Search found 143 matches
- Sat Mar 13, 2021 3:45 pm
- Forum: Experimental Details
- Topic: textbook 7.17
- Replies: 3
- Views: 784
Re: textbook 7.17
I believe its because C has the lowest activation energy.
- Sat Mar 13, 2021 3:41 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: "Thermodynamically Stable" vs "Kinetically Stable"
- Replies: 10
- Views: 1774
Re: "Thermodynamically Stable" vs "Kinetically Stable"
A thermodynamically stable reaction has a negative delta G, meaning that its spontaneous. A thermodynamically unstable reaction has a positive delta G, meaning that its nonspontaneous.
- Sat Mar 13, 2021 3:40 pm
- Forum: Balancing Redox Reactions
- Topic: Acidic and Basic Redox Reactions
- Replies: 7
- Views: 651
Re: Acidic and Basic Redox Reactions
This was very helpful, thank you!!! Dr. Lavelle also has some notes on his website that go over acid/base redox reactions and that really clarified a lot for me. I'll put the links down below: https://lavelle.chem.ucla.edu/wp-content/supporting-files/Chem14B/Balancing_Redox_Reactions_Acidic_Conditio...
- Sat Mar 13, 2021 3:37 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Textbook 4A.13
- Replies: 2
- Views: 334
Re: Textbook 4A.13
You can keep Celsius for this question because if the temperature rises by 2.49 degrees C, it also rises by 2.49 degrees K. For your second question, I believe you are right; q(reaction) = -q(calorimeter).
- Sat Mar 13, 2021 3:34 pm
- Forum: Student Social/Study Group
- Topic: Left/Right Electrode
- Replies: 9
- Views: 669
Re: Left/Right Electrode
The reaction on the right will be reduction (cathode) and the reaction of the left will be oxidation (anode). So, the equation for E*cell = E(cathode)* - E(anode)*.
- Fri Mar 05, 2021 2:07 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5I. 15 from Outline 1
- Replies: 3
- Views: 726
Re: 5I. 15 from Outline 1
When K is small, reactants are favored over products. When K is big, products are favored over reactants.
- Fri Mar 05, 2021 1:57 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Pseudo K
- Replies: 5
- Views: 275
Re: Pseudo K
I found a chem community post that explained this a few years back that was really helpful!!
Here is the link:
viewtopic.php?t=5450
Here is the link:
viewtopic.php?t=5450
- Fri Mar 05, 2021 1:55 pm
- Forum: Student Social/Study Group
- Topic: What do you miss / What are you looking forward to?
- Replies: 92
- Views: 10419
Re: What do you miss / What are you looking forward to?
I am looking forward to being on campus next year, especially after a long year not being able to go out and staying home! I also am very excited for in-person classes again, since it has been so long since we've had them! :)
- Thu Mar 04, 2021 12:22 pm
- Forum: Student Social/Study Group
- Topic: Playlist
- Replies: 86
- Views: 7589
Re: Playlist
I recommend So Good by Omar Apollo, Honey by Boy Pablo, Chinese Satellite by Phoebe Bridgers, Daydream by Luna Luna, and Don't Be a Fool by Dreamer Boy !! They all have a lot of underrated songs and are some of my favorite artists right now :)
- Thu Mar 04, 2021 12:18 pm
- Forum: Student Social/Study Group
- Topic: Moving to Westwood soon. Which restaurants should I visit?
- Replies: 45
- Views: 3008
Re: Moving to Westwood soon. Which restaurants should I visit?
I recommend Mochi Dochi!! It's a Korean hot dog restaurant in Sawtelle which is only 10-15 minutes away from campus :)
- Sun Feb 28, 2021 4:05 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Step up vs workshops
- Replies: 18
- Views: 1103
Re: Step up vs workshops
Step-up sessions usually cover conceptual concepts that we learned in lecture and then provide example problems that requires us to apply the conceptual knowledge. The workshops offer worksheets with more practice problems, similar to what we see in the textbook. I recommend attending step up sessio...
- Sun Feb 28, 2021 1:25 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Equation for E°
- Replies: 3
- Views: 265
Re: Equation for E°
The equation you use depends on what values of E° you are using. If you are given the standard reduction potentials, then you should use E° cell = E° cathode - E° anode. The negative sign before the E° anode basically switches the value to be the standard potential of the oxidation reaction. If you...
- Sun Feb 28, 2021 1:24 am
- Forum: General Science Questions
- Topic: Midterm 2 Reactions
- Replies: 79
- Views: 6575
Re: Midterm 2 Reactions
I am satisfied with my Midterm 2 score, but knowing that I did make simple errors does make me a little disappointed since the questions are worth a lot of points. I definitely wish I focused on conceptual concepts more, but for now I am just going to continue with the assigned textbook problems and...
- Sun Feb 28, 2021 1:04 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Equation for E°
- Replies: 3
- Views: 265
Equation for E°
I know there are two equations for solving E° --> (1). E° cell = E° cathode - E° anode and (2). E° cell = E° reduction + E° oxidation. But how do we know when we have to use either equation to solve for E° or does it not matter which equation we use?
- Thu Feb 25, 2021 10:20 am
- Forum: Student Social/Study Group
- Topic: Fave movie/show
- Replies: 67
- Views: 5007
Re: Fave movie/show
If you're willing to binge, Criminal Minds has 15 seasons!! I believe only the first 12 (I could be wrong) are on Netflix and the rest of the seasons are on Hulu.
- Thu Feb 25, 2021 10:14 am
- Forum: Balancing Redox Reactions
- Topic: Sapling Weeks 7 and 8 Question 3
- Replies: 3
- Views: 294
Re: Sapling Weeks 7 and 8 Question 3
I actually spent a lot of time on this problem (I had different molecules though!), but one of the concepts on the feedback on Sapling helped me solve this problem. One of the other methods for balancing in a basic solution is to skip the acidic rule step. If you need x atoms of Oxygen on one side o...
- Sun Feb 21, 2021 2:42 pm
- Forum: Balancing Redox Reactions
- Topic: Salt Bridge Diagram
- Replies: 8
- Views: 475
Re: Salt Bridge Diagram
For cell diagrams, I believe that anodes are typically on the left and cathodes are typically on the right. Electron flow is always from the anode (oxidation) to the cathode (reduction).
- Sat Feb 20, 2021 3:05 pm
- Forum: Student Social/Study Group
- Topic: Post Midterm 2 De-stressing
- Replies: 92
- Views: 7555
Re: Post Midterm 2 De-stressing
I plan on getting a lot of sleep this weekend and catching up on episodes of The Bachelor that I am behind on! Other than that, last night I treated myself by baking chocolate chip cookies :)
- Sat Feb 20, 2021 3:02 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Delta H and Delta S both positive
- Replies: 31
- Views: 8648
Re: Delta H and Delta S both positive
When Delta H is + and Delta S is +, the reaction is spontaneous only if Delta H is less than TDeltaS . When Delta H is + and Delta S -, the reaction is never spontaneous . When Delta H is - and Delta S is -, the reaction is spontaneous only if Delta H is greater than TDelta S . When Delta H is - and...
- Sat Feb 20, 2021 2:50 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Open vs closed
- Replies: 31
- Views: 2965
Re: Open vs closed
Here are some examples of open, closed, and isolated systems! Open: plants conducting photosynthesis, boiling water without a lid, an open cup of coffee Closed: boiling water with a lid, a closed water bottle, a cup of coffee with a lid Isolated: the universe, a thermos flask Something to remember a...
- Sat Feb 20, 2021 2:41 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Textbook 4A.11
- Replies: 6
- Views: 880
Re: Textbook 4A.11
Heat capacity can be defined as the amount of heat that is required to raise the temperature of a certain substance. The equation to calculate heat capacity is q/delta T.
- Thu Feb 18, 2021 11:58 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Textbook 4D.9
- Replies: 2
- Views: 269
Re: Textbook 4D.9
First, you need to find the overall enthalpy of formation of the entire reaction. To recap, that'd be the sum of the enthalpy of formation of products minus the sum of the enthalpy of formation of reactants. That answer should should be -13168 KJ/mol. Next, you need to divide the previous value by ...
- Sun Feb 14, 2021 5:26 pm
- Forum: Student Social/Study Group
- Topic: Balance / Self Care Tips
- Replies: 62
- Views: 3448
Re: Balance / Self Care Tips
I do mindfulness meditation every night before I go to sleep, because it helps me reflect on my day and identify the good parts of my day and places in which I need to improve :) I also value sleep a lot and I use a sleep calculator to help me plan out my nights! All you have to do is put in the tim...
- Sat Feb 13, 2021 2:18 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: qrev
- Replies: 27
- Views: 1666
Re: qrev
Hannah_Butler_2E wrote:Madeline Ogden 3B wrote:qrev is the amount of heat generated in a reversible process.
So does deltaS = q/T only apply to reversible expansions and compressions?
I believe you use this equation when you are at a constant temperature for a reversible process.
- Sat Feb 13, 2021 2:09 pm
- Forum: Student Social/Study Group
- Topic: Thoughts on Upcoming Midterm 2
- Replies: 41
- Views: 1780
Re: Thoughts on Upcoming Midterm 2
For now, I am being optimistic, because we still have a lot of time to study for Midterm 2 and many step-up and UA sessions to attend this week! There is still a lot to do in terms of studying, but I know we all can do it :) But for now, I am just completing textbook problems and practice problems, ...
- Sat Feb 13, 2021 2:04 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Sapling 8
- Replies: 2
- Views: 117
Re: Sapling 8
You have to approach this problem in three different steps. First, you should find the delta S when heating the water from 44.0 degrees C to 100 degrees C. Next you should find the delta S when cooling the water from 100 degrees C to 44.0 degrees C. You are already given the standard molar entropy o...
- Thu Feb 11, 2021 2:53 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Midterm 2 Coverage
- Replies: 6
- Views: 405
Re: Midterm 2 Coverage
Professor Lavelle hasn't informed us exactly what Midterm 2 will cover, but I'm assuming it will cover weeks 4-6 lectures! A good idea would be to to start reviewing lectures starting week 4, since we know for sure those will be on the midterm.
- Thu Feb 11, 2021 2:38 pm
- Forum: Student Social/Study Group
- Topic: Classes for next quarter?
- Replies: 165
- Views: 17073
Re: Classes for next quarter?
I plan on taking chem 14C next quarter along with anatomy and another course, but I am unsure of what my 3rd class should be. Do you guys have any recommendations? I heard Scand 50 was a good class but I also heard that it fills up fast. I was thinking of taking Scand 50 too! But I am also consider...
- Sun Feb 07, 2021 12:53 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Textbook 4D.7
- Replies: 1
- Views: 93
Re: Textbook 4D.7
You're going to use the equation (Delta u) = q + w. You are already given q (Delta H), so you should use the equation w = -deltanRT where n = 1.00 mol, R = 8.314 J x K^-1 x mol^-1, and T = 298K. Remember to convert this value from J to kJ. After you solve for w, all you have to do is plug your value...
- Sat Feb 06, 2021 5:27 pm
- Forum: Calculating Work of Expansion
- Topic: Sapling HW Week 3/4 #13
- Replies: 7
- Views: 432
Re: Sapling HW Week 3/4 #13
You should look at the number of the moles of gases when determining which are doing work and which are not. The correct answers would be the equations that produce more moles of gas than what is used up in the reaction, because this results in a negative w.
- Sat Feb 06, 2021 5:21 pm
- Forum: Calculating Work of Expansion
- Topic: Reversible Reactions
- Replies: 5
- Views: 291
Re: Reversible Reactions
Reversible reactions cause a very slow change in volume and occur when the system can restored to its initial state (in other words, it can be reversed).
- Fri Feb 05, 2021 2:42 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: How to find delt T when there's a transformation from an ice to liquid.
- Replies: 3
- Views: 197
Re: How to find delt T when there's a transformation from an ice to liquid.
What was the first step that you did in this problem? Given 53.8 g of ice, you have to convert this to mol ice and multiply it by delta H fusion (6.01 x 10^3 J/mol) to get the amount of heat required to melt the ice. Then you need to solve for q (ice) and q (water). So, your final equation to solve ...
- Fri Feb 05, 2021 2:34 pm
- Forum: Student Social/Study Group
- Topic: Sapling HW and exams
- Replies: 19
- Views: 839
Re: Sapling HW and exams
^ I agree with Tikva. Sapling problems are really helpful when you want more practice with problems that are similar to textbook problems! The solutions are also really helpful as well so you can see every step you need to solve a similar problem :)
- Fri Feb 05, 2021 2:12 pm
- Forum: Student Social/Study Group
- Topic: Midterm Review
- Replies: 7
- Views: 913
Re: Midterm Review
Textbook, textbook, textbook problems!!! In other words, redo textbook problems until you can finally get the answer without looking at the answer key or your notes. This will make sure that you KNOW the material in and out. I like to create notes for each outline too so I can just have all of the c...
- Sun Jan 31, 2021 3:40 pm
- Forum: Student Social/Study Group
- Topic: Worried About MT 1 Grades
- Replies: 39
- Views: 1910
Re: Worried About MT 1 Grades
I definitely understand where you're coming from, the midterm was definitely challenging! All you can do now is focus on the future and make sure to attend as many sessions as possible for the next midterm or final. There are plenty of opportunities to improve your grade :))
- Sun Jan 31, 2021 12:06 am
- Forum: General Science Questions
- Topic: Chem Jokes
- Replies: 28
- Views: 1831
Re: Chem Jokes
I lost an electron. You really have to keep an ion them :(
- Sat Jan 30, 2021 12:42 am
- Forum: Student Social/Study Group
- Topic: Go treat yourself after MT1!
- Replies: 75
- Views: 5539
Re: Go treat yourself after MT1!
I took a FAT nap after the midterm and then I decided to bake some brownies to celebrate finishing off the first week of midterms for this quarter! I decided to just rest for the day, so that I wouldn't overwhelm myself :)
- Sat Jan 30, 2021 12:33 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Enthalpy solving methods
- Replies: 7
- Views: 355
Re: Enthalpy solving methods
I believe there's a recommended method we should use for certain problems. For example, we use Hess's Law when we have to find the reaction enthalpy for a specific chemical equation, given a few chemical equations and their delta Hs that we have to manipulate to get the desired chemical equation. We...
- Sat Jan 30, 2021 12:22 am
- Forum: Administrative Questions and Class Announcements
- Topic: 14 C enrollment
- Replies: 2
- Views: 134
Re: 14 C enrollment
The Daily Bruin actually has a section that gives you the estimations of how quickly a class fills up at UCLA! You can test it out here:
https://stack.dailybruin.com/2020/02/05/class-fill-ups/
https://stack.dailybruin.com/2020/02/05/class-fill-ups/
- Fri Jan 29, 2021 9:18 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Taking the Anti-Log
- Replies: 37
- Views: 2669
Re: Taking the Anti-Log
You will take the anti-log whenever you are given pKa and you need Ka, pKb and you need Kb, pOH and you need [OH-], and pH and you need [H3O+]! The anti-log is as simple as taking 10^-(value you are given). Hope that makes sense!
- Fri Jan 29, 2021 8:49 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Ka and Kb
- Replies: 9
- Views: 440
Re: Ka and Kb
When determining whether or not to use Ka or Kb when calculating the pH of a salt solution, you should determine if you have a more acidic solution or a more basic solution. In situations in which you are given Kb yet your solution is more acidic, you can use the simple equation Kw = Ka x Kb and you...
- Sun Jan 24, 2021 11:24 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: How to predict relative strengths of acids and bases
- Replies: 5
- Views: 306
Re: How to predict relative strengths of acids and bases
Ka and pKa are two ways to measure the strength of an acid. A high Ka and a low pKa indicates a strong acid. Kb and pKb are two ways to measure the strength of a base. A high Kb and a low pKb indicates a strong base.
- Sat Jan 23, 2021 1:11 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: D.19
- Replies: 3
- Views: 216
Re: D.19
That is the correct concentration of CH3NH3Cl. Did you set up your equation as 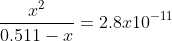 ? When you set it up like this, you should get a value for x and then you have to plug that value in -log(x) to get the pH.
? When you set it up like this, you should get a value for x and then you have to plug that value in -log(x) to get the pH.
- Sat Jan 23, 2021 1:04 pm
- Forum: Student Social/Study Group
- Topic: Favorite Music
- Replies: 113
- Views: 12445
Re: Favorite Music
I really like indie pop music! Some of my favorite songs at the moment include Lovesong by Charlie Burg, Sofia by Clairo, Puppy Dog by Dreamer Boy, and Sunshine and [censored] are no Longer Enough by Luna Luna. One of my anthems this year has been Good Days by SZA though :))
- Fri Jan 22, 2021 11:55 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Week 2 Sapling #5
- Replies: 9
- Views: 277
Re: Week 2 Sapling #5
You can solve this problem by finding the pOH of the solution (pOH = 14 - pH) and then using the pOH to solve for [OH-]. Then you can work backwards to solve for the unknown concentrations.
- Fri Jan 22, 2021 11:48 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Week 2 Sapling #9
- Replies: 4
- Views: 242
Re: Week 2 Sapling #9
I like to break the molecule up into two parts. For example, for LiClO4, we can break this up into Li and ClO4. Based on the chart for strong acids and strong bases, we know that Li comes from the strong base, LiOH, and ClO4 comes from the strong acid, HClO4. Since they are both strong, they cancel ...
- Thu Jan 21, 2021 12:02 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Midterm 1
- Replies: 9
- Views: 462
Re: Midterm 1
In case you can't find it, here's the link for the new constants and formulas sheet! I'd be sure to have this printed with a periodic table and some scratch paper before the midterm :)
https://lavelle.chem.ucla.edu/wp-conten ... ations.pdf
https://lavelle.chem.ucla.edu/wp-conten ... ations.pdf
- Thu Jan 21, 2021 11:46 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: steam causing burns
- Replies: 40
- Views: 1407
Re: steam causing burns
Yes, steam causes severe burns because it is releasing heat. Dr. Lavelle explained in lecture that this occurs due to water's large enthalpy of vaporization / large negative enthalpy of condensation.
- Sun Jan 17, 2021 4:31 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Typo in 6A.19
- Replies: 2
- Views: 179
Re: Typo in 6A.19
For future reference, Lavelle has this document on his website with any errors in the solution manual for textbook problems!!
https://lavelle.chem.ucla.edu/wp-conten ... rs_7Ed.pdf
https://lavelle.chem.ucla.edu/wp-conten ... rs_7Ed.pdf
- Sat Jan 16, 2021 3:02 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kw constant
- Replies: 25
- Views: 853
Re: Kw constant
I was curious about this question too. But I looked at my lecture notes, and since [H2O] is in large excess, we consider the concentration to be unchanged and therefore, we don't include it in the equation.
- Sat Jan 16, 2021 2:53 pm
- Forum: Ideal Gases
- Topic: Sapling Question #4
- Replies: 11
- Views: 555
Re: Sapling Question #4
To start this problem, you should create an ICE table. PCl3 Cl2 PCl5 I 0 0 0.0250 C +x +x -x E x x 0.0250 -x Then plug in these values in to the Kp expression to solve for X. Kp = \frac{P(PCl5)}{P(PCl3)P(Cl2)} = 393 Solve for x and then plug that answer back into the equilibr...
- Fri Jan 15, 2021 10:16 pm
- Forum: Ideal Gases
- Topic: Equilibrium Constant (Q and K)
- Replies: 13
- Views: 666
Re: Equilibrium Constant (Q and K)
Q is the reaction quotient and K is the equilibrium constant. When determining the direction a reaction is going to proceed, you have to compare your Q and K values. So, If Q is less than K, then the forward reaction is favored. If Q is greater than K, then the reverse reaction is favored. When Q is...
- Fri Jan 15, 2021 10:12 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Midterm
- Replies: 7
- Views: 389
Re: Midterm
I would assume that the our midterms for 14B are structured in a similar way as they were in 14A, so I believe that they would be multiple choice. However, I don't think Lavelle has officially announced whether or not it is multiple choice so that would definitely be subject to change :)) Also yes, ...
- Thu Jan 14, 2021 9:16 pm
- Forum: Student Social/Study Group
- Topic: Study Habits
- Replies: 96
- Views: 6768
Re: Study Habits
Personally, I set a specific schedule of what I need to get done each day and I make sure that I am motivated to keep up with this schedule to make sure that I am getting my work done. Another thing I like to do is to redo textbook problems until I am able to solve them without looking at the soluti...
- Thu Jan 14, 2021 8:49 pm
- Forum: Ideal Gases
- Topic: Thermodynamics in Equilibirum
- Replies: 6
- Views: 247
Re: Thermodynamics in Equilibirum
I'm pretty sure that Outline #4 is dedicated to thermodynamics.
https://lavelle.chem.ucla.edu/wp-conten ... namics.pdf
https://lavelle.chem.ucla.edu/wp-conten ... namics.pdf
- Sun Jan 10, 2021 7:24 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Week 1 Assignment
- Replies: 17
- Views: 751
Re: Week 1 Assignment
Sapling homework is always mandatory. I believe the Sapling problems for the chemical equilibrium outline are due next Sunday (1/17). However, textbook problems aren't necessarily mandatory, but HIGHLY recommended to complete. I took 14a last quarter with Lavelle and I found the textbook problems to...
- Sat Jan 09, 2021 4:32 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: 5I.13
- Replies: 1
- Views: 84
Re: 5I.13
For part a, you should first form a balanced equation and find the initial concentration of Cl2 (g). The balanced equation is then Cl2 (g) -> 2Cl (g) and the initial concentration of Cl2 (g) would be 0.0020 mol Cl2 / 2.0 L to get 0.0010 M. The ice table is then set up like this: concentration Cl2 2C...
- Sat Jan 09, 2021 4:07 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Textbook 5H.3
- Replies: 3
- Views: 106
Re: Textbook 5H.3
Faith St Amant 3D wrote:Here's how my TA worked through it. You just need to add the two reactions shown from the table together, and then multiply their K values.
Is there a specific reason why you have to multiply their K values?
- Fri Jan 08, 2021 8:36 pm
- Forum: Ideal Gases
- Topic: Ideal Gas Definition
- Replies: 10
- Views: 655
Re: Ideal Gas Definition
The ideal gas is a theoretical concept and is used to guide us with calculations and make estimations. Khan Academy has a video that I found to be really helpful in explaining the definition and application, I recommend watching it! Here's the link: https://www.khanacademy.org/science/class-11-chemi...
- Fri Jan 08, 2021 8:32 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Calculator Trick for ICEbox Calculations
- Replies: 13
- Views: 798
Re: Calculator Trick for ICEbox Calculations
Tanya Bearson 2K wrote:Thank you all for the tips! Are we allowed to use TI-84 calculators on exams?
If I am correct, I believe we are able to use any calculator for tests.
- Thu Jan 07, 2021 11:30 am
- Forum: Ideal Gases
- Topic: Ideal vs real gases
- Replies: 12
- Views: 667
Re: Ideal vs real gases
Ideal gases are exactly that: they're ideal and theoretical and they follow all of the gas laws regardless of the conditions they're in. I believe that the ideal gas law really helps us with calculations about properties and behaviors of gases.
- Thu Jan 07, 2021 11:25 am
- Forum: Student Social/Study Group
- Topic: Learning Sessions
- Replies: 24
- Views: 1217
Re: Learning Sessions
I would attend as many as you can in the beginning to see which UA is best for your learning style! After you figure out who works best, I'd suggest attending one or two per week. When midterms come around, I would recommend doubling that just to get extra practice if you need it :)
- Wed Dec 16, 2020 4:55 pm
- Forum: Student Social/Study Group
- Topic: Changing Study Habits
- Replies: 35
- Views: 1488
Re: Changing Study Habits
To study for the midterms, I would write outlines of what was covered to help me study. I think I am going to try to start on these earlier so that I am always reviewing the material from the previous lectures :) Also attending more UA sessions if I'm ever unclear on anything.
- Wed Dec 16, 2020 4:53 pm
- Forum: Student Social/Study Group
- Topic: Plans for Relaxing After Finals
- Replies: 98
- Views: 16448
Re: Plans for Relaxing After Finals
I plan on taking lots of naps to catch on sleep and catching up on tv shows I stopped watching when school started! I also plan on making more playlists to expand my music library :)
- Sat Dec 12, 2020 9:38 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Strong Bases
- Replies: 1
- Views: 131
Strong Bases
Are bases made from Group 1 elements stronger or weaker than bases made from Group 2 elements?
- Sat Dec 12, 2020 10:53 am
- Forum: Student Social/Study Group
- Topic: Plans for Relaxing After Finals
- Replies: 98
- Views: 16448
Re: Plans for Relaxing After Finals
I plan on doing self-care meditation to really just relax all the stress that's been building up over this week for this final :) And also binge watching Netflix and Disney+ since I am so behind on a lot of the shows I like to watch!
- Sat Dec 12, 2020 10:51 am
- Forum: Student Social/Study Group
- Topic: Winter Break
- Replies: 44
- Views: 2400
Re: Winter Break
I made my own outline notes of each outline that we covered in class, and I'm probably going to just review them as often as I can to make sure that I retain all of the information! Also doing practice problems over break could help too. Personally, I am going to focus on acids and bases a little bi...
- Fri Dec 11, 2020 3:40 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: UA wkst answers
- Replies: 1
- Views: 211
Re: UA wkst answers
Here's the Google Drive folder he gave us with all of his previous worksheets :)) https://drive.google.com/drive/folders/ ... sp=sharing
I believe each worksheet has a link to to their corresponding key.
I believe each worksheet has a link to to their corresponding key.
- Fri Dec 11, 2020 3:37 pm
- Forum: Lewis Structures
- Topic: HClO3 Lewis Structure [ENDORSED]
- Replies: 4
- Views: 1410
Re: HClO3 Lewis Structure [ENDORSED]
H is bonded to O in HClO3 because that's the most stable structure. With this structure, each atom has a formal charge of 0. If H was bonded to the Cl, the O without the double bond would have a formal charge of 0 and the Cl would have a formal charge of +1. Also, I believe since HClO3 is an acid, t...
- Thu Dec 10, 2020 4:21 pm
- Forum: Bronsted Acids & Bases
- Topic: Lewis/Bronsted/Arrhenius
- Replies: 1
- Views: 122
Re: Lewis/Bronsted/Arrhenius
I would just understand the definitions of all of them to be the most prepared for the final! We've definitely covered more of Bronsted acids/bases and Lewis acids/bases in lecture and in textbook problems, though, I think it would just depend on what the question is asking.
- Thu Dec 10, 2020 4:16 pm
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: Textbook Problem 6B.3
- Replies: 1
- Views: 84
Re: Textbook Problem 6B.3
a) To find the desired pH, you have to take -log of the concentration. So solve -log(0.025) = the desired pH. b) To find the actual pH, you have to consider the mistake that was made by the laboratory technician. To find the concentration, you should take 0.025 x \frac{200 mL}{250 mL} . Then take th...
- Sun Dec 06, 2020 12:07 am
- Forum: Student Social/Study Group
- Topic: Evaluations
- Replies: 17
- Views: 875
Re: Evaluations
Our evaluations are anonymous, so I don't think there's a way for him to know whether or not we completed them. But also since he didn't say anything about it, it's likely a no
- Sun Dec 06, 2020 12:04 am
- Forum: Bronsted Acids & Bases
- Topic: Acids strong vs not
- Replies: 3
- Views: 321
Re: Acids strong vs not
Here's a PDF I found that lists the most common strong acids and strong bases! I think it's helpful to use this as a resource when deciding :) https://www2.palomar.edu/users/ngeetha/110labhandouts/TABLE%20OF%20STRONG%20ACIDS%20and%20bases.pdf Another thing to consider is the shape of the molecule. T...
- Sat Dec 05, 2020 12:50 pm
- Forum: Naming
- Topic: Roman Numeral for Transition Metal
- Replies: 7
- Views: 760
Re: Roman Numeral for Transition Metal
The roman numeral for a transition metal is its charge/oxidation state. To figure it out, you can use this equation my TA provided us in discussion last week: (# of metal atoms)(oxidation # of metal) + sum of (# of each ligand)(charge of ligand) = charge of ion and then you can just rearrange the fo...
- Sat Dec 05, 2020 12:45 pm
- Forum: Student Social/Study Group
- Topic: How are you?
- Replies: 154
- Views: 15033
Re: How are you?
A little bit stressed for finals! But I've been meditating and taking time for myself as well to ensure that I don't overwhelm myself too. Hope everyone is doing well :)
- Sat Dec 05, 2020 12:38 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Tetrahedral v Square Planar
- Replies: 6
- Views: 319
Re: Tetrahedral v Square Planar
I don't think we've learned how to do this in Chem14A, I believe we'd just put tetrahedral or square planar for the compound shape. I was looking back at a few practice problems I did at a Step-Up session and that's what the UA put as the shape of a compound with a coordination number of 4 !
- Fri Dec 04, 2020 5:48 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: chelate
- Replies: 7
- Views: 413
Re: chelate
The sigma bonds allow for rotation in a chelate ! So yes, you're correct. I believe sigma bonds are able to do this because the electrons are located in a cylindrically symmetrical cloud along the axis while pi bonds do not allow the atoms to rotate.
- Fri Dec 04, 2020 5:22 pm
- Forum: Bronsted Acids & Bases
- Topic: HClO3 Strength for 14A
- Replies: 3
- Views: 145
Re: HClO3 Strength for 14A
In this situation, I'd follow what the textbook says. HClO3 is a strong acid.
- Sun Nov 29, 2020 9:35 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Sigma and Pi Bonds
- Replies: 5
- Views: 438
Re: Sigma and Pi Bonds
I'm not sure if we need to know exactly which bond in a double or triple bond is sigma or pi, but I'd suggest memorizing the patterns of sigma and pi bonds. So,
Single Bond = 1 sigma bond
Double Bond = 1 sigma bond + 1 pi bond
Triple Bond = 1 sigma bond + 2 pi bonds
Single Bond = 1 sigma bond
Double Bond = 1 sigma bond + 1 pi bond
Triple Bond = 1 sigma bond + 2 pi bonds
- Sun Nov 29, 2020 9:31 pm
- Forum: Octet Exceptions
- Topic: PCl5 expanded octet question
- Replies: 6
- Views: 1031
Re: PCl5 expanded octet question
In PCl5, P has 10 bonding electrons, thus an expanded octet. Typically elements in Period 3 or higher are allowed to have an expanded octet because they have d orbitals that can accommodate any extra electrons.
- Sat Nov 28, 2020 4:02 pm
- Forum: Student Social/Study Group
- Topic: Midterm/Final Success?
- Replies: 17
- Views: 835
Re: Midterm/Final Success?
Attend step-up sessions if you're confused! I attended one before MT2 and before I did, I. was so confused on intermolecular forces but after, it was really helpful for me to understand :) Something I like to do is go over my lecture notes again and condense them onto one document where I include th...
- Sat Nov 28, 2020 3:52 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Which shapes to memorize
- Replies: 11
- Views: 816
Re: Which shapes to memorize
Here's another helpful document that I've been using to help me study molecular shape!
https://www.angelo.edu/faculty/kboudrea ... _VSEPR.pdf
https://www.angelo.edu/faculty/kboudrea ... _VSEPR.pdf
- Sat Nov 28, 2020 3:51 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Step Up/UA Sessions
- Replies: 10
- Views: 526
Re: Step Up/UA Sessions
I personally find them really helpful! I recently started going to Hannah's step up session on Tuesdays from 2 to 4pm and she's been really helpful in explaining the concepts. She usually posts a worksheet that in the chat from google drive and I just copy that onto my iPad so I can write directly o...
- Fri Nov 27, 2020 7:07 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Outlines for the Final
- Replies: 11
- Views: 677
Re: Outlines for the Final
They should be covered before the final, I believe that we just started Outline 5 with Wednesday's lecture :)
- Fri Nov 27, 2020 7:04 pm
- Forum: Octet Exceptions
- Topic: Question on Radicals
- Replies: 7
- Views: 499
Re: Question on Radicals
I believe that they are interchangeable, radicals (or free radicals) are atoms with at least one unpaired valence electron.
- Fri Nov 27, 2020 6:41 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Textbook 2E #7
- Replies: 1
- Views: 93
Re: Textbook 2E #7
First, you should draw the Lewis structure to help you answer these questions! For part A: The shape of a thionyl chloride molecule is trigonal pyramidal. We know this because there are 3 bonding pairs and 1 lone pair when we draw the Lewis structure of SOCl2. For part B: We know that the angles are...
- Sun Nov 22, 2020 3:37 pm
- Forum: Hybridization
- Topic: HW Question
- Replies: 3
- Views: 140
Re: HW Question
Lillian Ma 1L wrote:I believe that it is a sp3 hybridization.
How can we figure out the hybridization of an atom? Do we just need to determine the shape through the VSEPR model?
- Sun Nov 22, 2020 3:29 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Visualizing VSEPR Shapes
- Replies: 5
- Views: 193
Re: Visualizing VSEPR Shapes
Maybe try looking online for good diagrams of how VSEPR shapes look and then put those pictures in your notes so you can always refer back to them when you're studying. I definitely get where you are coming from though, visuals are so much more helpful in helping us remember these shapes!
- Sun Nov 22, 2020 3:27 pm
- Forum: Student Social/Study Group
- Topic: Exercising Our Minds and Bodies
- Replies: 120
- Views: 21335
Re: Exercising Our Minds and Bodies
I enjoy going on walks around sunset so I can watch the sunset go down! It's so calming, especially since there's a nice view from my house :) During the day, I enjoy playing volleyball with my friends just to take a break from any school work that I have too!
- Sat Nov 21, 2020 3:00 pm
- Forum: Lewis Structures
- Topic: Identifying Lewis Acids and Bases
- Replies: 8
- Views: 505
Re: Identifying Lewis Acids and Bases
Lewis bases donate electron pairs, which means that they are more likely to have a lone pair of electrons, such as NH3 or OH. In most cases, anions are Lewis bases. Lewis acids accept electron pairs, and I believe that in most cases, cations are Lewis acids, such as H+.
- Sat Nov 21, 2020 2:53 pm
- Forum: Trends in The Periodic Table
- Topic: Book Problem 1F3
- Replies: 5
- Views: 527
Re: Book Problem 1F3
We know that the ionic radius follows the same trends as the atomic radius: the ionic radius decreases across a period and increases down a group. Since these ions are isoelectronic, we have to look at the size of each one to determine increasing size. Following the ionic radius trend, we know that ...
- Sat Nov 21, 2020 2:45 pm
- Forum: Ionic & Covalent Bonds
- Topic: Covalent Character
- Replies: 14
- Views: 2425
Re: Covalent Character
I went to Hannah Chew's Step Up session last Tuesday and she was really helpful in explaining covalent character. Basically, the smaller the cation, the greater covalent character and the larger the anion, the greater covalent character as well. I'll explain some of the few examples she had us do! B...
- Sat Nov 21, 2020 2:27 pm
- Forum: General Science Questions
- Topic: bond lengths
- Replies: 10
- Views: 1104
Re: bond lengths
I don't believe that it'll always be that specific in every situation, but I'd just try to always remember that in order of increasing length, bond lengths are: triple bonds < double bonds < single bonds.
- Sat Nov 21, 2020 2:09 pm
- Forum: Student Social/Study Group
- Topic: Study Tips for Final Exam
- Replies: 57
- Views: 2964
Re: Study Tips for Final Exam
Attend Step-Up sessions! I went to one this past Tuesday and it was VERY helpful in helping me understand intermolecular forces more. Also, make sure to memorize the conceptual concepts, especially since a lot of the last couple of outlines have been conceptual. I'd suggest redoing practice problems...
- Sun Nov 15, 2020 1:40 pm
- Forum: Coordinate Covalent Bonds
- Topic: Sapling Week 5/6 #13
- Replies: 5
- Views: 343
Re: Sapling Week 5/6 #13
There are 8. In addition to the 4 Hydrogen bonds that can be formed from the hydrogen atoms bonded to nitrogen, nitrogen has one pair of unpaired electrons and can form a hydrogen bond from that pair. Because there are two nitrogen atoms, 2 hydrogen bonds can be formed. Oxygen has two pairs of unpai...
- Sun Nov 15, 2020 1:34 pm
- Forum: Dipole Moments
- Topic: London Dispersion forces
- Replies: 14
- Views: 598
Re: London Dispersion forces
I believe that all molecules have london dispersion forces. The strength of these forces increase with the number of electrons (larger molecules have stronger forces).
- Sat Nov 14, 2020 11:57 am
- Forum: Student Social/Study Group
- Topic: Midterm 2
- Replies: 22
- Views: 879
Re: Midterm 2
I am going to be focusing on textbook problems and making sure I can independently do each problem without looking at my notes. I also plan on doing the same for Sapling. Good luck!
- Sat Nov 14, 2020 11:50 am
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Polarizability
- Replies: 9
- Views: 751
Re: Polarizability
Veronica Macias 3C wrote:Does polarizability have anything to do with electronegativity?
Polarizability decreases as an atom / ion gets smaller and more electronegative. So when you are looking at a periodic table, it increases down a group and decreases across a period, which is the opposite trend of electronegativity.
- Fri Nov 13, 2020 4:07 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Textbook 3F.3
- Replies: 2
- Views: 165
Textbook 3F.3
For which of the following molecules will dipole-dipole interactions be important: (a) CH4; (b) CH3Cl; (c) CH2Cl2; (d) CHCl3; (e) CCl4?
How can we figure out whether or not a molecule has dipole-dipole interactions? I understand that (a) and (e) are no, but how can we identify the other three?
How can we figure out whether or not a molecule has dipole-dipole interactions? I understand that (a) and (e) are no, but how can we identify the other three?
- Fri Nov 13, 2020 10:33 am
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Polarizable vs. Polarizability
- Replies: 5
- Views: 517
Re: Polarizable vs. Polarizability
According to the textbook, highly polarizable atoms and ions are usually large anions, because there would be a greater distortion on its electron cloud. So, polarizability would follow the opposite trends as electronegativity. Polarizability is more defined as how easily an atom's electron cloud is...

