Oct 19, 2020 11:59 am
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Atomic Spectroscopy Post-Assessment Question
- Replies: 6
- Views: 279
Re: Atomic Spectroscopy Post-Assessment Question
Once atoms form molecules, they have a specific energy transitions and wavelengths that it absorbs which lets it have a unique spectral fingerprint as well. Hope this helps! Just like what Sophie said, both atoms and molecules have a unique "fingerprint" which can be depicted through spec...
- Wed Oct 14, 2020 2:55 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Lyman Series, Balmer Series, and Infrared
- Replies: 4
- Views: 275
Re: Lyman Series, Balmer Series, and Infrared
The Paschen series is what you are asking about. This series includes transitions from n >= 4 to n = 3 state.
- Wed Oct 14, 2020 2:06 pm
- Forum: Einstein Equation
- Topic: Wave Model Question
- Replies: 5
- Views: 298
Re: Wave Model Question
The wave model predicts that the amplitude of the wave (the intensity) determines how much energy the wave carries. But this classical model does not apply to light. It can explain its wavelike appearance properties but does not accurately explain its energy properties. This is because the photoelec...
- Wed Oct 14, 2020 1:55 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: atomic spectroscopy question
- Replies: 2
- Views: 222
Re: atomic spectroscopy question
A good way to think about this is that the energy levels have to match up. If there is not enough energy, it will not match the energy needed to jump the gap, so it will pass through because it is not useful to the atom. But when the energy is enough and matches what is required, the atom will absor...
- Mon Oct 12, 2020 1:34 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: high intensity light
- Replies: 3
- Views: 262
Re: high intensity light
We learned today that increasing the intensity/amplitude of a light (making it brighter) does not have any effect on the electrons emitted. This is because the energy required to emit an electron has not been met. To meet this required energy, a specific photon with a high frequency is necessary. To...
- Mon Oct 12, 2020 12:24 pm
- Forum: Properties of Light
- Topic: Quanta and Photons
- Replies: 19
- Views: 397
Re: Quanta and Photons
I once heard someone describe quanta as pennies of the quantum world. If you want to transfer energy, quanta are the smallest units that you can do so in. This analogy is super helpful! Thank you for sharing because it makes something so abstract seem as tangible as everyday coins. Definitely incre...
- Mon Oct 05, 2020 10:54 am
- Forum: Molarity, Solutions, Dilutions
- Topic: Dilutions
- Replies: 10
- Views: 309
Re: Dilutions
When doing a dilution, you usually pour more water in or pour a more concentrated solution in. You don't usually pour more solute in. For instance, if I had a bowl with 10 blueberries and 10 strawberries, I could reduce the ratio of blueberries by putting more strawberries. I didn't change the amou...
- Mon Oct 05, 2020 10:48 am
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Accuracy vs Precision
- Replies: 20
- Views: 689
Re: Accuracy vs Precision
Yes, I agree. That was super helpful!! For me the way I try to remember is "precision means consistent" and "accuracy means correct".
- Mon Oct 05, 2020 10:42 am
- Forum: Empirical & Molecular Formulas
- Topic: Emprical Formulas Ever Larger than Molecular? [ENDORSED]
- Replies: 26
- Views: 1974
Re: Emprical Formulas Ever Larger than Molecular? [ENDORSED]
The empirical formula is always the most basic, simplified ratio. Thus, the molecular formula will either be the same or a multiple of that base ratio depending on the molar mass.
- Mon Oct 05, 2020 10:34 am
- Forum: Administrative Questions and Class Announcements
- Topic: Advice from a Medical Student [ENDORSED]
- Replies: 297
- Views: 424323
Re: Advice from a Medical Student [ENDORSED]
Thank you so much for your encouraging advice. I appreciate you taking your time to post this, and now I am even more motivated to take notes during lectures and discussions. Best of luck in Med School!!

